Abstract
In this study, we have employed graphene oxide as a matrix to simultaneously and directly quantify serum nonesterified and esterified fatty acids (FAs) using matrix-assisted laser/desorption ionization-Fourier transform ion cyclotron resonance mass spectrometry (MALDI-FTICR MS). Twelve serum nonesterified FAs combined with their individual esterified FAs (i.e., C16:0, C16:1, C18:0, C18:1, C18:2, C18:3, C20:2, C20:3, C20:4, C20:5, C22:5, and C22:6) were quantified based on their calibration curves with the correlation coefficients of >0.99, along with the analytical time of <1 min each sample. As a result, serum levels of twelve total FAs (TFAs) in 1440 serum samples from 487 healthy controls (HCs), 479 patients with benign lung diseases (BLDs) and 474 patients with lung cancer (LC) were determined. Statistical analysis indicated that significantly increased levels of C16:0, C16:1, C18:0, C18:1, C18:3, C20:3, and C22:6 and decreased levels of C20:5 were observed in LC patients compared with BLDs. Receiver operating characteristic (ROC) analysis revealed that panel a (C18:2, C20:3, C20:4, C20:5, C22:5, and C22:6), panel b (C18:0, C20:4, C20:5, and C22:6), and panel c (C16:1, C18:0, C18:1, C20:3, and C22:6) have exhibited good diagnostic ability to differentiate BLDs from LC relative to clinical uses of tumor markers (CEA and Cyfra 21-1).
Similar content being viewed by others
Introduction
Lung cancer (LC) is the leading cause of cancer-related death worldwide for both men and women1, and a majority of LC patients present with inoperable at diagnosis and poor prognosis2. Despite the great progress made in recent years against LC, LC patients still have a low 5-year survival rate of approximately 18% in USA3. Currently, LC diagnosis and screening mainly depend on biopsy and high-resolution (or low-dose) computed tomography4,5. However, biopsy and computed tomography are not desirable to frequently detect tumors due to their invasive and high-cost. In addition, several circulating tumor markers, such as carcinoembryonic antigen (CEA) and cytokeratin 19 fragment antigen 21-1 (Cyfra 21-1), are the best known tumor markers for LC6, show poor sensitivities of 11~69% for LC detection2,6,7. Therefore, it is necessary to screen economic, simple, and noninvasive biomarkers for differentiating LC from benign lung diseases (BLDs).
Fatty acids (FAs) are the major structural components of lipids that involve in many biological functions, such as energy storage, signaling molecules8, and cellular messengers9. In addition, FAs have diverse structure and function in metabolism, cell, and tissue responses10,11. The sum of circulating free FAs (or nonesterified FAs) and esterified FAs from the circulating lipids may reflect the overall metabolism of endogenous and dietary FAs12,13. Previous studies have shown that serum total fatty acids (TFAs) including nonesterified FAs and esterified FAs not only generate great impact on human health but also involve in many biological functions, such as inflammatory response14,15. Emerging evidence indicates that free FAs are closely associated with insulin resistance and cancers16,17,18,19 and the esterified FAs play essential roles in regulating membrane fluidity, stability, and permeability, as well as in influencing the behaviors of membrane-bound enzymes and receptors20,21. The esterified FAs are associated with cancers22,23,24 and serum levels of TFAs are closely related with nutritional and metabolic disorders13,25.
Traditional analysis of circulating TFAs is usually performed using gas chromatography-mass spectrometry or liquid chromatography-mass spectrometry, both of which require complicated and time-consuming sample preparation26,27,28. Lipids analysis by matrix-assisted laser desorption/ionization -mass spectrometry (MALDI-MS) presents a lot of advantages, such as high detection speed, simplicity, and high salt tolerance. Recently, MALDI-MS has been applied to quantify metabolites in vivo without chromatographic separation16,29.
In this study, we have employed graphene oxide (GO) with efficient energy transfer, large thermal conductivity, and excellent electronic transportation30 as a matrix to ionize lipids in combination with collision induced dissociation to fragment phospholipids so that they become a novel powerful approach to simultaneously and directly quantify serum TFAs using MALDI-Fourier transform ion cyclotron resonance mass spectrometry (FTICR MS). Finally, twelve serum TFAs (i.e., C16:0, C16:1, C18:0, C18:1, C18:2, C18:3, C20:2, C20:3, C20:4, C20:5, C22:5, and C22:6) were simultaneously quantified. Statistical analysis indicated that serum levels of TFAs are closely associated with physiopathological states and that the combination of C18:2, C20:3, C20:4, C20:5, C22:5, and C22:6, the combination of C18:0, C20:4, C20:5, and C22:6, and the combination of C16:1, C18:0, C18:1, C20:3, and C22:6 have shown good diagnostic capability to differentiate BLD patients from LC patients compared with CEA and Cyfra 21-1.
