Abstract
Hydrogen permeation membranes are a key element in improving the energy conversion efficiency and decreasing the greenhouse gas emissions from energy generation. The scientific community faces the challenge of identifying and optimizing stable and effective ceramic materials for H2 separation membranes at elevated temperature (400–800 °C) for industrial separations and intensified catalytic reactors. As such, composite materials with nominal composition BaCe0.8Eu0.2O3-δ:Ce0.8Y0.2O2-δ revealed unprecedented H2 permeation levels of 0.4 to 0.61 mL·min−1·cm−2 at 700 °C measured on 500 μm-thick-specimen. A detailed structural and phase study revealed single phase perovskite and fluorite starting materials synthesized via the conventional ceramic route. Strong tendency of Eu to migrate from the perovskite to the fluorite phase was observed at sintering temperature, leading to significant Eu depletion of the proton conducing BaCe0.8Eu0.2O3-δ phase. Composite microstructure was examined prior and after a variety of functional tests, including electrical conductivity, H2-permeation and stability in CO2 containing atmospheres at elevated temperatures, revealing stable material without morphological and structural changes, with segregation-free interfaces and no further diffusive effects between the constituting phases. In this context, dual phase material based on BaCe0.8Eu0.2O3-δ:Ce0.8Y0.2O2-δ represents a very promising candidate for H2 separating membrane in energy- and environmentally-related applications.
Similar content being viewed by others
Introduction
Hydrogen permeation membranes are a key element to reach high energy conversion efficiency and decreasing the greenhouse gas emissions from power generation and energy-intensive industries, i.e. by capturing and utilizing CO2 or moving towards hydrogen-based systems by extracting highly pure H2 from gas mixtures1,2,3. In this context, the integration of high performance H2-permeation ceramic membranes with competitive manufacturing cost and life time (>3 years) in industrial processes can significantly intensify them by (i) maximizing products yield and energy efficiency and (ii) promoting the reaction rates4. Such integrative membrane approach of in situ H2 extraction (used as fuel) or consumption (as raw material for chemical production) in a proton conductor-based membrane reactor would shift the thermodynamic equilibrium towards the product side, hence boosting process efficiency, saving energy and reducing final product cost- all factors of high environmental and economic impact. Proton conducting ceramic membranes may therefore be of interest for integrated gasification process5,6, and more specifically in water gas shift reactors at elevated temperatures (600–900 °C)7, in catalytic membrane reactors (CMRs) for accomplishing chemical and petro-chemical reactions (e.g. ammonia synthesis8,9, non-oxidative de-hydrogenation reaction10).
Although every specific application field sets particular requirements in terms of membrane stability, as a general rule, a good ceramic candidate has to remain phase and chemically stable at elevated temperatures in atmospheres possibly containing H2, H2O, CO, CO2, CH4, SOx, H2S, and different levels of ash. Such atmospheres represent extreme operating environments for the majority of H2 selective material classes, leading to material decomposition, membrane disintegration and performance degradation. Specifically, formation of undesired phases (carbonates, hydrates, sulfates) is critical for ceramic based membranes, while hydrogen-induced embrittlement and sulfur poisoning are particularly critical for Pd-based membranes7,11.
Properties like high selectivity for H2, significant durability, mechanical and hydrothermal stability in reducing environments, as well as operating temperature ranges above 500 °C, make dense ceramic mixed proton-electronic conductors a promising alternative to precious metals and related alloys (Ag-Pd)12, polymers13, metal-organic frameworks (MOFs)14,15, zeolite16 or other ceramic microporous materials17,18.
Apart from the stability issues which emerge to be solved, performance target for H2-flux of 1–2 mLn·min−1·cm−2 at 600–700 °C has been set for H2-separation dense ceramic materials (based on the technical targets for dense metallic membranes for H2 separation and purification). However, these values have not been yet achieved.
In the context of above, scientific community still faces the challenge of identifying highly performing and at the same time stable mixed proton-electronic conducting ceramic materials and to explore and utilize them as membranes in a number of chemical reactions and H2 separation tasks at elevated temperatures.
In search for promising candidates for H2 separation applications, electrical and proton transport properties of large number of oxide ceramics from several structural classes have been explored with particular intensity in the last years. Hence, a wide range of state-of-the art perovskite-based proton conductors19,20 to materials with less trivial structures and relatively novel to H2 extraction application, as defective fluorites21,22,23,24,25,26, pyrochlores27, fergusonites28,29,30,31,32 has been covered. A comprehensive review on the electrical properties of a number of proton conductors could be found in ref. 33, while Table 1 presents a comparison of thickness-normalized H2 permeation flux values from literature. As it could be inferred from the table, H2-permeation rates still remain lower than the milestone value at the target temperature.
The largest number of studies is however dedicated to the perovskite-based oxide ceramic materials, in particular with large lattice constant of the type SrCeO3, BaCeO3 and BaZrO3 (Table 1). Substituted BaCeO3 exhibits the highest levels of proton thermodynamic stability in the crystal structure remaining fully hydrated up to temperatures of about 600 °C but it is unstable in acidic gas environments due to its alkaline character19,66,67,68.
Apart from their good proton conduction, perovskite-based materials e.g. of the type BaCe1-xMxO3-δ exhibit also electronic conduction upon doping/substitution with M-cations with mixed valence40,42,69. However, the levels of electronic conduction are significantly low relative to the ionic conduction. Indeed, a raise in the level of electronic conductivity can be pursued by adding a second electronically conducting crystalline phase to the pristine proton conductor. As reported by Elangovan et al.61, a successful approach to obtain H2-permeating material with high performance, is to combine (i) a protonic conducting perovskite phase BaCe1-xMxO3-δ and (ii) an electronic conducting fluorite phase Ce1-yMyO2-δ (M is metal dopant) into the so called dual-phase ceramic material, which at the end possesses increased ambipolar conductivity. The two crystalline phases form the cer-cer composite with certain degree of percolation, which provides efficient pathways for proton and electronic transport across the membrane. They are furthermore in a close contact at high temperature and reducing environments, therefore sufficient chemical and thermal compatibility between them is required. Elangovan et al.61, Ricote et al.70, Medvedev et al.71 and Rebollo et al.63 have shown the chemical compatibility and stability of systems consisting of BaCe1-xLnxO3-δ:Ce1-xLnxO2-δ (x = 0.1–0.2) and BaCe0.65Zr0.2Y0.15O3-δ:Ce0.85Ln0.15O2-δ (Ln = Y, Gd), while Huang et al.72 reported the appearance of minor additional phase in the grain boundaries when Zr is doped in the perovskite phase. Furthermore, the addition of the doped ceria phase would enhance the stability of otherwise easily attacked by CO2 and H2O-containing environments BaCeO3 phase due to a shift in thermodynamic equilibrium towards the reactant side73. This leads to overall suppression of BaCeO3 decomposition to BaCO3, Ba(OH)2 and CeO2.
