Abstract
We performed the biotransformation of ferulic acid to vanillin using Bacillus subtilis (B. subtilis) in the stirring packed-bed reactors filled with carbon fiber textiles (CFT). Scanning electron microscope (SEM), HPLC, qRT-PCR and ATP assay indicated that vanillin biotransformation is tightly related to cell growth, cellar activity and the extent of biofilm formation. The biotransformation was affected by hydraulic retention time (HRT), temperature, initial pH, stirring speed and ferulic acid concentration, and the maximum vanillin production was obtained at 20 h, 35 °C, 9.0, 200 rpm, 1.5 g/L, respectively. Repeated batch biotransformation performed under this optimized condition showed that the maximum productivity (0.047 g/L/h) and molar yield (60.43%) achieved in immobilized cell system were 1.84 and 3.61 folds higher than those achieved in free cell system. Therefore, the stirring reactor packed with CFT carrier biofilm formed by B. subtilis represented a valid biocatalytic system for the production of vanillin.
Similar content being viewed by others
Introduction
Vanillin (4-hydroxy-3-methoxybenzaldehyde), the major compound of vanilla flavor, is widely used in foods, beverages, perfumes, and pharmaceuticals1. It can be produced by many technologies including chemical synthesis, hydrolysis and biotransformation2. Among them, biotransformation is believed to be most promising eco-friendly method because it can meet the rising demand for healthy and natural vanillin1,2. Many efforts have been made to biotransform vanillin using natural abundant substrates including lignin, phenolic stilbenes, isoeugenol, eugenol, ferulic acid, vanillic acid, sugars, aromatic amino acids creosol, vanillyl amine, and waste residues1.
As one of the most excellent precursors, ferulic acid is a nearly ubiquitous, readily available substance and can be naturally released by a combination of physical and enzymatic processing3. Biotransformation of ferulic acid to vanillin have been assessed using various microorganisms, including Rhodococcus ssp., Actinomycetes spp., Corynebacterium glutamicum, Saccharomyces cerevisiae, Rhodotorula rubra, Debaryomyces hanseni, Halomonas elongata, Schizophyllum commune, Bacillus licheniformis, Bacillus coagulans, Bacillus subtilis, Pycnoporus cinnabarinus CGMCC1115, Pseudomonas sp. EF3, Pseudomonas fluorescens, Pseudomonas putida, Escherichia coli JM109/pBB1, Aspergillus niger CGMCC0774, Lactic acid bacteria, Amycolatopsis sp. HR167, Streptomyces sp. V-1, Streptomyces setonii ATCC39116, Streptomyces sannanensis, Streptomyces halstedii GE107678, and genetically engineered microbes1,2,3. However, vanillin yield was low mostly due to the inhibitory effect of vanillin on free microbial cells and/or the genetic instability of the recombinant strains during the transformation2. Additionally, expensive microbial culture, long-time operation, and complicated following treatments would lead to high cost of biotransformation using free microorganisms4.
Immobilization of microbial cells has been attracting worldwide attention for their excellent biological compatibility, high cell concentrations, storage and operational stabilities and the tolerance against harsh conditions4. Among immobilization carriers, CFT are regarded as effective microbial cell attached materials because of their surface-hydrophobicity and porous character5. CFT have been widely applied in the field of biocatalysts and have proved beneficial for successful operation at high substrate concentration and biotransformation of artificial liquid medium6. However, there is a remarkable lack of information about the biotransformation of ferulic acid to vanillin in the stirring reactors packed with CFT.
In the present paper, the production of vanillin from ferulic acid by B. subtilis using stirring fixed-CFT reactor was reported for the first time. SEM, HPLC, qRT-PCR and ATP assay were used to investigate the time course of transformation in relation to biofilm formation, vanillin titer in broth, cell growth and cellar activity during immobilizing, respectively. The influences of various parameters, including HRT, temperature, initial pH, stirring speed, and ferulic acid concentration, on vanillin yield in this type of reactor were investigated. The repeated batch biotransformation using immobilized cells or free cells was also performed.
Results and Discussion
The sequence of biofilm formation on CFT carrier in different time intervals (0, 10, 20, 30 and 40 h) could be clearly seen in SEM images (Fig. 1a–e). It can be observed that the CFT carrier at 0 h has a furrowed surface with interstices, pleads and cavities, quite suitable for colonization and apparently offered a larger surface area (Fig. 1a). After 10 h of continuous operation of the packed bed-stirred fermentor, bacterial cells appeared to penetrate into the CFT carrier as shown in Fig. 1b. The co-existence of immobilized- and suspended-growth profile provided the complex hydrodynamic and substrates uptaking circumstances, resulted in overgrowth of bacteria on the interior and surface of CFT carrier7. Some holes and channels on CFT carrier surface indicated biofilm formation and extracellular polymeric substances (EPS) production8. The EPS play an important role in the immobilization of cells to the carrier, positively correlated with the biofilm development and possibly by protecting the individual cells in the biofilm against detrimental environment9. After 20 h, the biofilm structure became denser and thicker (Fig. 1c). It also can be seen from Fig. 1c that CFT carriers were colonized almost exclusively in crevices and pleats. After 30 h, the pleats and some holes were fully colonized showing a dense biofilm, as can be seen in Fig. 1d. The biofilm showed several clearly different layers. This might be attributable to the presence of an area with a lower number of cells and probably more EPS. SEM were taken after 40 h of operation and showed the CFT carrier was completely covered with biofilm (Fig. 1e).