Results
Generating serum TFAs using optimal MS condition
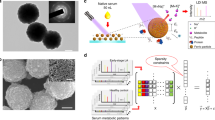
To establish an optimal MS condition for fragmenting phospholipids and generating serum TFAs using the QC sample as a model sample, we first optimized two important parameters: MALDI laser power and the skimmer 1 voltage of FTICR MS using GO as a MALDI matrix31. When the laser power was set to 30%, 50%, or 90% at both the skimmer 1 voltage of −10 V and−45 V, respectively, the intensities of different serum phospholipids were decreased gradually, while those of serum TFAs were increased (Fig. 1A–D). It should be noted that when the skimmer 1 voltage was set up to −100 V, the intensities of serum TFAs were dramatically decreased and phospholipids almost disappeared (Fig. 1E). Finally, it is found that most of the serum phospholipids were fragmented and abundant serum TFAs were generated with laser power of 90% and the skimmer 1 voltage of −45 V (Fig. 1D). As a result, the MS condition was optimal as laser power of 90% and the skimmer 1 voltage of −45 V.
To assess the effect of different MALDI matrixes on the degree of the fragmentation of different phospholipids, several standard phospholipids (i.e., PA(18:1/18:1), PE(18:1/18:1), PC(18:1/18:1), PI(16:0/18:2) and PG(16:0/16:0)) were selected as model compounds. As shown in Fig. 2, a comparison of commonly used matrixes (i.e., 9-AA, DMAN, and NEDC) and GO shows that GO matrix has exhibited the best performance to fragment all of the above-mentioned standard phospholipids, along with the fragmentation degree of >80%, while NEDC matrix shows the lowest degree of fragmentation for all above-mentioned standard phospholipids compared with other matrixes. Therefore, GO was selected as the MALDI matrix to analyze serum extracts.
Simultaneous quantification of serum TFAs
Based on the working standard solutions, the calibration curves of C16:0, C16:1, C18:0, C18:1, C18:2, C18:3, C20:4, and C22:6 were constructed with their correlation coefficients of >0.99 (Table 1), and their linearity ranges, LODs, and spike-and-recovery are also listed in Table 1. The reproducibility of the eight FAs is less than 18.0% (Table 1). Their intraday RSDs were from 6.2% to 10.7% and their interday RSDs were from 9.5% to 15.1% (Supplementary Table S1). The spike-and-recovery in triplicate at three different concentrations of all eight FAs were between 78.8% and 110.0%.
Representative mass spectra of serum TFAs from one HC, one BLD patient, and one LC patient are shown in Fig. 3. Serum TFAs quantified in this study were identified based on their observed accurate m/z values relative to the theoretical values with a mass error of <±0.00025 Da and reliable isotopic distributions with the RSDs of <2.0% between the observed and theoretical intensities (Supplementary Table S2). The levels of serum TFAs were calculated based on their respective calibration curves (Table 1) and the resulting data are shown in Fig. 4.
Associations of the levels of serum TFAs with gender and age
Comparison of the levels of serum TFAs between female and male in each physiopathological state (For HCs, females: n = 212, age: 47.3 ± 10.3 years old; males: n = 275, age: 48.5 ± 10.1 years old. For BLD patients, females: n = 212, age: 55.7 ± 9.0 years old; males: n = 234, age: 55.2 ± 9.5 years old. For LC patients, females: n = 238, age: 57.5 ± 8.4 years old; males: n = 236, age: 57.9 ± 8.3 years old) and all participants (females: n = 745, age: 53.4 ± 10.2 years old; males: n = 695, age: 53.7 ± 10.2 years old) was performed using Mann-Whitney U test. The statistical analysis indicated that there is no statistical significance between the levels of serum TFAs and gender in each physiopathological state and between three different states (p > 0.05, Supplementary Table S3).