In the present work composite ceramic membrane material with nominal composition BaCe0.8Eu0.2O3-δ:Ce0.8Y0.2O2-δ (labeled BCEO:CYO) at 50:50 vol.% ratio was explored as H2 permeation membrane in the temperature range 600–700 °C. This ratio was selected to ensure the largest degree of interaction between the two phases and sufficient transport pathways both for protons and electrons. In the context of above, acceptor substitution with 20 mol. % Eu3+ in BaCeO3 and the same amount of Y3+ in CeO2 was undertaken as a strategy to pursue an improvement and fine-tuning of transport properties of the composite ceramic material.
For the selection of the two substituents several factors were accounted. BaCeO3 doped by Gd from rare earths (RE) in addition to Y, exhibits the highest ionic (protonic) conductivity among solid solutions BaCe1-xRExO3-δ74,75,76. Eu3+ (4f6) and Gd3+ (4f7) have the same valence state, nearly the same ionic radii and good geometrical compatibility with Ce as B-site ion in Ba-cerate. The ionic radius increases in the row Gd→Eu→Sm and decreased protonic conductivity has been found for Sm due to the increased lattice distortion. However, similar lattice distortions and conductivities are expected to be produced by Eu3+ and Gd3+ in BaCeO3. As demonstrated elsewhere77, slightly higher values of the total conductivity in H2 environments were determined for BaCe0.85Gd0.15O3-δ in comparison with BaCe0.85Eu0.15O3-δ. Furthermore, there was practically no difference in conductivities under H2/N2 and air atmospheres measured for BaCe0.85Eu0.15O3-δ, while for BaCe0.85Tb0.15O3-δ largely higher values were recorded under reducing environments. According to Radojkovic78, Eu3+ substitution in BaCeO3 leads to clear advantages over that with Y3+ or Gd3+ reported as the best proton conductors. One may highlight (i) the lower sintering temperature (reported to be below 1500 °C); (ii) the larger conductivity under wet H2 conditions (1.21·10−2 S·cm−1 at 600 °C) due to the larger unit cell volume and lattice distortion favoring intra-octahedral proton migration of lower activation energy; (iii) improved grain boundary conductivity ascribed to a decrease in band gap energy related to the transition between O 2p valence band to Ce 4f conduction band; and (iv) dominating proton conductivity under OCV conditions at 650 °C and 700 °C.
On the other hand, in reducing atmospheres, Y-substituted CeO2 exhibits predominantly electronic conduction via small polaron hopping mechanism due to Ce4+/3+ variable valence. Besides, detailed structural and phase characterization via Rietveld refinement on XRD will help to evaluate the phase formation and phase stability of the cer-cer composite supported by microstructural study via SEM and HR-TEM. Electrical conductivity properties of the composite material and its constituting phases were studied to elucidate the relative contributions of each phase in the composite. In addition, hydrogen permeation of the composite membrane with thickness of 500 μm was characterized as a function of the temperature, the hydration conditions and the hydrogen concentration in the feed. Membrane stability in CO2-containing atmospheres was studied in situ with H2 permeation measurements to conclude on eventual phase, microstructural and performance degradation effects.
Results and Discussion
Structural and phase composition study
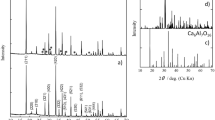
Figure 1 shows the XRD patterns of BCEO and CYO synthesized via the conventional solid-state route at 1400 °C, and the composite pattern resulting from mixing them in a 50:50 vol.% ratio and sinter for 10 h at 1600 °C. Rietveld refinement on the XRD performed on the BCEO and CYO separately showed that the single phases were not formed after 6 h of calcination. XRD patterns revealed a mixture of tetragonal and orthorhombic perovskite phases for BCEO and two fluorite phases with different Y content (details are summarized in the Table 2). An extra thermal treatment of 20 h (in total 26 h) allows single phase samples with sharp defined peaks to be obtained. Besides, once the pressed pellet is sintered at 1600 °C, the observed splitting of peaks disappear and XRD reveals that a single BCEO perovskite phase and a single CYO fluorite were formed. The presence of other oxides was not observed.
As it can be observed from Table 2, the lattice parameters of the two phases constituting the dual phase ceramics disclosed certain deviation from the nominal stoichiometry. The ceria phase shows a cubic fluorite structure with a lattice parameter of 5.411 (1) Å, clearly shifted from the theoretical value of 5.405 Å estimated by Kim’s empirical formula79 for 20 mol.% yttria substitution in CeO2 (and from the 5.405 Å obtained experimentally for the single doped ceria phase). The calculation took into account the ionic radii in 8-fold coordinated Ce4+ and Y3+ cations, which are 0.97 and 1.019 Å, respectively. Since the ionic radii of Eu3+ is 1.066 Å, the substitution of Ce4+ cations by Eu3+ is suggested. By using the experimental cell parameter obtained for the fluorite phase, Kim’s formula can be reversely used to estimate to which extent Eu migrated to the ceria phase. As there are not yttrium related impurities, the yttrium content in the fluorite was fixed to 20 mol.%, which gives a value of x = 10.5 mol.% for Eu3+ and corresponds to stoichiometry Ce0.695Y0.2Eu0.105O2-δ. This value indicates that practically all the Eu introduced by the perovskite phase has migrated to the fluorite phase. The mass balance by ICP-OES (Supplementary information, Table S1) indicates that the barium was not deficient after the sintering (see Experimental Section, Sample preparation paragraph). The unit cell volumes of pure BaCeO3, BaCe0.9Eu0.1O2.95 according to78, BaCe0.8Eu0.2O2.9 after 26 h at 1400 °C and for Ba-cerate phase in the composite material BaCe0.8Eu0.2O2.9:Ce0.8Y0.2O1.9 after 10h at 1600 °C are respectively: 340 Å3, 341.79 Å3, 342.4 Å3 and 340.73 Å3, showing that (i) with increasing the Eu amount the volume expands as expected; and (ii) Ba-cerate in the composite is practically Eu-depleted.
Microstructural characterization
FE-SEM analysis of the polished cross-section of the BCEO:CYO membrane revealed a high relative density (>98%), although some occluded porosity is still present (Fig. 2a–f). The thermal expansion coefficient (TEC) of the composite material in air (12.4·10−6 K−1) shows identical behavior during heating and cooling cycles in air (Supplementary information, Figure S2). No cracks related to the TEC thermochemical cycles were detected due to the good thermal compatibility of the two ceramic components. BSE-SEM (Fig. 2c–e) and EDS analysis (Fig. 2f) show dark grains corresponding to the CYO phase, as well as a bright phase corresponding to BCEO. In addition, Fig. 2c,f shows the EDS linescan analysis of a fracture cross-section of the membrane and the corresponding Ba and Y concentration profiles. Neither Ba (in Fig. 2f the blue line) nor Y (in Fig. 2f the red line) interdiffusion between both phases occurs. The topography and distinct textures observed in the polished cross-section for each phase originates from the difference in hardness, i.e., the dark phase (CeO2) is harder, and this effect was previously reported for BaCeO3:CeO2 composites70.