To understand the time course of transformation in relation to immobilization and biofilm formation, the vanillin concentration in fermented broth, the cell growth and cellular activity in CFT carrier were monitored during immobilizing. The qRT-PCR was able to measure growth of B. subtilis in terms of the 16S rDNA gene number. ATP is present in all living microorganisms as the basic energy molecule, it could be applied to determine cellular activity and measure active biomass10,11. As shown in Fig. 2, the cell numbers of B. subtilis varied in a big range of 7.90 × 106–2.75 × 1011 copies/g. It appears that B. subtilis had a lag phase at beginning of immobilization. B. subtilis attaching to CFT carrier need to acclimate to immobilized medium and carrier niche by incubation for 10 h after inoculation. It can be observered that the amount of B. subtilis rapidly increased from 3.19 × 109 copies/g at 10 h to the maximum value at 30 h, and then decreased to 2.39 × 1011 copies/g (Fig. 2). A similar trend was observed in time course of ATP concentration (Fig. 2). These indicated that B. subtilis successfully immobilized, grown, maintained cellar activity, and formed biofilm on CFT carrier within 40 h. For B. subtilis, the amount of ATP is 1.00 × 10−8–6.00 × 10−8 μg/cell, and the number of 16S rDNA genes per genome is reported to be 9 or probably 10 12. Accordingly, values of cell number obtained by ATP assay were lower when compared to qRT-PCR values. The main reason for this discrepancy was that the qRT-PCR values included potentially extracellular DNA, DNA from viable and non-viable cells, whereas ATP values only contained viable cells.
Concerning vanillin concentration, the value increased from 0 to 0.18 g/L during immobilizing. The increase pattern of vanillin concentration was similar to cell growth and cellar activity before hour 30 (Fig. 2). This suggested that the immobilized cells on CFT carrier could successfully and efficiently transform ferulic acid to vanillin. After hour 30, vanillin concentration maintained stable while cell growth and cellar activity decreased (Fig. 2). This may indicated that, the ferulic acid exhausted and transformed to vanillin within 30 h, leading to termination of vanillin transformation and decline of cell numbers and ATP content. These results suggested that vanillin production is closely related to the growth and activity of cells on CFT carrier biofilm.
Batch experiments were performed at various HRTs (10, 20, 30, and 40 h) to assess the effect of HRT on the vanillin molar yield. Other conditions such as temperature, initial pH, stirring speed and ferulic acid concentration were kept constant at 35 °C, 9.0, 200 rpm and 1.5 g/L, respectively.
Figure 3 shows that the maximal production performance, as peak vanillin molar yield of 58.26% at HRT of 20 h. It can be observed that the vanillin concentration in reactor increased significantly from 10 h HRT (0.36 g/L) to 20 h HRT (0.68 g/L), however, decreased to 0.67 g/L at 30 h HRT. Lowering HRT could lead to a decrease in biotransformation due to the availability of less substrate frequently to microorganisms13. It has been reported that highering HRT could improve the production performance because more substrate was solubilized and consumed under the longer HRTs14. However, it would result in the high cellular toxicity of vanillin and further degradation of vanillin13. It also can be seen form Fig. 3 that the vanillin molar yield declined continually when HRT was over 20 h. This was attributed to the limitation of bacterial growth by the low substrate concentration supplied at higher HRTs. Thus, shortening the HRT to 20 h was sufficient to obtain the highest vanillin molar yield and reduce the product inhibition in biotransformation.
As an important factor in fermentation, temperature has great effect on the bacterial growth, the product generation, and the rheological properties of fermentation broth15. To investigate the influence of temperature on biotransformation, the incubation temperature was varied at 25, 30, 35, and 40 °C. The HRT, initial pH, stirring speed and ferulic acid concentration were fixed at 20 h, 9.0, 200 rpm and 1.5 g/L, respectively.
As illustrated in Fig. 4a, a great difference in the production of vanillin was observed when biotransformation was performed at different temperatures. It can be seen that the production of vanillin was sharply increased with the increase of temperature from 25 to 35 °C. However, further increase of temperature resulted in a sharp decrease in the yield of vanillin. The maximum amount of vanillin production was observed at 35 °C and 0.66 g/L vanillin was produced with the molar yield of 55.83% (Fig. 4a).
As we all know, mesophilic temperature fermentation not only reduce the energy consumption for heating and cooling, but also maintains the stability of substrate16. Generally, Bacillus species have an optimal fermentation temperature range of 30–40 °C to produce products15. Only 0.23 g/L vanillin was produced with the molar yield of 19.13% at 25 °C. The primary reason might be that the exchange of nutrients and the diffusion rate of dissolved gases were greatly retarded at low temperature conditions17. Additionally, low temperature could negatively influence bacterial growth and biofilm formation in CFT carrier. The elevating temperature could significantly improve the vanillin production due to several reasons. Firstly, the escalating temperature can change the physiological activity of cells, impact the EPS production and change the surface charge of cells18,19. These would promote the attachment of cells and the biofilm formation during biotransformation. It has been reported that even a small increase in temperature increases the biofilm thickness significantly20. Secondly, increasing temperature may influence the secretion and activity of enzyme related to vanillin production such as decarboxylase1,15. Lastly, escalations in temperature could increase substrate solubility, improve mass transfer due to increased diffusion rates and declined viscosity15. It has been stated that a higher temperature would lead to a higher collision rate and higher average kinetic energy of molecules20.