The effect of age on the levels of serum TFAs for HCs was also analyzed based on four different age groups (i.e., group 1, 30~39 years old (n = 114); group 2, 40~49 years old (n = 157); group 3, 50~59 years old (n = 138), and group 4, 60~70 years old (n = 78)) using one-way ANOVA with LSD test after data were transformed to normal distribution. It was found that the levels of C18:2, C20:2, C20:3, C20:4, C20:5, C22:5, and C22:6 were significantly correlated with age (p < 0.05, Supplementary Table S4). However, there is no statistical difference except for C18:3 between group 3 and group 4 for BLD patients (p = 0.032, Supplementary Table S5) and LC patients (Supplementary Table S6).
Association of changes in the levels of serum TFAs with physiopathological states
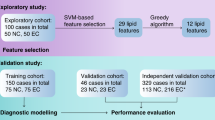
Based on the above-mentioned results, the 914 age-matched participants, including 304 HCs, 311 BLD patients, and 299 LC patients were selected to screen biomarkers for differentiating different physiopathological states (Table 2). In order to obtain high accurate diagnostic biomarkers, these participants were further classified randomly into a training set and a validation set (Fig. 5). In the training set study, as shown in Fig. 4, the levels of C18:2, C20:2, C20:3, C20:4, C20:5, C22:5, and C22:6 in BLD patients were significantly decreased relative to HCs. Significant increase in the levels of C16:0, C18:0, C18:1, and C18:3 and decrease in the levels of C20:2, C20:4, C20:5, C22:5, and C22:6 in LC patients were observed compared with HCs. However, remarkable increase in the levels of C16:0, C16:1, C18:0, C18:1, and C18:3 and decrease in the levels of C20:3 and C22:6 in LC patients were detected compared with BLD patients. In addition, an independent validation set also proved the above-mentioned change trends of serum TFAs in different physiological states (Fig. 4), and all p values are listed in Supplementary Table S7.
Diagnostic ability of serum TFAs
The AUC values, sensitivities, specificities, and cut-off values of serum TFAs panels are listed in Table 3. For the training set, a combination of C18:2, C20:3, C20:4, C20:5, C22:5, and C22:6, namely panel a, has shown a powerful capability to differentiate HCs from BLD patients, with the AUC value of 0.863. A combination of C18:0, C20:4, C20:5, and C22:6, namely panel b, has a powerful ability to differentiate HCs from LC patients, with the AUC value of 0.729. A combination of C16:1, C18:0, C18:1, C20:3, and C22:6, namely panel c, is a good predictor for distinguishing BLD from LC patients, with the AUC value of 0.752. To validate the diagnostic ability of the above-mentioned panels, an independent validation study was performed. As shown in Table 3, the panels a, b, and c all have good capability to differentiate between HCs, BLDs, and LC based on the cut-off values obtained in the training set, with the AUC values of 0.781, 0.759, and 0.703, respectively. In addition, based on these cut-off values, each of three panels has shown a good capability to differentiate HC from BLDs plus LC, with the AUC values of >0.74 (Table 4), and it should be noted that the AUC values of three individual panels to distinguish HC plus BLDs from LC were still more than 0.64 (Table 4).
Levels of serum tumor markers
In this study, serum tumor markers, CEA and Cyfra 21-1, were also measured in accordance with the manufacturer’s instructions and their median and ranges are listed Table 5. It is found that both CEA and Cyfra 21-1 were significantly increased in BLD or LC patients relative to HC (p < 0.001). However, no statistical differences in the level of serum Cyfra 21-1 was observed between BLD patients and LC patients.
Comparison of diagnostic ability between serum tumor markers and serum TFA panels
Based on the cut-off values of serum CEA of 5.0 ng/mL, Cyfra 21-1 of 3.5 ng/mL and a combination of CEA and Cyfra 21-1 of 0.5, their AUC values were calculated to differentiate HC from BLDs, HC from LC or BLDs from LC. As shown in Table 6, CEA and Cyfra 21-1 present a similar AUC values as the serum TFA panels to differentiate HCs from BLDs patients or LC patients, along with high specificities and low sensitivities for CEA and Cyfra 21-1 compared with serum TFA panels. It is worth noting that serum TFA panels with the AUC values of 0.706~0.732 have shown high diagnostic accuracy to differentiate BLDs from LC relative to serum tumor markers with the AUC values of 0.521~0.588. In addition, ROC analysis indicated that the diagnostic ability of CEA, Cyfra 21-1 and a combination of CEA and Cyfra 21-1 with the AUC values of 0.739~0.829 is similar to that of serum TFA panels with the AUC values of 0.735~0.760 between HC and BLDs plus LC (Table 7), while serum TFA panels have exhibited a slightly better diagnostic ability to differentiate HC plus BLDs from LC compared with serum tumor markers (Table 7).