Further detailed insight in composite microstructure was achieved by the TEM and high resolution scanning transmission electron microscope with high-angle annular dark-field imaging (HRSTEM-HAADF) (Fig. 3). A low-magnification TEM image acquired from the composite sample as-sintered at 1600 oC is shown in Fig. 3a (Composite sample, nominally labeled BCEO:CYO, was actually named BaCeO:CeYEuO in the TEM image after its actual composition with Eu predominantly located in the CYO phase). The sample is composed of randomly oriented, well-connected and packed grains with sizes ranging from 0.5 μm to 2 μm. The grains have a polygonal shape, which minimizes their surface energy. Two types of grains were observed: some grain’s interiors were decorated with dotty/fuzzy contrast, while other grains surface was even. Furthermore, dotty contrast inside grains is sensitive to the grains relative orientation with respect to the electron beam. The slight tilt of the grain could enhance or diminish the dotty contrast significantly (Fig. 3b,c). HR-TEM investigation does not reveal any interface phases at the grain boundaries (not shown). STEM–HAADF images acquired using strong elemental contrast (Z-contrast) conditions are shown in Fig. 3d. The dotty/fuzzy contrast is evidenced under such imaging conditions as well. EDX analysis (Fig. 3d,e) revealed that dotty/fuzzy contrast rich grains are composed of Ce, Y, Eu and O elements, while other grains consist of Ba, Ce and O. The dotty pattern contrast might be attributed to Eu presence in CYO grains, which is associated with high point-defect density caused by incorporation of Eu into this phase. Furthermore, no migration of Y from the fluorite to the perovskite could be evidenced (Fig. 3(e)). HR-STEM-HAADF images recorded from the two grain types in range of crystallographic projections exhibit single crystal structure as shown in Fig. 3f. Dotty contrast is absent in HRSTEM-HAADF imaged for CYO (Ce-Y-Eu-O) grain due to channeling contrast mechanisms dominant at those imaging conditions. By correlating selected area electron diffraction (SAED) and HRSTEM-HAADF images with crystal structure data, the CYO (actually CYEuO) grains showed Fm-3m space group, while BCEO (actually BCO) grains exhibit Pmcn space group crystal structure, both in agreement with the Rietveld refinement made on the XRD patterns. As a summary, the microstructural analysis confirms that Eu diffused from the BCEO perovskite to the CYO fluorite when exposed to high temperature, as previously shown in the XRD section with no evidence for Y diffusion from the fluorite to the perovskite.
(a) Overview TEM image acquired from BCEO:CYO sample sintered at 1600 °C for 10 h; (b)-(c) shows the grain contrast dependence with distinct sample orientation (tilt); (d) Overview STEM-HAADF acquired from BCEO:CYO samples together with corresponding elemental EDX areal maps; (e) integrated EDX spectrum from the CYO and BCEO grains; and (f) High-resolution STEM-HAADF images acquired from CYO (Ce-Y-Eu-O) grain (left column) and BCEO (Ba-Ce-O) grain (right column) in the BCEO:CYO dual phase sample utilizing different crystallographic projections.
Since the composition and microstructure of both phases is pure, without grain boundary segregations electrical and electrochemical study was performed in order to evaluate the transport properties of this dual-phase material.
Electrochemical characterization
Figure 4 displays the total conductivity corresponding to the single phase materials BaCe0.8Eu0.2O3-δ (BCEO) and Ce0.8Y0.2O2-δ (CYO), and the composite material BCEO:CYO with nominal composition BaCe0.8Eu0.2O3-δ:Ce0.8Y0.2O2-δ. The samples have been measured as a function of inverse temperature under H2, H2+H2O, D2 and D2+D2O atmospheres. Within the studied range of temperatures (from 800 °C to 400 °C), BCEO exhibits mainly protonic conductivity, ascertained from the H/D isotopic and hydration effect, i.e. lower conductivity in D2 than in H2 atmospheres and higher conductivity in wet than in dry atmospheres. Above 750 °C, the different conductivity curves converge due to the progressive oxide dehydration with temperature, leading to significant drop in proton concentration. On the other hand, CYO possesses higher conductivity in dry atmospheres than in wet conditions (wet atmospheres signify less reducing conditions than dry atmospheres) suggesting that electronic transport prevails under these conditions. Finally, BCEO:CYO composite shows prevailing n-type conduction behaviour and conductivity values similar to CYO, indicating proper percolation of this electron conducting component, as previously suggested by the SEM analysis.
In order to disclose the predominant transport of these compounds, pO2 effect on the conductivity was also studied under wet reducing conditions (Fig. 5). The BCEO behavior as a prevailing proton conductor deduced from the observed H/D isotopic effect is confirmed by the relationship σ∝pO20 in all studied temperatures i.e., the conductivity is independent on the pO2 values. On the other hand, CYO exhibits predominant n-type electronic conduction following a power law of σ∝pO2−1/4 as it has been reported previously for CYO material80,81. Finally, from the relationship σ∝pO2−1/6 observed for the composite BCEO:CYO, it can be assumed an intermediate behaviour, i.e. it is mainly n-type electronic conductor but presents a significant ionic contribution, arising from the physical mixture of the two different conductors.
Temperature programmed reduction (TPR) measurements (Figure S3 in Supplementary Information) were performed in order to study the redox behavior of the single compounds and the composite, and to correlate with the conductivity data. The CYO sample shows the maximum of its reduction peak at 725 °C, ascribed to the reducible bulk Ce4+. This reduction temperature agrees with previous studies on doped CeO282. Regarding BCEO, a broad reduction peak is observed between 300–700 °C, which can be attributed to the Ce4+ reduction and some oxygen release. The reduction of Eu3+ to Eu2+ is not expected in the considered temperature range as it was observed previously for Eu doped NdWO compound83. Finally, the Ce4+ reduction in the sintered BCEO:CYO samples is shifted to higher temperatures presenting its maximum at 800 °C. The lower reducibility of Ce4+ in this sample could be ascribed to the different stoichiometry of the BCEO and doped ceria grains as compared with the separate single material, as this is previously stated for instance in heavily doped cerias84.
Hydrogen permeation measurements
Permeation properties were thoroughly studied by analyzing the influence of the environment humidification and temperature on the H2 permeation. Three different configurations, depicted in Fig. 6a, were selected in order to study the effect of humidification: (A) only feed side humidified, (B) both membrane sides humidified and (C) only sweep side humidified. 50 vol.% H2 in He was employed as feed gas and Ar was used as sweep gas. Figure 6b plots the H2 permeation behavior through the composite membrane as a function of the temperature in the three configurations. When only the feed side is humidified (configuration A), H2 flow is below 0.05 mL·min−1·cm−2 due to the low concentration of protons as a result of the large pH2O gradient across the membrane. When both sides are humidified (configuration B), the obtained H2 flow is one order of magnitude higher than in configuration A (0.4 mL·min−1·cm−2 at 700 °C). This important increase is attributed to the faster proton transport through the membrane, as a consequence of the higher hydration of the material and hence the higher concentration of protonic defects63. In addition to proton transport, H2 is also generated at the sweep side by water splitting reaction mediated by the oxygen ion diffusion from the high pO2 side (Ar sweep) to the lower pO2 side (H2 feed). The oxygen transport takes place preferentially through the CYO phase, since this phase presents significant oxygen ion conductivity at elevated temperatures80, although it is still one order of magnitude lower than the electronic conductivity in reducing conditions. Finally, for humidified sweep gas (configuration C), the H2 flow increases further, reaching values of 0.61 mL·min−1·cm−2 at 700 °C. This increase in H2 production stems from the higher magnitude of water splitting process since a higher pO2 gradient is imposed across the membrane as compared to configuration B.