It was observed that only 0.56 g/L vanillin was produced with the molar yield of 47.67% at 40 °C (Fig. 4a). This indicated that the production of vanillin from ferulic acid using B. subtilis immobilized on CFT carrier was not always enhanced at elevated temperatures. Previous study also revealed that EPS production and biofilm formation did not take place at extreme temperature but did so at intermediary temperature20.
It is well known that the system pH plays a crucial role in biotransformation. Generally, variation in pH can affect both the ionic state of substrate and enzymes involved in the biochemical reaction21. To verify the effect of pH on the biotransformation, experiments were carried out at pH ranging from 8.0 to 9.5. The HRT, temperature, stirring speed, and ferulic acid concentration were kept at 20 h, 35 °C, 200 rpm, and 1.5 g/L, respectively.
As shown in Fig. 4b, increasing the pH from 8.0 to 8.5 led to enhance the production of vanillin, a maximum molar yield of 54.73% was obtained with 0.64 g/L vanillin at pH of 8.5. Bacillus species can survive in a wide range of pH 6.0–12.0 but they need an alkaline pH of 8.0–10.0 for growth and enzyme secretion22. The negative charge density on the surface of cells would increase with the increase of pH, resulting in a tight cell attachment on CFT carrier23. Additionally, as the pH increased, the solubility of ferulic acid increased and more ferulic acid molecules become negatively charged due to its low pKa of 4.824.
However, as is evident from the figure, pH higher than 8.5 tended to have negative effect on the vanillin production. The vanillin molar yield at pH 8.5 declined to 53.5% at pH 9.0 and 53.1% at pH 9.5, respectively (Fig. 4b). It has been stated that the optimal pH for the activity of protease from B. subtilis was between 8.5 and 9.025. Increasing pH led to the reduction of activity of enzyme involved in biotransformation. Furthermore, high pH usually results in reducing the production of EPS which have an important effect on biofilm formation26. In reactor, biofilm formation on CFT carrier is not an isolated phenomenon, but an equilibrium process between the planktonic and sessile states of bacteria27. Differences in the physicochemical properties of planktonic cells at different pH levels would change their cell wall composition, which could have resulted in negative effect on the equilibrium biofilm thickness. Moreover, the increase in electrostatic repulsion between the cell and ferulic acid due to the increase of pH might lead to the decrease of vanillin production.
Oxygen is an indispensable raw material in aerobic fermentation. Oxygen levels could affect the transcription and/or synthesis of different enzymes, resulting in the changes of cell metabolism, product yield and productivity28. The supply of oxygen can be achieved by means of mechanical stirring. Generally, aerobes including B. subtilis require large amounts of oxygen for NAD(P)H or FADH2 reoxidization and ATP generation during cell growth and proliferation29. It has been stated that stirring speed was an important factor for B. subtilis and its products production in bioreactor30. In order to determine the effect of stirring speed on biotransformation, the reactor was operated at a HRT of 20 h with temperature of 35 °C, initial pH of 9.0, and initial ferulic acid concentration of 1.5 g/L. The stirring speed was varied from 150 to 300 rpm.
It can be seen form Fig. 5a that increased the stirring speed from 150 to 200 rpm resulted in an increase in vanillin production. The vanillin yield peaked quickly. Based on the maximum vanillin production at 0.63 g/L with a molar yield of 53.86%, 200 rpm was found to be the optimum speed. This indicated that the stirring speed less than 200 rpm did not benefit the production of vanillin. The explanation was related to the oxygen transfer rate in the reactor. The stirring speed was closely related to the oxygen partial pressure which has a major effect on the oxygen solubility and the mass transfer driving force in the liquid31. At lower stirring speed, the oxygen partial pressure was lower and led to a low oxygen solubility32. Hence, the vanillin yield is lower. Previous literature revealed that low stirring speed can cause a drastic reduction in the protease production by Bacillus sp32.
When the stirring speed increased, the oxygen solubility was higher and resulted in a higher oxidative capacity of the broth. Surprisingly, the vanillin production did not increase and was found to be negatively influenced by variations in stirring speed beyond 200 rpm (Fig. 5a). Stirring is crucial for better oxygen and nutrient transfer during biotransformation. However, very high dissolved oxygen could hamper the production of the target product due to the suppression of enzyme activities required in producing target product28. Meanwhile, high dissolved oxygen concentration would result in the oxidization of vanillin to further products1. Therefore, the overall vanillin yield decreased. Additionally, higher stirring speed might cause shear stress on bacterial cells resulting in the reduction of biomass32. Furthermore, stirring might change the hydrodynamic condition which was defined as the transport of cells, nutrients, and oxygen from the fluid to the biofilm. The hydrodynamic condition could affect the structure, physiological composition and metabolic characteristics of biofilm33. High stirring speed would lead to the reduction of biofilm thickness, volumetric density and cell density, resulting in a decrease of vanillin yield. Moreover, rapid stirring could cause foam in B. subtilis fermentations, which led massive overflow of broth30. Power consumption during stirring is a significant fraction of the total operating cost. Hence, increasing the stirring speed would increase product cost.