Discussion
The detection of serum TFAs is usually performed using gas chromatography-mass spectrometry, along with a complicated and time-consuming sample preparation: lipid extraction, hydrolysis, and methylation32. In the present study, we developed a highly efficient method to generate esterified FAs and to detect directly and simultaneously serum nonesterified and esterified FAs through optimizing the skimmer 1 voltage of MS in combination with GO as a MALDI matrix. The calibration equations of C16:0, C16:1, C18:0, C18:1, C18:2, C18:3, C20:4, and C22:6 were constructed with the correlation coefficients of >0.99 based on their mixture standard working solutions. Their LODs were between 0.1 μM and 2.2 μM. 144 mass spectra of the QC sample were analyzed with a relative standard deviation of 18% for all analytes. The spike-and-recovery experiments on the basis of three different concentrations of FAs indicate that their recoveries were in the range from 78.8% to 110.0%. Our data indicate that the stability and precision of this method are acceptable for complex biological sample analysis. In term of the established method, twelve TFAs from 1440 serum samples were rapidly and simultaneously quantified and the measured concentrations of serum TFAs in the present study are similar to the previously published results obtained using gas chromatograph-mass spectrometry13,33.
Statistic analysis indicated that gender-specific difference was observed neither in the levels of twelve serum TFAs in each physiopathological state nor between three physiopathological states (Supplementary Table S3). It is worth noting that age-specific differences were only detected in the levels of some TFAs including C18:2, C20:2, C20:3, C20:4, C20:5, and C22:6 in HCs and C18:3 in BLD patients (Supplementary Tables S4 and S5), while for LC patients, no age-specific differences was observed, which are in line with previous reports19,34. Our findings indicate that different metabolic mechanisms between HC, BLDs, and LC might exist. Based on the age-matched samples (Table 2), statistic analysis indicated that the changes in the levels of serum TFAs between three different physiological states still present significantly statistical differences (Supplementary Table S7), further indicating the different metabolic mechanisms of serum TFAs between three physiopathological states.
Previous studies indicate that FA synthase has been found to be a hyperactivity in many cancers including LC35,36. Increase in the levels of C16:0, C18:0, and C18:1 in LC patients may be ascribed to the overexpression of FA synthase to sustain the increasing demand of energy during cancer cell proliferation37. It is found that α-linolenic acid (C18:3 n-3) can reduce the levels of reactive oxygen species produced by macrophages, resulting in the imbalance between oxidant and antioxidant systems in LC patients38. Decreased levels of C20:2, C20:3, C20:4, C20:5, C22:5, and C22:6 in both BLD and LC patients are in agreement with previous studies26,27,39, which may result in decreased membrane fluid to protect cancer cells from oxidative stress. In addition, C20:2, C20:3, C20:4, C20:5, C22:5, and C22:6 are the precursors of eicosanoid which are associated with inflammation, autoimmunity, and cancer15,40,41, and inflammation could influence tumor lipid metabolism42,43. Under inflammatory conditions, tumor cells may decrease the levels of endogenous inflammatory molecules to escape from immune attack and cell apoptosis44, which is in agreement with the decreased levels of C20:2, C20:3, C20:4, C20:5, C22:5, and C22:6 in both BLDs and LC.
These results further prove that lipid metabolism in HC and BLDs is significantly different from that in LC. The human body can produce all FAs except for C18:2 n-6 and C18:3 n-345. C18:2 n-6, which affects gene expression46, is the precursor of n-6 series of FAs. Lipid mediators (e.g., prostaglandins, thromboxanes, and leukotrienes) play proinflammatory and angiogenic functions and involve in several pathologic progresses, which are generated primarily through oxidative pathways from C20:428. In the present study, decreased C18:2 in BLDs and decreased C20:4 in both BLDs and LC might be associated with changes in inflammatory response. In addition, the combinations of serum TFAs have exhibited powerful diagnostic ability to differentiate BLDs from LC with the AUC values of 0.706~0.732 relative to serum tumor markers with the AUC values of 0.521~0.588 (Tables 6 and 7), indicating that lipid mechanisms are closely correlated with different physiological states.