H2 permeation was also studied as a function of the hydrogen concentration (pH2) in the feed side (Fig. 6c,d). Irrespective of the humidification conditions, H2 flow rises with increasing pH2 as it is postulated by the Wagner equation, which describes the transport of both protons and oxygen ions. Furthermore, H2 permeation was investigated by using lower pH2O at the sweep side, pH2O = 0.0094 atm instead of 0.042 atm. Results are shown in Fig. 7a–c. H2 flows obtained at 700 °C sweeping pH2O = 0.042 atm are 19% and 8% higher than the values obtained for pH2O = 0.0094 atm, in configurations B and C, respectively (Fig. 7a). Assuming that the partial conductivities are constant in the studied pO2 and pH2O ranges, and that H2 transport can be described by Wagner’s, the H2 flow could be expressed as it is indicated in eq. 1, where the first term corresponds to the H2 permeating through the membrane and the second one responds to the H2 produced by water splitting.

With pH2O = 0.042 atm,  is 1.285 and 1.121 times higher than the corresponding to pH2O = 0.0094 atm in configuration B and C, respectively. On the other hand, the variation in the term
is 1.285 and 1.121 times higher than the corresponding to pH2O = 0.0094 atm in configuration B and C, respectively. On the other hand, the variation in the term by changing the pH2O is almost negligible. From these results, it could be concluded that an important contribution to the H2 flow observed comes from the water splitting and the changes observed when pH2O decreases are mainly ascribed to the reduction of the oxygen transport through the membrane.
by changing the pH2O is almost negligible. From these results, it could be concluded that an important contribution to the H2 flow observed comes from the water splitting and the changes observed when pH2O decreases are mainly ascribed to the reduction of the oxygen transport through the membrane.
Step-changes from configurations C to A were monitored for both conditions (Fig. 7b,c). Hydrogen fluxes decrease changing from configuration C to B and A for pH2O = 0.042 atm (Fig. 7b) and they increase changing from configuration A to B and C for pH2O = 0.0094 atm (Fig. 7c). The quick response for both conditions is mainly related to the important changes in H2 production by water splitting11.
In the light of the previous reports on similar cercer composites63,64,85, the effect of Pt layer applied on the surface of the tested membrane on water splitting reaction must be also taken into consideration. According to62, no contribution of water splitting to the H2 permeation could be evidenced at lower water vapor pressure at the sweep side due to omitting Pt catalyst layer. However, cercer composites were just recently identified as promising candidates for H2 permeation membranes, therefore detailed studies have not been yet done to assign different reaction contributions to the overall H2 flux.
Stability tests
The assessment of the H2 permeation stability under CO2 atmospheres was carried out at 700 °C during 6 days by using 15 vol.% CO2 in Ar as sweep gas, 50 vol.% H2 in He as feed gas and humidifying both sides of the membrane (configuration B). Figure 8a shows that the H2 flow obtained under CO2 containing atmosphere is significantly lower than that obtained by using pure Ar, around 0.15 mL·min−1·cm−2. This drop in the H2 flux by using CO2 could be ascribed to the CO2/H2 and/or CO2/O2 competitive adsorption on the membrane surface11,63 that slows down the gas exchange. Besides, the flux equilibration in these conditions takes more than 2 days.
TG analysis was performed under 5 vol.% CO2 balanced with Ar by using crushed samples sintered at 1600 °C. Figure 8b indicates that CYO sample does not suffer any CO2 uptake under CO2 containing atmospheres within the studied temperature range. On the other hand, a mass gain is observed for BCEO sample above 500 °C, which is ascribed to the carbonation of the sample37,66,73. However, the mass of the composite BCEO:CYO does not increase indicating that no carbonation process is taking place. It can be concluded that the addition of ceria to the cerate allows shifting the carbonation equilibrium and consequently improves the stability of the composite.
The structural stability of BCEO:CYO membrane after 13 days on stream (total duration of the permeation measurements, including CO2 stability measurement) was verified by XRD (Fig. 9a) and FE-SEM (Fig. 9b–e) analysis. Figure 9a depicts the XRD patterns recorded on dual-phase BCEO:CYO sintered samples before permeation (labeled BP-1600/10 h) and after the H2-permeation tests (labeled AP). Both the feed and sweep sides of the membrane were investigated to detect any phase changes or formation of carbonates (denoted with AP-F and AP-S for feed and sweep side of the membrane, respectively). As reference, the peak positions and related intensities for BaCeO3 and Ce0.8Y0.2O1.9 are presented at the bottom of the figure. Up to the analytical limits of the XRD device, no changes in the structure were found and both fluorite and perovskite phases are well distinguished. The peaks marked by a diamond correspond to Pt traces from the catalytic layer. The XRD pattern of the sweep side of the membrane after permeation shows broader peaks, which could indicate some modification in the surface microstructure.
(a) Diffraction patterns for BCEO:CYO dual phase ceramic samples: as-sintered sample (BP-1600/10 h) and sample after H2-permeation tests (feed side AP-F and sweep sides AP-S). Peak positions corresponding to Pt traces from the catalytic layer are denoted by symbol (♦). As a reference (bottom): peak positions and their intensities for BaCeO3 and Ce0.8Y0.2O1.9. (b) SEM and (c,d) BSE-SEM micrographs and (e) EDS linescan analysis of the fractured cross-section of the BCEO:CYO membrane after the permeation measurements.
Figure 9b–e presents the FE-SEM (b), and the BSE-SEM (c,d) micrographs and EDS linescan (e) analysis of the membrane after the permeation measurements (note that the membrane is the same specimen for all the H2 permeation measurements described in the work). Neither morphological nor structural changes were detected.
Finally, stability of dual phase composite material was checked after running the sintered membranes in continuous electrical tests for 670 h under 4% H2-containing dry reducing conditions, including several cycling steps from room temperature up to 900 °C and 1000 °C with holding times at these temperatures of 48 h and 20 h, respectively. TEM investigation of a reference sample and of post-treatment specimens is shown in Figure S4 in Supplementary Information. No morphological differences between the reference and post-treatment membranes and no grain boundary segregations were detected resulting from the 670 h continuous operation under reducing conditions.
Conclusions
We have presented a dual phase material based on perovskite and fluorite exhibiting both high electronic and H+/O−2 co-ionic conductivity. Nominal BaCe0.8Eu0.2O3-δ (BCEO) and Ce0.8Y0.2O2-δ (CYO) were mixed in a 50:50 vol.% ratio. Eu3+ cations originally present in BCEO diffused to CYO during the sintering step (Ce0.695Y0.2Eu0.105O2-δ: CYEuO) as revealed by TEM analysis. DC-conductivity using a deuterium tracer showed that the composite is mixed protonic-electronic conductor (MPEC). Indeed, BCEO behaves as a prevailing proton conductor and CYO (CYEuO) as a mixed oxygen ionic and electronic conductor.