Increasing substrate concentration required to obtain high-yield of product so that the process is profitable. The following experiments were aimed at evaluating the effect of initial ferulic acid concentration on biotransformation. Experiments were carried out at pH 9.0, HRT 20 h, temperature 35 °C, stirring speed 200 rpm, and different ferulic acid concentration: 0.5, 1.0, 1.5 and 2.0 g/L. It can be clearly observed from Fig. 5b that 0.5 g/L of initial ferulic acid concentration resulted 0.20 g/L of vanillin together with a molar yield of 51.93%. Both the vanillin molar yield and the total amount of vanillin increased correspondingly with increasing initial concentration of ferulic acid. A maximum yield of 0.70 g/L vanillin with molar yield of 59.20% appeared at initial concentration of 1.5 g/L (Fig. 5b). However, the vanillin production declined if initial ferulic acid concentration exceeded 1.5 g/L.
These revealed that ferulic acid at a concentration under 1.5 g/L could have no adverse effect, whereas a higher ferulic acid concentration might negatively affected biotransformation. It has been stated that high concentrations of ferulic acid were toxic for microbial cells and could inhibit the growth of microbes2. In this study, a ferulic acid concentration higher than 1.5 g/L might inhibit the growth of planktonic cell, which in turn led to the break of equilibrium process between the planktonic and sessile states of bacteria during biofilm formation27. Higher concentration of ferulic acid also led to an increase in viscosity of the fermentation broth, which in turn negatively influenced the volumetric oxygen mass transfer, ultimately resulting in lower yield of protease and EPS32. Thus, low protease production rates would lead to a limited enzymatic biotransformation. Meanwhile, the reduction of EPS could not only impair the structural integrity of the biofilm, but lessen the nutrient reserve which ensure cell survival under famine conditions34, resulting in low vanillin production.
To understand the time course of biotransformation by free and immobilized cells of B. subtilis, the repeated batch process was performed varying the length of each batch in function of maximum vanillin production. Figure 6 depicts the results of the repeated batch biotransformation carried out in the stirring reactor using free cells. In batch 1, fermentation exhibited a lag phase of 10 h, which was longer than that of the two subsequent batches (5 h) (Fig. 6). The shortened lag phase was because the cells from batch 1 were reused to inoculate the next batch35. After 35 h fermentation, a maximum vanillin titer of 0.37 g/L was obtained. The cell growth and the cellar activity increased continuously during biotransformation. The maximum values for 16S rDNA copy number and ATP concentration were 5.10 × 107 copies/g and 0.017 μg/g at 35 h, respectively (Fig. 6). The fermentation time for batch 2 and 3 was the same, both were 30 h. A gradual increase of cell growth and cellar activity were observed in batch 2 and 3. Regarding vanillin production, similar values obtained in batch 2 and 3, in the range of 0.35–0.38 g/L, were achieved (Fig. 6).
Figure 7 reports the repeated batch biotransformation carried out using immobilized B. subtilis cells under the optimized conditions. It can be found that the sustainable continuous increases of cell growth and cellar activity among the three experiments (repeated batch 1–3) occurred on the production of vanillin (Fig. 7). The lag phase of batch 1 was about 5 h, and was eliminated in batch 2 and 3. Consequently, the fermentation time of batch 2 and 3 was only 15 h. Similar values of 16S rDNA copy number obtained in all batches, ranging from 3.69 × 1011 to 3.72 × 1011 copies/g, were achieved (Fig. 7). The concentration of ATP obtained in batch 2 and 3 was the same, or higher, than that of batch 1. It also can be seen that the vanillin titer of each repeated batch cycle was more or less constant, ranging from 0.68 to 0.71 g/L (Fig. 7).
During repeated batch biotransformation, the cell growth and cellar activity in liquid were also detected. The copy numbers of 16S rDNA varied in a small range of 4.17 × 106–3.12 × 107 copies/g, maintaining relatively stable throughout the repeated batch period. Accordingly, similar values of ATP titer in the range of 0.004–0.022 μg/g, were achieved in the three experiments. These revealed that very little amount of free cells (approximately 10−5–10−6 fold less than the values for immobilized cells) grew during each batch fermentation in the stirring fixed-CFT reactor immobilized B. subtilis, and probably its contribute to the biotransformation was minimal. After stopping fermentation, the biomass was obtained from liquid sample of the immobilized cell system, and its concentrations for batch 1, 2 and 3 were 0.29, 0.32 and 0.34 g/L, respectively. The low values of free biomass allowed easy transfer operations from a batch to the following and vanillin recovery36. In order to understand the adsorption of vanillin to biomass, the vanillin in the biomass precipitate was analyzed. Results showed that vanillin titer obtained from the three batch biotransformation was the same or lower than 0.004 g/L. This indicated that vanillin adsorbed to cell biomass (ca. 1.1%) was very scare and its effects on vanillin titer of liquid sample were negligible.
Comparing Fig. 6 with Fig. 7, the fermentation period of the immobilized cell system was shorter than that of free cell system. This was because that the immobilized cell system only required as a one-off seed culture, which would shorten the fermentation period significantly37. It was evident that immobilized cell system was able to obtain maximum vanillin production after only 15 h while the free cells needed double time (30 h). It is worth mentioning that both 16S rDNA copy number and cellar activity in the immobilized cell system were higher than those from the free cell system. The reason was because the CFT carrier act as a protection barrier for access of the inhabited factors to the cell38. The vanillin molar yield (58.70%, 60.43% and 59.59% for batch 1, 2 and 3, respectively) and productivity (0.034, 0.047 and 0.046 g/L/h for batch 1, 2 and 3, respectively) from the immobilized cell system were higher than those from the free cell system (31.30%, 32.81% and 30.00% of molar yield for batch 1, 2 and 3, respectively; 0.011, 0.013 and 0.012 g/L/h of productivity for batch 1, 2 and 3, respectively). This might be attributed to the synergistic effect of cell reuse and microorganism acclimation35.