Our study has some limitations. First, due to the lack of the detailed clinical information on LC staging, early stage diagnostic ability of serum TFAs could not be preformed. Second, the location of the double bonds of each unsaturated FAs has not been designed due to no gas chromatography separation and corresponding standard compounds. Finally, non complete fragmentation of serum phospholipids may affect the diagnostic capability of serum TFAs.
Conclusions
In the present study, based on the optimized parameters of MS in combination with the physicochemical properties of GO as a MALDI matrix, we developed a rapid and simultaneous quantification method of 12 serum FAs including nonesterified and esterified FAs via the direct fragmentation of phospholipids using MALDI-MS without hydrolysis and methylation of FAs. The correlation coefficients of the calibration curves were large than 0.99, with a linear dynamic range of 3 orders of magnitude and the LODs of 0.1~2.2 μM. The levels of serum TFAs in 1440 serum samples from three different physiopathological states reveal that lipid metabolic mechanisms are closely correlated with the physiopathological states. ROC analysis indicated that three different TFA panels have exhibited good diagnostic capability to differentiate among three different physiopathological states with the AUC values of >0.7 compared with serum tumor markers. Especially for differentiating BLDs from LC, the AUC values of serum TFA panels are 0.706~0.732, while those of serum tumor markers are 0.521~0.588. Taken together, our findings indicate that lipid metabolism is deeply involved in changes in physiological state, and our data may offer a stepping stone of new biomarker panels for differentiating BLDs from LC.
Methods
Chemicals and reagents
Pentadecanoic acid (C15:0), palmitic acid (C16:0), palmitoleic acid (C16:1), heptadecanoic acid (C17:0), stearic acid (C18:0), oleic acid (C18:1), linoleicacid (C18:2), linolenic acid (C18:3), nonadecanoic acid (C19:0), arachidonic acid (C20:4), heneicosanoic acid (C21:0), docosahexaenoic acid (C22:6), L-α-phosphatidylinositol sodium salt (PI, PI(16:0/18:2)), 9-aminoacridine (9-AA), 1,8-bis(dimethylamino)naphthalene (DMAN), and N-(1-naphthyl) ethylenediamine dihydrochloride (NEDC) were purchased from Sigma-Aldrich Chemicals (St. Louis, MO, USA). 1,2-di-(9Z-octadecenoyl)-sn-glycero-3-phosphate (sodium salt) (PA(18:1(9Z)/18:1(9Z))), 1,2-di-(9E-octadecenoyl)-sn-glycero-3-phosphocholine (PC(18:1(9E)/18:1(9E))), 1,2-di-(9E-octadecenoyl)-sn-glycero-3-phosphoethanolamine (PE(18:1(9E)/18:1(9E))) and 1,2-dipalmitoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (sodium salt) (PG(16:0/16:0)) were purchased from Avanti Polar lipids (Alabaster, AL, USA). GO was from Nanjing JCNANO Technology Co., Ltd (Nanjing, China). HPLC-grade isopropanol and hexane were from Fisher Scientific (Pittsburg, PA, USA). Ultrapure water was purified by a Milli-Q system (Millipore, MA, USA).
Participants and study design
In this study, a total of 1440 overnight (more than 10 hours) fasting serum samples were collected in Peking Union Medical College Hospital (Beijing, China). Serum samples are the remaining sera after routine physical examination or clinical examination. All samples were stored under −80 °C until use. Healthy controls (HCs) without any aberrant clinical appearance and pathomorphology were included. BLDs were diagnosed based on the clinical diagnostic criterion and LC was further confirmed by cytological or histological examination of tumor tissue. Study design is shown in Fig. 5. Age-unmatched participants (i.e., 183 HCs, 168 BLD patients, and 175 LC patients) were excluded, and the clinical characteristics of the age-matched participants are shown in Table 2. Informed consent were obtained from all participants and the study was approved by the Ethics Review Board at the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. All experiments were performed in accordance with relevant guidelines and regulations.
The levels of CEA and Cyfra 21-1
Serum tumor marker levels were determined with a CEA and Cyfra 21-1 test kit (Roche Diagnostic GmbH, Mannheim, Germany) using a Cobas e601 analyzer. The cut-off values were 5.0 ng/mL for CEA and 3.5 ng/mL for Cyfra 21-1 as recommended by the manufacture’s instruction.