H2 permeation through a thick planar BCEO:CYO membrane was measured in different gas hydration configuration. The reached H2 fluxes are among the highest reported for ceramic membranes up to date 0.4 and 0.61 mL·min−1·cm−2 at 700 °C under configuration B and C, respectively (or thickness-normalized values of 0.02 and 0.0305 mL·min−1·cm−1 at 700 °C under configuration B and C, respectively). This remarkably high permeability originates from the transport of protons as well as from the hydrogen generated by water splitting on the permeate side (the high oxygen partial pressure side) of the membrane due to the oxygen ion transport in ceria. The membrane material was stable after 670 h continuous operation under 4% H2-containing dry reducing conditions, as well as in 15% CO2 containing atmosphere, although the resulting flux was lower than in pure Ar due to the competitive adsorption in the surface between H2/O2 and CO2. The stability in CO2 is tentatively attributed to the protective effect of the ceria phase over the cerate phase.
Experimental Section
Sample preparation
Materials with nominal composition BaCe0.8Eu0.2O3-δ (BCEO) and Ce0.8Y0.2O2-δ (CYO) were synthesized via the conventional solid-state route. Stoichiometric amounts of high-purity BaCO3 and CeO2, Eu2O3, Y2O3 (Sigma Aldrich) were weighted, mixed and ball-milled in ethanol. The solid-state synthesis for both compounds took place at 1400 °C for 6 h in air. A second calcination step (20 h) was required for the complete formation of the perovskite single phase, and resulting products were milled in ethanol. After drying, the two powder products were mixed in 50:50 vol.% ratio and were homogenized 24 h in ethanol, dried and sieved. Uniaxially pressed samples were sintered at 1600 °C for 10 h leading to formation of dual phase BCEO:CYO material with nominal stoichiometry BaCe0.8Eu0.2O3-δ:Ce0.8Y0.2O2-δ. Samples were grinded to remove the defective top layer and no Ba-deficiency or secondary oxide exclusions compensating eventual Ba evaporation were detected by ICP-OES. Relative density of more than 98% was determined via the Archimedes approach on sintered dual phase composite samples.
Characterization techniques
Inductively coupled plasma optical emission spectrometry (ICP-OES) analysis was used to monitor chemical composition of the dual phase ceramics by quantification of the cation content. 50 mg sample powder was mixed with 2 mL HClO4 (NORMATOM®, VWR Chemicals, Germany) and heated for 30 min to fuming. After cooling, 1 mL H2O2 (NORMAPUR) and 1 mL HCl (Merck Suprapur, Germany) were added. Samples were heated at 70 °C for 30 min until complete dissolution of solid content and finally 50 mL volumes were prepared. Samples were measured at dilution 1:20 with a Thermo Scientific iCAP 7600 dual-view spectrometer, each sample was measured twice and the mean result of three emission lines per element was used for quantification. External calibration was performed with standards prepared by dilution of Merck Certipur® certified plasma emission standards with diluted acids. Relative standard deviation is 1–3%.
Powder X-ray diffraction (XRD) patterns of BCEO, CYO and composite BCEO:CYO as prepared and sintered were recorded in the 2 theta range from 10° to 80° using D4 ENDEAVOR diffractometer by Bruker AXS with CuKα radiation (λ = 1.54 Å). XRD patterns of BCEO:CYO membrane after permeation tests were recorded in the 2 theta range from 20° to 90° using PANalytical Cubix fast diffractometer with CuKα1,2 radiation and an X’Celerator detector in Bragg-Brentano geometry. Phase identification was carried out with ICDD PDF2-Database (Release 2004) and X′Pert Highscore Plus (by PANalytical). The TOPAS V4 software (Bruker AXS) was used to determine the lattice parameters and for Rietveld refinements. The stoichiometry of each phase is fixed for the Rietveld refinement and the starting crystal structure model was obtained from the Inorganic Crystal Structure Database (ICSD). The final weighted R-factor (Rwp, %) for each refinement is listed in Table 2.
Microstructural and chemical analyses of BCEO and CYO, as well as of the dual phase samples were performed by means of field emission scanning electron microscopy (FE-SEM) (Zeiss Ultra 55) equipped with energy-dispersive X-ray spectroscopy (EDS) (INCA, Oxford). Transmission electron microscopy (TEM) analysis was carried out using a Tecnai TF 20 UT equipped with EDS and a Gatan’s imaging filter (GIF) operated at 200 kV. High-resolution scanning TEM high angle annular dark field (STEM-HAADF) imaging was performed by using Cs probe corrected FEI-Titan 80–300 STEM instrument operating at 300 kV. Electron-transparent specimens were prepared from as-sintered pellets using the traditional approach which includes ultrasonic drilling, mechanical polishing and Ar+ ion milling at 5 keV.
Thermal expansion coefficient (TEC) of the dual phase ceramics was measured on sintered bar samples with dimensions 25 × 4 × 4 mm3 from 30 to 1400 °C (heating rate 3 °C/min) in air using Netzsch Dil 402C. Linear thermal expansion coefficients α under heating and cooling were calculated using Eq. (2), where L0 and dL are respectively the initial length and the length change of the sample, while T0 and T are the starting and the final temperature, respectively:

Electrical conductivity measurements were carried out by the standard four-point DC technique on sintered bars. Rectangular bar specimens were prepared from the resulting powders through uniaxial pressing at 100 MPa and subsequently sintered at 1600 °C. Silver paste and wire were used for contacting. The constant current was supplied by a programmable current source (Keithley 2601), while the voltage drop through the sample was detected by a multimeter (Keithley 3706). The voltage was measured by the current in both forward and reverse directions, in order to eliminate the thermal effect and to avoid non-ohmic responses. H/D isotopic effect and hydration effect for the separate materials and the dual phase material BCEO:CYO, were studied under reducing conditions: 5 vol.% H2 in He and 5 vol.% D2 in He both dry and moist (H2 and D2 humidified with H2O and D2O at room temperature, respectively). The influence of the pO2 on total conductivity was also evaluated under wet reducing atmospheres by using: wet 0.05 vol.%, 5 vol.% and 100 vol.% H2 and wet 5 vol.% D2.
Temperature-programmed reduction (TPR) was performed using 2910 Micromeritics equipment on crushed and ground samples sintered at 1600 °C. Powders were degassed under a dry Ar flow for 1 h and then were subjected to reduction under a dry H2/Ar (1/9) flow, and a heating rate of 10 °C·min−1 until 900 °C. H2 consumption was measured by a TCD (thermal conductivity detector).