To date, several studies have been carried out to biotechnologically produce vanillin from ferulic acid using various organisms1,2,39,40,41,42,43,44,45,46,47,48. The reported maximum vanillin productivity and molar yield obtained using free mutant Pseudomonas putida, engineered Pseudomonas fluorescens, recombinant Escherichia coli were much higher than those of B. subtilis B7-S in this study (Table 1). Although the production of vanillin can be increased through genetic manipulation of these bacteria mentioned above, the cost of these methods could be too high due to the complex operation49. Additionally, the genetic instability of these recombinant strains might occur during biotransformation2. The maximum vanillin productivities for free Amycolatopsis sp. HR167, Streptomyces sp. V-1 reported by previous studies reached 0.061 and 0.34 g/L/h, respectively (Table 1), which much higher than 0.047 g/L/h in this work. However, by the immobilized cell strategy here, the maximum molar yield is at least 1.11 fold higher than that of free cells mentioned above. This suggested that the stirring bioreactor packed with CFT carrier biofilm formed by B. subtilis could efficiently transform ferulic acid to vanillin. Although the reported maximum molar yield obtained using free Streptomyces halstedii GE 107678 and mix culture of Aspergillus niger and Pycnoporus cinnabarinusi was higher than that of immobilized B. subtilis B7-S in this study, the immobilized cell system could shorten the fermentation time and obtain a higher value of the maximum vanillin productivity (Table 1). The current work was most effective in producing vanillin (immobilized repeated batch system) compared to the closest counterpart (immobilized batch or fed-batch system) in Table 1. Additionally, the immobilized cell system reached the highest vanillin production as compared to previous fermentations carried using frees of B. subtilis45. Therefore, the reactor packed with CFT biofilm of B. subtilis in this study is a stable and highly efficient process to produce vanillin from ferulic acid.
In conclusion, biotransformation of ferulic acid to vanillin by B. subtilis in a stirring packed-bed reactor filled with CFT was investigated. Results obtained from SEM, HPLC, qRT-PCR and ATP assay during immobilizing indicated that B. subtilis could be successfully immobilized and form biofilm on the surface of CFT carrier, and biotransformation was closely related with the extent of biofilm formation. Among parameters relating to biotransformation, temperature, stirring speed and initial ferulic acid concentration were found to have a significant influence on vanillin production. Repeated batch biotransformation indicated the maximum vanillin molar yield (60.43%) and productivity (0.047 g/L/h) from the immobilized cell system was higher than those from the free cell system (molar yield of 32.81%, productivity of 0.013 g/L/h). These results revealed that the reactor packed with CFT biofilm of B. subtilis possesses a good application potential in producing vanillin from ferulic acid.
Methods
Chemicals
Vanillin standard was purchased from Sigma-Aldrich (Shanghai, China). Analytical-grade ferulic acid was obtained from Aladdin (Shanghai, China). Solvents used in high-performance liquid chromatography (HPLC) analysis were of HPLC grade. Other chemicals were of analytical grade.
Bacterium and medium
B. subtilis CCTCC M2011162 was used for the biotransformation of ferulic acid. It has been proved that this strain is able to transform ferulic acid to vanillin with relatively high yield of vanillin50. Seed medium (pH 8.0) contained 5 g/L glucose, 10 g/L peptone, 3 g/L yeast extract powder, and 5 g/L NaCl. Immobilized medium (pH 8.0) contained 10 g/L peptone, 3 g/L yeast extract powder, 5 g/L NaCl and 0.5 g/L ferulic acid. Biotransformation medium (pH 8.0–9.5) contained 10 g/L peptone, 3 g/L yeast extract powder, 5 g/L NaCl and 0.5–2.0 g/L ferulic acid. The pH of media mentioned-above were adjusted by 5 M NaOH.
Bioreactor and cell immobilization
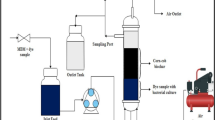
The stirred stainless steel fermenters packed with active carbon fiber (Xintong Activated Carbon Fiber Co., Ltd., Nantong, China) as the biofilm carrier were used in this study (Fig. 8). The fermenter has an effective volume of 10 L (21 cm outside diameter and 30 cm height). A frustum of a cone composed of eight stainless steel wires and two rings was laterally packed by CFT. This conical frustum (12 cm top diameter, 16 cm bottom diameter, and 25 cm height) was placed into the reactor as biofilm carriers.
The reactor containing 7.6 L of immobilized medium without ferulic acid was steam sterilized. Then the filtered ferulic acid solution (dissolved in 1 M NaOH solution, pH 8.0) was directly added to the rector. The B. subtilis was cultured in seed medium. After reaching stationary growth phase, 400 mL of cultures were pumped by a peristaltic pump into the reactor with immobilized medium. The immobilization was performed in this rector at 150 rpm and 35 °C for about 40 h. An air pump with 0.22 μm filter was used to provide sufficient air and ensure the system was never oxygen limited (Fig. 8). Approximately 20 mL liquid sample and 4 cm2 CFT biofilm carrier sample were taken at 10 h intervals during immobilizing. The extent of biofilm formation, cell growth, cellular activity, and vanillin concentration were detected.