Preparation of stock standard solutions
C17:0 and C21:0 were selected as internal standards (ISs), and their mixed stock solution was prepared in isopropanol at the concentrations of 375 μM C17:0 and 75 μM C21:0. The standard solutions of 6.60 mM C16:0, 1.65 mM C16:1, 10.00 mM C18:0, 10.00 mM C18:1, 10.00 mM C18:2, 0.826 mM C18:3, 3.30 mM C20:4, and 1.65 mM C22:6 were prepared in isopropanol, respectively. Then equal volume of the above-mentioned standard solutions was pooled into the mixed standard solution followed by 1-fold dilution as the stock standard solution at the final concentrations of 412.5 μM C16:0, 103.1 μM C16:1, 625.0 μM C18:0, 625.0 μM C18:1, 625.0 μM C18:2, 51.6 μM C18:3, 206.3 μM C20:4, and 103.1 μM C22:6.
Sample preparation
Lipids were extracted from serum sample on the basis of the approach used by Hara et al.47, with a slight modification. Briefly, after thawing at 4 °C, 10 μL of serum sample was transferred into a 1.5 mL eppendorf tube containing 10 μL ISs (37.5 μM C17:0 and 7.5 μM C21:0), followed by the addition of 135 μL of hexane/isopropanol (2:1, v:v) and 45 μL ultrapure water. The resulting mixture was vortexed for 1 min and then stored at −20 °C overnight. After the mixture was centrifuged at 19,000 × g for 30 min at 4 °C, 20 μL of the supernatant was transferred into a new tube and air-dried. The dried sample was stored at −80 °C until use. 10 μL of isopropanol/water (1:1, v:v) was added to re-dissolve the air-dried samples for mass spectrometric analysis.
Mass spectrometry analysis
The GO solution (0.5 mg/mL in water) was sonicated for 2 h, followed by the centrifugation at 13,000 × g to remove the unexfoliated GO particles. Then the supernatant was collected for further use as a MALDI matrix. 0.3 μL of the GO solution was first pipetted on the MTP AnchorChipTM plate (Bruker Daltonics, Billerica, MA, USA) and air-dried prior to the addition of 0.3 μL of the redissolved sample onto the GO matrix for mass spectrometric analysis.
All experiments were performed using a 9.4 T Apex-ultraTM hybrid Qh-FTICR MS (Bruker Daltonics, Billerica, MA, USA) equipped with a 355 nm Nd:YAG Smartbeam II 200 Hz laser in negative ion mode. Instrument calibration was performed using a mixture of C15:0 at m/z 241.21730, C17:0 at m/z 269.24860, C19:0 at m/z 297.27990, and C21:0 at m/z 325.31120 in negative ion mode. Mass spectrum of each sample was acquired over the m/z range of 150~400 with the resolution of 200,000 at m/z 400, along with 100 laser shots per scan and the skimmer 1 voltage of −45 V in negative ion mode. The fragmentation degree of the model compounds was calculated based on the equation (1).

Statistical analysis
Mass spectral data were obtained using ApexControl 3.0.0 (Bruker Daltonics). After isotopic deconvolution, the resulting data were transferred to Microsoft Excel, and the half of baseline strength in each spectrum was adopted as their intensities of missing serum TFAs. Univariate analysis was performed using non-parametric Mann-Whitney U test. One-way analysis of variance (ANOVA) with Fisher’s least significant test was used to evaluate the effect of age on the levels of serum TFAs. Non-normally distributed data were transformed into normal distribution before statistical analysis. Receiver operating characteristics (ROC) curve analysis was used to calculate the area under the ROC curve (AUC), cut-off values, sensitivities, and specificities. The prediction model was further confirmed by an independent validation set based on the cut-off values obtained in the training set. Statistical analyses were performed using SPSS software (version 16.0, Chicago, IL, USA). In all cases, a p value less than 0.05 was considered to be statistically significant.
Identification of serum TFAs
Serum TFAs were identified as previously described19. Briefly, serum TFAs were identified based on their observed accurate m/z values relative to theoretical values of <±0.00025 Da and their observed distributions of isotopic abundance relative to theoretical distributions of <2.0%.
Method validation for quantitative analysis
The reliability of MALDI-MS for quantitative analysis of FAs was validated based on the linearity, limit of detection (LOD), stability, precision, and spike-and-recovery experiments.