H2 permeation measurements were performed in a multistep continuous process using a BCEO:CYO dense disk with a diameter of 15 mm and thickness of 500 μm sintered at 1600 °C. Both disk surfaces were coated with a 20 μm screen printed Pt porous layer aiming to improve the surface catalytic activity and evaluate predominantly the bulk H2 transport. Permeation measurements were performed on a sealed sample in double chamber quartz reactor following the methodology reported elsewhere25,86. Three different hydration conditions were evaluated: (A) feed side humidified and sweep side dry; (B) both sides humidified; (C) only sweep side humidified, where humidification means pH2O = 0.042 atm and dry corresponds to pH2O = 2·10−5 atm (bottle dry). Ar was used as sweep gas and a 50 vol.% H2 in He mixture was employed as feed (1.18 atm absolute pressure) under all the mentioned conditions. The flow rates used were 100 mL·min−1 for feed and 150 mL·min−1 for sweep and they were controlled using mass flow controllers (MFCs). The hydrogen content in the permeate side was analyzed using micro-GC Varian CP-4900 equipped with Molsieve5A and PoraPlot-Q glass capillary modules. Sealing was obtained using a silver ring and applying a spring load. Sealing was confirmed by continuous monitoring the He concentration in the permeate stream and it was considered adequate when the helium concentration was lower than 5% of the H2 permeated.
In order to evaluate the stability in operation of the composite under CO2 containing atmospheres, H2 permeation measurements were also performed at 700 °C for 6 days by using 15 vol.% CO2 in Ar as sweep gas. After permeation measurements, integrity of the sample was evaluated by means of XRD and SEM analysis. Scheme of the steps of the hydrogen permeation measurements in a continuous process is given in Supplementary information, Figure S1.
Additionally, TG measurements were carried out on the BCEO, CYO and the composite BCEO:CYO by using crushed samples sintered at 1600 °C. TG was performed in a flow of dry 5% CO2 in Ar from 25 to 1000 °C with a heating ramp of 10 K·min−1 by using a Mettler-Toledo StarE balance.
Additional Information
How to cite this article: Ivanova, M. E. et al. Hydrogen separation through tailored dual phase membranes with nominal composition BaCe0.8Eu0.2O3-δ:Ce0.8Y0.2O2-δ at intermediate temperatures. Sci. Rep. 6, 34773; doi: 10.1038/srep34773 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Meulenberg, W. A., Ivanova, M. E., Serra, J. M. & Roitsch, S. In Advanced Membrane Science and Technology for Sustainable Energy and Environmental Applications. 541–567 (2011).
Sørensen, B. Hydrogen and Fuel Cells. (2012).
Dincer, I. & Zamfirescu, C. Advanced Power Generation Systems (2014).
Drioli, E., Stankiewicz, A. I. & Macedonio, F. Membrane engineering in process intensification-An overview. J. Memb. Sci. 380, 1–8 (2011).
Franz, J. & Scherer, V. Impact of ceramic membranes for CO2 separation on IGCC power plant performance. Energy Procedia 4, 645–652 (2011).
Higman, C. & van der Burgt, M. In Gasification (Second Edition) 91–191 (Gulf Professional Publishing, 2008).
van Holt, D. et al. Ceramic materials for H2 transport membranes applicable for gas separation under coal-gasification-related conditions. J. Eur. Cer. Soc. 34, 2381–2389 (2014).
Stoukides, M. Solid-Electrolyte Membrane Reactors: Current Experience and Future Outlook. Catal. Rev. - Science and Engineering 42, 1–70 (2000).
Marnellos, G., Zisekas, S. & Stoukides, M. Synthesis of ammonia at atmospheric pressure with the use of solid state proton conductors. J. Catal. 193, 80–87 (2000).
Kjølseth, C. & Vestre, P. C. Proton conducting membrane (2011).
Escolastico, S., Solis, C., Scherb, T., Schumacher, G. & Serra, J. M. Hydrogen separation in La5.5WO11.25δ membranes. J. Memb. Sci. 444, 276–284 (2013).
Basile, A., Gallucci, F. & Tosti, S. In Membrane Science and Technology Vol. 13, 255–323 (2008).
Franz, J. & Scherer, V. An evaluation of CO2 and H2 selective polymeric membranes for CO2 separation in IGCC processes. J. Memb. Sci. 359, 173–183 (2010).
Wang, N., Mundstock, A., Liu, Y., Huang, A. & Caro, J. Amine-modified Mg-MOF-74/CPO-27-Mg membrane with enhanced H2/CO2 separation. Chem. Eng. Sci. 124, 27–36 (2015).
Wang, N. et al. Polydopamine-based synthesis of a zeolite imidazolate framework ZIF-100 membrane with high H2/CO2 selectivity. J. Mater. Chem. A 3, 4722–4728 (2015).
Korelskiy, D. et al. Efficient ceramic zeolite membranes for CO2/H2 separation. J. Mater. Chem. A 3, 12500–12506 (2015).
Ngamou, P. H. T. et al. Tailoring the structure and gas permeation properties of silica membranes via binary metal oxides doping. RSC Adv. 5, 82717–82725 (2015).
Van Gestel, T., Sebold, D., Hauler, F., Meulenberg, W. A. & Buchkremer, H.-P. Potentialities of microporous membranes for H2/CO2 separation in future fossil fuel power plants: Evaluation of SiO2, ZrO2, Y2O3–ZrO2 and TiO2–ZrO2 sol–gel membranes. J. Memb. Sci. 359, 64–79 (2010).
Kreuer, K. D. In Annual Review of Materials Research Vol. 33, 333–359 (2003).
Medvedev, D. et al. BaCeO3: Materials development, properties and application. Prog. Mater. Sci. 60, 72–129 (2014).
Shimura, T., Fujimoto, S. & Iwahara, H. Proton conduction in non-perovskite-type oxides at elevated temperatures. Solid State Ionics 143, 117–123 (2001).
Meulenberg, W. A. et al. (US Patent App. 13/810,296, 2011).
Haugsrud, R. Defects and transport properties in Ln6WO12 (Ln = La, Nd, Gd, Er). Solid State Ionics 178, 555–560 (2007).
Seeger, J. et al. Synthesis and Characterization of Nonsubstituted and Substituted Proton-Conducting La6-xWO12-y . Inorg Chem 52, 10375–10386 (2013).
Escolastico, S. et al. Enhanced H2 separation through mixed proton-electron conducting membranes based on La5.5W0.8M0.2O11.25-δ . ChemSusChem 6, 1523–1532 (2013).
Deibert, W., Ivanova, M. E., Meulenberg, W. A., Vaßen, R. & Guillon, O. Preparation and sintering behaviour of La5.4WO12-δ asymmetric membranes with optimised microstructure for hydrogen separation. J. Memb. Sci. 492, 439–451 (2015).
Phair, J. W. & Badwal, S. P. S. Review of proton conductors for hydrogen separation. Ionics 12, 103–115 (2006).
Haugsrud, R. & Norby, T. Proton conduction in rare-earth ortho-niobates and ortho-tantalates. Nat. Mater. 5, 193–196 (2006).
Mather, G. C., Fisher, C. A. J. & Islam, M. S. Defects, dopants, and protons in LaNbO4 . Chem. Mater. 22, 5912–5917 (2010).
Mokkelbost, T. et al. High-temperature proton-conducting lanthanum ortho-niobate-based materials. Part II: Sintering properties and solubility of alkaline earth oxides. J. Amer. Ceram. Soc. 91, 879–886 (2008).