Optimization of reaction parameters
Bioconversion experiments were carried out in the immobilized reactor described above. The sterile biotransformation media without ferulic acid and the filtered ferulic acid solution were pumped into the reactors. In order to obtain the maximum yield of vanillin in the stirring fixed-CFT reactor immobilized with B. subtilis, different HRTs (10–40 h), temperature (25–40 °C), initial pH (8.0–9.5), stirring speed (150–300 rpm), and ferulic acid concentration (0.5–2.0 g/L) were evaluated in this study. Approximately 20 mL liquid sample was withdrawn at preestablished intervals for vanillin determination.
Repeated batch biotransformation
Biotransformation were conducted both in the stirring fixed-CFT reactor immobilized B. subtilis (immobilized cells) and in the stirring reactor inoculated B. subtilis without CFT carrier (free cells) under the optimized condition obtained by the above-mentioned single factor experiments. Repeated batch biotransformation were performed removing the exhausted medium at the time of maximum vanillin production and, after washing the CFT carrier to remove free cells and residue twice with sterile distilled water, adding 8 L fresh biotransformation medium. Repeated batch operations were carried out with three cycles. For each biotransformation run, approximately 20 mL liquid sample and 4 cm2 CFT biofilm carrier sample were withdraw every 5 h for vanillin analysis, cell growth determination, and cellular activity detection. After stopping each batch biotransformation, the liquid sample obtained from the immobilized cell systems were centrifuged for biomass measurement and vanillin analysis.
Analysis
The concentration of vanillin was determined by HPLC as described in (Information S1 in the Electronic Supplementary Materials). Vanillin molar yield was calculated as the ratio between the produced vanillin (mM) and the initial ferulic acid (mM)2. The extent of biofilm formation were examined by a scanning electron microscope (EVO-18, Carl Zeiss, Germany). The sample was cut from the carriers and dried naturally. All samples were mounted and sputtered together to ensure there should be no difference in coating thickness. Bacterial cell growth was measured using real-time fluorescent quantitative PCR as described in (Information S2 in the Electronic Supplementary Materials). The ATP content was used as an indicator to evaluate the cellular activity of B. subtilis in different systems. It was measured using an ATP assay kit (Beyotime, Nanjing, China) according to the manufacturer’s instructions based on the bioluminescence technique (Information S3 in the Electronic Supplementary Materials). Biomass was measured using dry weight method reported by previous study51. All experiments were carried out in triplicate and the average values were reported. A t-test was used to compare the batch to batch variation using SPSS 19 statistical software (SPSS Inc., Chicago, USA).
Additional Information
How to cite this article: Yan, L. et al. Biotransformation of ferulic acid to vanillin in the packed bed-stirred fermentors. Sci. Rep. 6, 34644; doi: 10.1038/srep34644 (2016).
References
Kaur, B. & Chakraborty, D. Biotechnological and molecular approaches for vanillin production: a review. Applied Biochemistry and Biotechnology 169, 1353–1372 (2013).
Di Gioia, D. et al. Metabolic engineering of Pseudomonas fluorescens for the production of vanillin from ferulic acid. Journal of Biotechnology 156, 309–316 (2011).
Ashengroph, M., Nahvi, I., Zarkesh-Esfahani, H. & Momenbeik, F. Novel strain of Bacillus licheniformis SHL1 with potential converting ferulic acid into vanillic acid. Annals of Microbiology 62, 553–558 (2012).
Feng, C. et al. Effective bioconversion of sophoricoside to genistein from Fructus sophorae using immobilized Aspergillus niger and Yeast. World Journal of Microbiology and Biotechnology 31, 187–197 (2015).
Sasaki, K., Morita, M., Hirano, S.-i., Ohmura, N. & Igarashi, Y. Decreasing ammonia inhibition in thermophilic methanogenic bioreactors using carbon fiber textiles. Applied Microbiology and Biotechnology 90, 1555–1561 (2011).
Sasaki, K., Morita, M., Hirano, S.-i., Ohmura, N. & Igarashi, Y. Effect of adding carbon fiber textiles to methanogenic bioreactors used to treat an artificial garbage slurry. Journal of Bioscience and Bioengineering 108, 130–135 (2009).
Sun, F. et al. Hybrid biofilm-membrane bioreactor (Bf-MBR) for minimization of bulk liquid-phase organic substances and its positive effect on membrane permeability. Bioresource Technology 198, 772–780 (2015).
Guilbaud, M., Piveteau, P., Desvaux, M., Brisse, S. & Briandet, R. Exploring the diversity of Listeria monocytogenes biofilm architecture by high-throughput confocal laser scanning microscopy and the predominance of the honeycomb-like morphotype. Applied and Environmental Microbiology 81, 1813–1819 (2015).
Anjaneya, O. et al. Decolourization of Amaranth dye by bacterial biofilm in batch and continuous packed bed bioreactor. International Biodeterioration & Biodegradation 79, 64–72 (2013).
Liu, H. et al. Effect of electro-stimulation on activity of heterotrophic denitrifying bacteria and denitrification performance. Bioresource Technology 196, 123–128 (2015).
Vang, Ó. K., Corfitzen, C. B., Smith, C. & Albrechtsen, H.-J. Evaluation of ATP measurements to detect microbial ingress by wastewater and surface water in drinking water. Water Research 64, 309–320 (2014).
Cilia, V., Lafay, B. & Christen, R. Sequence heterogeneities among 16S ribosomal RNA sequences, and their effect on phylogenetic analyses at the species level. Molecular Biology and Evolution 13, 451–461 (1996).