Ivanova, M. E. et al. Functional properties of La0.99X0.01Nb0.99Al0.01O4-δ and La0.99X0.01Nb0.99Ti0.01O4-δ proton conductors where X is an alkaline earth cation. J. Eur. Ceram. Soc. 35, 1239–1253 (2015).
Huse, M., Norby, T. & Haugsrud, R. Proton conductivity in acceptor-doped LaVO4 . J. Electrochem. Soc. 158 (2011).
Ivanova, M. et al. In Doping: Properties, Mechanisms and Applications 221–276 (2013).
Escolastico, S., Vert, V. B. & Serra, J. M. Preparation and Characterization of Nanocrystalline Mixed Proton-Electronic Conducting Materials Based on the System Ln6WO12 . Chem. Mater. 21, 3079–3089 (2009).
Escolastico, S., Solis, C. & Serra, J. M. Hydrogen separation and stability study of ceramic membranes based on the system Nd5LnWO12 . Int J Hydrogen Energ 36, 11946–11954 (2011).
Escolastico, S., Solis, C. & Serra, J. M. Study of hydrogen permeation in (La5/6Nd1/6)5.5WO12-δ membranes. Solid State Ionics 216, 31–35 (2012).
Escolastico, S., Somacescu, S. & Serra, J. M. Tailoring mixed ionic-electronic conduction in H2 permeable membranes based on the system Nd5.5W1-xMoxO11.25-σ . J. Mater. Chem. A 3, 719–731 (2015).
Escolastico, S., Somacescu, S. & Serra, J. M. Solid State Transport and Hydrogen Permeation in the System Nd5.5W1-xRexO11.25-δ . Chem. Mater. 26, 982–992 (2014).
Matsumoto, H. et al. Protonic-electronic mixed conduction and hydrogen permeation in BaCe0.9-xY0.1RuxO3-α . J. Electrochem. Soc. 152 (2005).
Cai, M. et al. Preparation and hydrogen permeation of BaCe0.95Nd0.05O3-δ membranes. J. Memb. Sci. 343, 90–96 (2009).
Escolastico, S. et al. Improvement of transport properties and hydrogen permeation of chemically-stable proton-conducting oxides based on the system BaZr1-x-yYxMyO3-δ . RSC Adv. 2, 4932–4943 (2012).
Cheng, S. G., Gupta, V. K. & Lin, J. Y. S. Synthesis and hydrogen permeation properties of asymmetric proton-conducting ceramic membranes. Solid State Ionics 176, 2653–2662 (2005).
Qi, X. W. & Lin, Y. S. Electrical conduction and hydrogen permeation through mixed proton-electron conducting strontium cerate membranes. Solid State Ionics 130, 149–156 (2000).
Kniep, J. & Lin, Y. S. Effect of Zirconium Doping on Hydrogen Permeation through Strontium Cerate Membranes. Ind. Eng. Chem. Res. 49, 2768–2774 (2010).
Wei, X., Kniep, J. & Lin, Y. S. Hydrogen permeation through terbium doped strontium cerate membranes enabled by presence of reducing gas in the downstream. J. Memb. Sci. 345, 201–206 (2009).
Zhan, S. et al. Preparation and hydrogen permeation of SrCe0.95Y0.05O3-δ asymmetrical membranes. J. Memb. Sci. 340, 241–248 (2009).
Hamakawa, S., Li, L., Li, A. & Iglesia, E. Synthesis and hydrogen permeation properties of membranes based on dense SrCe0.95Yb0.05O3-α thin films. Solid State Ionics 148, 71–81 (2002).
Song, S. J., Wachsman, E. D., Rhodes, J., Dorris, S. E. & Balachandran, U. Hydrogen permeability of SrCe1-xMxO3-δ (x = 0.05, M = Eu, Sm). Solid State Ionics 167, 99–105 (2004).
Liang, J., Mao, L., Li, L. & Yuan, W. Protonic and Electronic Conductivities and Hydrogen Permeation of SrCe0.95-xZrxTm0.05O3-δ (0 < = x < = 0.40) Membrane. Chinese J. Chem.Eng. 18, 506–510 (2010).
Xing, W. et al. Hydrogen permeability of SrCe0.7Zr0.25Ln0.05O3-δ membranes (Ln = Tm and Yb). J. Memb. Sci. 473, 327–332 (2015).
Oh, T., Yoon, H., Li, J. & Wachsman, E. D. Hydrogen permeation through thin supported SrZr0.2Ce0.8-xEuxO3-δ membranes. J. Memb. Sci. 345, 1–4 (2009).
Meng, X. et al. Ni-BaCe0.95Tb0.05O3-δ cermet membranes for hydrogen permeation. J. Memb. Sci. 401, 300–305 (2012).
Kim, H. et al. Microstructural adjustment of Ni–BaCe0.9Y0.1O3−δ cermet membrane for improved hydrogen permeation. Ceram. Int. 40, 4117–4126 (2014).
Song, S. J., Moon, J. H., Lee, T. H., Dorris, S. E. & Balachandran, U. Thickness dependence of hydrogen permeability for Ni-BaCe0.8Y0.2O3-δ . Solid State Ionics 179, 1854–1857 (2008).
Wei, Y. et al. Enhanced stability of Zr-doped Ba(CeTb)O3-δ-Ni cermet membrane for hydrogen separation. Chem. Comm. 51, 11619–11621 (2015).
Zuo, C., Dorris, S. E., Balachandran, U. & Liu, M. Effect of Zr-doping on the chemical stability and hydrogen permeation of the Ni-BaCe0.8Y0.2O3-α mixed protonic-electronic conductor. Chem. Mater. 18, 4647–4650 (2006).
Fang, S., Brinkman, K. S. & Chen, F. Hydrogen permeability and chemical stability of Ni-BaZr0.1Ce0.7Y0.1Yb0.1O3-δ membrane in concentrated H2O and CO2 . J. Memb. Sci. 467, 85–92 (2014).
Fang, S. et al. CO2-Resistant Hydrogen Permeation Membranes Based on Doped Ceria and Nickel. J. Phys. Chem. C 114, 10986–10991 (2010).
Balachandran, U. et al. Dense cermet membranes for hydrogen separation. Sep. Purif. Technol. 121, 54–59 (2014).
Escolastico, S., Solis, C., Kjolseth, C. & Serra, J. M. Outstanding hydrogen permeation through CO2-stable dual-phase ceramic membranes. Energ Environ Sci 7, 3736–3746 (2014).
Elangovan, S., Nair, B. G. & Small, T. A. Ceramic mixed protonic/electronic conducting membranes for hydrogen separation. (2007).
Rosensteel, W. A., Ricote, S. & Sullivan, N. P. Hydrogen permeation through dense BaCe0.8Y0.2O3-δ - Ce0.8Y0.2O2-δ composite-ceramic hydrogen separation membranes. Int J Hydrogen Energ 41, 2598–2606 (2016).
Rebollo, E. et al. Exceptional hydrogen permeation of all-ceramic composite robust membranes based on BaCe0.65Zr0.20Y0.15O3-δ and Y- or Gd-doped ceria. Energ Environ Sci 8, 3675–3686 (2015).