Ma, X. k. & Daugulis, A. J. Transformation of ferulic acid to vanillin using a fed‐batch solid–liquid two‐phase partitioning bioreactor. Biotechnology Progress 30, 207–214 (2014).
Damaraju, S., Singh, U. K., Sreekanth, D. & Bhandari, A. Denitrification in biofilm configured horizontal flow woodchip bioreactor: effect of hydraulic retention time and biomass growth. Ecohydrology and Hydrobiology 15, 39–48 (2015).
Zeng, W. et al. Metabolic studies of temperature control strategy on poly (γ-glutamic acid) production in a thermophilic strain Bacillus subtilis GXA-28. Bioresource Technology 155, 104–110 (2014).
Kardos, L. et al. Comparing of mesophilic and thermophilic anaerobic fermented sewage sludge based on chemical and biochemical tests. Applied Ecology and Environmental Research 9, 293–302 (2011).
Jeong, H.-H. et al. Effect of temperature on biofilm formation by Antarctic marine bacteria in a microfluidic device. Analytical Biochemistry 446, 90–95 (2014).
Ahmed, U. & Vafai, K. Analysis of biofilm growth in the presence of osmotic pressure and temperature effects. International Journal of Heat and Mass Transfer 55, 5709–5721 (2012).
Asadishad, B., Olsson, A. L. J., Dusane, D. H., Ghoshal, S. & Tufenkji, N. Transport, motility, biofilm forming potential and survival of Bacillus subtilis exposed to cold temperature and freeze–thaw. Water Research 58, 239–247 (2014).
Park, Y., Uehara, H., Teruya, R. & Okabe, M. Effect of culture temperature and dissolved oxygen concentration on expression of α-amylase gene in batch culture of spore-forming host, Bacillus subtilis 1A289. Journal of Fermentation and Bioengineering 84, 53–58 (1997).
Surkatti, R. & El-Naas, M. H. Biological treatment of wastewater contaminated with p-cresol using Pseudomonas putida immobilized in polyvinyl alcohol (PVA) gel. Journal of Water Process Engineering 1, 84–90 (2014).
Johnvesly, B. & Naik, G. R. Studies on production of thermostable alkaline protease from thermophilic and alkaliphilic Bacillus sp. JB-99 in a chemically defined medium. Process Biochemistry 37, 139–144 (2001).
Liu, Y.-g. et al. Biosorption of copper (II) from aqueous solution by Bacillus subtilis cells immobilized into chitosan beads. Transactions of Nonferrous Metals Society of China 23, 1804–1814 (2013).
Tao, Y., Chen, Z., Zhang, Y., Wang, Y. & Cheng, Y. Immobilized magnetic beads based multi-target affinity selection coupled with high performance liquid chromatography–mass spectrometry for screening anti-diabetic compounds from a Chinese medicine “Tang-Zhi-Qing”. Journal of Pharmaceutical and Biomedical Analysis 78, 190–201 (2013).
Sathishkumar, R., Ananthan, G. & Arun, J. Production, purification and characterization of alkaline protease by ascidian associated Bacillus subtilis GA CAS8 using agricultural wastes. Biocatalysis and Agricultural Biotechnology 4, 214–220 (2015).
Oliveira, R., Melo, L., Oliveira, A. & Salgueiro, R. Polysaccharide production and biofilm formation by Pseudomonas fluorescen: effects of pH and surface material. Colloids and Surfaces B: Biointerfaces 2, 41–46 (1994).
Nguyen, H. D. N., Yang, Y. S. & Yuk, H. G. Biofilm formation of Salmonella Typhimurium on stainless steel and acrylic surfaces as affected by temperature and pH level. LWT-Food Science and Technology 55, 383–388 (2014).
Song, P. et al. Two-stage oxygen supply strategy for enhanced lipase production by Bacillus subtilis based on metabolic flux analysis. Biochemical Engineering Journal 71, 1–10 (2013).
Masuo, S. et al. Global gene expression analysis of Aspergillus nidulans reveals metabolic shift and transcription suppression under hypoxia. Molecular Genetics and Genomics 284, 415–424 (2010).
Yánez-Mendizábal, V. et al. Production of the postharvest biocontrol agent Bacillus subtilis CPA-8 using low cost commercial products and by-products. Biological Control 60, 280–289 (2012).
Araújo, J. D. P., Grande, C. A. & Rodrigues, A. E. Structured packed bubble column reactor for continuous production of vanillin from Kraft lignin oxidation. Catalysis Today 147, S330–S335 (2009).
Potumarthi, R., Ch, S. & Jetty, A. Alkaline protease production by submerged fermentation in stirred tank reactor using Bacillus licheniformis NCIM-2042: effect of aeration and agitation regimes. Biochemical Engineering Journal 34, 185–192 (2007).
Lemos, M., Mergulhão, F., Melo, L. & Simões, M. The effect of shear stress on the formation and removal of Bacillus cereus biofilms. Food and Bioproducts Processing 93, 242–248 (2015).
Fang, H. H. P., Xu, L.-C. & Chan, K.-Y. Effects of toxic metals and chemicals on biofilm and biocorrosion. Water Research 36, 4709–4716 (2002).
Zhang, Y. et al. Improving lactic acid productivity from wheat straw hydrolysates by membrane integrated repeated batch fermentation under non-sterilized conditions. Bioresource Technology 163, 160–166 (2014).