Fish, J. S., Ricote, S., O’Hayre, R. & Bonanos, N. Electrical properties and flux performance of composite ceramic hydrogen separation membranes. J. Mater. Chem. A 3, 5392–5401 (2015).
Unemoto, A. et al. Hydrogen permeability and electrical properties in oxide composites. Solid State Ionics 178, 1663–1667 (2008).
Scholten, M. J., Schoonman, J., Vanmiltenburg, J. C. & Oonk, H. A. J. Synthesis of Strontium and Barium Cerate and Their Reaction with Carbon-Dioxide. Solid State Ionics 61, 83–91 (1993).
Dauter, J., Maffei, N., Bhella, S. S. & Thangadurai, V. Studies on Chemical Stability and Electrical Properties of Proton Conducting Perovskite-Like Doped BaCeO3 . J. Electrochem. Soc. 157, B1413–B1418 (2010).
Brandao, A. et al. Guidelines for improving resistance to CO2 of materials for solid state electrochemical systems. Solid State Ionics 192, 16–20 (2011).
Matsumoto, H., Shimura, T., Yogo, T., Iwahara, H. & Katahira, K. (Google Patents, 2003).
Ricote, S., Manerbino, A., Sullivan, N. P. & Coors, W. G. Preparation of dense mixed electron- and proton-conducting ceramic composite materials using solid-state reactive sintering: BaCe0.8Y0.1M0.1O3−δ–Ce0.8Y0.1M0.1O2−δ (M = Y, Yb, Er, Eu). J Mater Sci 49, 4332–4340 (2014).
Medvedev, D. et al. Structural, thermomechanical and electrical properties of new (1 – x)Ce0.8Nd0.2O2−δ–xBaCe0.8Nd0.2O3−δ composites. J. Power Sources 267, 269–279 (2014).
Huang, J., Zhang, L., Wang, C. & Zhang, P. CYO–BZCYO composites with enhanced proton conductivity: Candidate electrolytes for low-temperature solid oxide fuel cells. Int J Hydrogen Energ 37, 13044–13052 (2012).
Ryu, K. H. & Haile, S. M. Chemical stability and proton conductivity of doped BaCeO3–BaZrO3 solid solutions. Solid State Ionics 125, 355–367 (1999).
Matskevich, N. I. & Wolf, T. A. The enthalpies of formation of BaCe1-xRExO3-δ (RE = Eu, Tb, Gd). J. Chem. Thermodyn. 42, 225–228 (2010).
Amsif, M. et al. Influence of rare-earth doping on the microstructure and conductivity of BaCe0.9Ln0.1O3-δ proton conductors. J. Power Sources 196, 3461–3469 (2011).
Bonanos, N., Ellis, B., Knight, K. S. & Mahmood, M. N. Ionic-conductivity of gadolinium-doped barium cerate perovskites. Solid State Ionics 35, 179–188 (1989).
Wachsman, E. D. & Jiang, N. Ionic conductor useful as hydrogen gas permeation membrane or electrode material comprises a perovskite-type oxide. EP1048613-A1; CA2307005-A1; US2001001379-A1; US6296687-B2; EP1048613-B1; DE60020772-E; DE60020772-T2; CA2307005-C.
Radojkovic, A. et al. Structural and electrical properties of BaCe0.9Eu0.1O2.95 electrolyte for IT-SOFCs. Electrochimica Acta 161, 153–158 (2015).
Kim, D.-J. Lattice Parameters, Ionic Conductivities, and Solubility Limits in Fluorite-Structure MO2 Oxide [M = Hf4+, Zr4+, Ce4+, Th4+, U4+] Solid Solutions. J. Amer. Ceram. Soc. 72, 1415–1421 (1989).
Guan, X., Zhou, H., Wang, Y. & Zhang, J. Preparation and properties of Gd3+ and Y3+ co-doped ceria-based electrolytes for intermediate temperature solid oxide fuel cells. J. Alloy. Compd. 464, 310–316 (2008).
Wang, S. R., Kobayashi, T., Dokiya, M. & Hashimoto, T. Electrical and ionic conductivity of Gd-doped ceria. J. Electrochem. Soc. 147, 3606–3609 (2000).
Balaguer, M., Solis, C., Roitsch, S. & Serra, J. M. Engineering microstructure and redox properties in the mixed conductor Ce0.9Pr0.1O2-δ + Co 2 mol%. Dalton Transactions 43, 4305–4312 (2014).
Escolastico, S., Schroeder, M. & Serra, J. M. Optimization of the mixed protonic-electronic conducting materials based on (Nd5/6Ln1/6)5.5WO11.25-δ . J. Mater. Chem. A 2, 6616–6630 (2014).
Balaguer, M., Solis, C. & Serra, J. M. Structural-Transport Properties Relationships on Ce1-xLnxO2-δ System (Ln = Gd, La, Tb, Pr, Eu, Er, Yb, Nd) and Effect of Cobalt Addition. J. Phys. Chem. C 116, 7975–7982 (2012).
Escolástico, S., Kjølseth, C. & Serra, J. M. Catalytic activation of ceramic H2 membranes for CMR processes. J. Memb. Sci. 517, 57–63 (2016).
Escolastico, S. & Serra, J. M. Nd5.5W1-xUxO11.25-δ system: Electrochemical characterization and hydrogen permeation study. J. Memb. Sci. 489, 112–118 (2015).
Acknowledgements
This work has been conducted with the financial support by the Helmholtz Association under the Research Programme Energy Efficiency, Materials and Resources. Financial funding from the Spanish Government (ENE2014-57651 and SEV-2012-0267 grants) is also gratefully acknowledged. ZEA-3 at FZJ is gratefully acknowledged for performing the ICP-OES analysis.
Author information
Authors and Affiliations
Contributions
M.E.I., W.A.M., O.G., S.E. and J.M.S. designed the project and lead the interpretation and writing of the manuscript. M.E.I. and S.E. performed the electrochemical measurements. J.P. and J.M. carried out the microstructural analysis, Y.J.S. and M.B. contributed to the structural characterization. All authors contributed to the concept and analysis of results, and revised the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ivanova, M., Escolástico, S., Balaguer, M. et al. Hydrogen separation through tailored dual phase membranes with nominal composition BaCe0.8Eu0.2O3-δ:Ce0.8Y0.2O2-δ at intermediate temperatures. Sci Rep 6, 34773 (2016). https://doi.org/10.1038/srep34773
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep34773
This article is cited by
-
Crystal structure investigation of La5.4W1−yMoyO12−δ for gas separation by high-resolution transmission electron microscopy
Scientific Reports (2019)
-
PS-PVD Processing of Single-Phase Lanthanum Tungstate Layers for Hydrogen-Related Applications
Journal of Thermal Spray Technology (2019)
-
Heat capacity by differential scanning calorimetry and thermodynamic functions of BaCe0.8Gd0.1Y0.1O2.9 in the temperature range of 166–790 K
Journal of Thermal Analysis and Calorimetry (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.