Fenice, M., Federici, F., Selbmann, L. & Petruccioli, M. Repeated-batch production of pigments by immobilised Monascus purpureus. Journal of Biotechnology 80, 271–276 (2000).
Wan, W. A. A. Q. I., Latif, N. A., Harvey, L. M. & McNeil, B. Production of exopolysaccharide by Ganoderma lucidum in a repeated-batch fermentation. Biocatalysis and Agricultural Biotechnology 6, 91–101 (2016).
Xie, G.-J. et al. Enhanced photo-H2 production by Rhodopseudomonas faecalis RLD-53 immobilization on activated carbon fibers. Biomass and Bioenergy 44, 122–129 (2012).
Tripathi, U., Rao, S. R. & Ravishankar, G. Biotransformation of phenylpropanoid compounds to vanilla flavor metabolites in cultures of Haematococcus pluvialis. Process Biochemistry 38, 419–426 (2002).
Brunati, M. et al. Biotransformations of cinnamic and ferulic acid with actinomycetes. Enzyme and Microbial Technology 34, 3–9 (2004).
Lee, E. G. et al. Directing vanillin production from ferulic acid by increased acetyl‐CoA consumption in recombinant Escherichia coli. Biotechnology and Bioengineering 102, 200–208 (2009).
Hua, D. et al. Enhanced vanillin production from ferulic acid using adsorbent resin. Applied Microbiology and Biotechnology 74, 783–790 (2007).
Sarangi, P. K. & Sahoo, H. P. Enhancing the rate of ferulic acid bioconversion utilizing glucose as carbon source. Science 6, 115–117 (2010).
Achterholt, S., Priefert, H. & Steinbüchel, A. Identification of Amycolatopsis sp. strain HR167 genes, involved in the bioconversion of ferulic acid to vanillin. Applied Microbiology and Biotechnology 54, 799–807 (2000).
Chen, P. et al. A microbial transformation using Bacillus subtilis B7-S to produce natural vanillin from ferulic acid. Scientific Reports 6, 20400 (2016).
Tilay, A., Bule, M. & Annapure, U. Production of biovanillin by one-step biotransformation using fungus Pycnoporous cinnabarinus. Journal of Agricultural and Food Chemistry 58, 4401–4405 (2010).
Gallage, N. J. & Møller, B. L. Vanillin–bioconversion and bioengineering of the most popular plant flavor and its de novo biosynthesis in the vanilla orchid. Molecular plant 8, 40–57 (2015).
Johnson, T. S., Ravishankar, G. & Venkataraman, L. Biotransformation of ferulic acid and vanillylamine to capsaicin and vanillin in immobilized cell cultures of Capsicum frutescens. Plant cell, Tissue and Organ Culture 44, 117–121 (1996).
Yan, L. et al. Optimization of magnetosome production by Acidithiobacillus ferrooxidans using desirability function approach. Materials Science and Engineering: C 59, 731–739 (2016).
Chen, P. et al. Draft genome sequence of Bacillus subtilis type strain B7-S, which converts ferulic acid to vanillin. Genome Announcements 2, e00025–00014 (2014).
Basuki, T., Van Laake, P., Skidmore, A. & Hussin, Y. Allometric equations for estimating the above-ground biomass in tropical lowland Dipterocarp forests. Forest Ecology and Management 257, 1684–1694 (2009).
Acknowledgements
This work was supported by Gansu Province Science Foundation for Distinguished Young Scholars (Grant No. 1308RJDA014), Longyuan Support Project for Young Creative Talents (Grant No. GANZUTONGZI [2014] no. 4), Technology Program of Gansu Province (Grant No. 1205TCYA034 Grant No. 1604FKCA110), Technology Program of Lanzhou City (Grant No. 2013-4-115 Grant No. 2015-3-142, 2015-3-97 and 2015-3-93), The Fundamental Research Funds for the Central Universities of China (Grant No. lzujbky-2015-57), University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (UNPYSCT-2015086), National Natural Science Foundation of China (41471201, 31100006) and Scientific Research Staring Foundation in HBAU (XZR2014-15).
Author information
Authors and Affiliations
Contributions
L.Y. initiated and supervised the study. L.Y., P.C. and S.Z. conceived and conducted the experiments. S.L., H.L. and L.Y. draft the manuscript and performed the project. X.Y., N.W. and N.L. revised the manuscript. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yan, L., Chen, P., Zhang, S. et al. Biotransformation of ferulic acid to vanillin in the packed bed-stirred fermentors. Sci Rep 6, 34644 (2016). https://doi.org/10.1038/srep34644
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep34644
This article is cited by
-
A comprehensive review of eclectic approaches to the biological synthesis of vanillin and their application towards the food sector
Food Science and Biotechnology (2024)
-
Production of ferulic acid from dried bamboo shoots for the biotransformation into vanillin using a novel microbe Enterobacter aerogenes
Biomass Conversion and Biorefinery (2023)
-
Transformation of agro-biomass into vanillin through novel membrane integrated value-addition process: a state-of-art review
Biomass Conversion and Biorefinery (2023)
-
Biotransformation of wheat straw into biovanillin by solid-state fermentation and optimization of conditions parameters through response surface methodology
Biomass Conversion and Biorefinery (2022)
-
Improvement of biovanillin production with two-stage pH control strategy from lemongrass leaves hydrolysates using Phanerochaete chrysosporium ATCC 24725 in batch culture
Biomass Conversion and Biorefinery (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.