To construct the calibration curves of each FA, the mixed stock standard solution was diluted to four different concentrations (i.e., 3.3, 6.6, 16.5, and 82.5-fold), respectively, and finally, five working standard solutions were obtained. The calibration curves between the intensity ratios of individual FAs to ISs (the final concentrations of 37.5 μM C17:0 and 7.5 μM C21:0) versus their corresponding concentration ratios were constructed based on the above-mentioned working standard solutions. C17:0 as an IS was for quantifying the levels of C16:0, C16:1, C18:0, C18:1, C18:2, and C18:3, and C21:0 as an IS was for quantifying the levels of C20:2, C20:3, C20:4, C20:5, C22:5, and C22:6. In addition, the calibration curve of C20:4 was also used for quantifying C20:2, C20:3, and C20:5 and the calibration curve of C22:6 was for quantifying C22:5 because their commercial standards are not available. Each of the working solution was analyzed three times and the results were shown as mean ± standard deviation. The LOD is defined as the concentration of each analytes at the signal-to-noise ratio of 3.
A pooled quality control (QC) serum sample obtained from the mixture of 5 HCs and 5 LC patients sera was analyzed once every 10 test samples. Finally, a total of 144 spectra of the QC sample were obtained in this study. The experimental stability and reproducibility were evaluated using the relative standard deviation (RSD) obtained based on the intensity ratios of the detected TFAs relative to their corresponding ISs. The experimental precision was assessed based on the intraday precision of three measured values of the QC sample on the same day and the interday precision of three measured values of the QC sample on three consecutive days.
To assess the extraction efficiency of serum phospholipids and FAs, the spike-and-recovery experiment was employed. Briefly, 10 μL of the QC serum sample in triplicate was mixed with 10 μL ISs, 127.5 μL hexane/isopropanol (2:1, v:v), and 42.5 μL water, and then three resulting solutions were spiked with 10 μL of three different concentrations of standard FAs, respectively. Finally, three different concentration solutions of the spiked FAs are listed as below. R1, the concentrations of the spiked FAs: 10.0 μM C16:0, 2.5 μM C16:1, 15.0 μM C18:0, 15.0 μM C18:1, 15.0 μM C18:2, 1.2 μM C18:3, 5.0 μM C20:4, and 2.6 μM C22:6; R2, the concentrations of the spiked FAs: 100.0 μM C16:0, 25.0 μM C16:1, 150.0 μM C18:0, 150.0 μM C18:1, 150.0 μM C18:2, 12.0 μM C18:3, 50.0 μM C20:4, and 26.0 μM C22:6; R3, the concentration of the spiked FAs: 150.0 μM C16:0, 37.5 μM C16:1, 225.0 μM C18:0, 225.0 μM C18:1, 225.0 μM C18:2, 18.0 μM C18:3, 75.0 μM C20:4, and 39.0 μM C22:6. Lipid extraction of these three solutions were performed as described at the section of sample preparation and each was analyzed in triplicate.
Additional Information
How to cite this article: Ren, J. et al. Simultaneous Quantification of Serum Nonesterified and Esterified Fatty Acids as Potential Biomarkers to differentiate benign lung diseases from lung cancer. Sci. Rep. 6, 34201; doi: 10.1038/srep34201 (2016).
References
Torre, L. A. et al. Global cancer statistics, 2012. CA: a cancer journal for clinicians 65, 87–108 (2015).
Okamura, K. et al. Diagnostic value of CEA and CYFRA 21-1 tumor markers in primary lung cancer. Lung cancer 80, 45–49 (2013).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2015. CA: a cancer journal for clinicians 65, 5–29 (2015).
National Lung Screening Trial Research, T. et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. The New England journal of medicine 365, 395–409 (2011).
Sox, H. C. Screening for lung cancer with chest radiographs. Jama 306, 1916–1918 (2011).
Chu, X. Y. et al. Diagnostic values of SCC, CEA, Cyfra21-1 and NSE for lung cancer in patients with suspicious pulmonary masses: a single center analysis. Cancer biology & therapy 11, 995–1000 (2011).
Grunnet, M. & Sorensen, J. B. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung cancer 76, 138–143 (2012).
Dinasarapu, A. R. et al. Signaling gateway molecule pages-a data model perspective. Bioinformatics 27, 1736–1738 (2011).
Eyster, K. M. The membrane and lipids as integral participants in signal transduction: lipid signal transduction for the non-lipid biochemist. Adv Physiol Educ 31, 5–16 (2007).
Tvrzicka, E., Kremmyda, L. S., Stankova, B. & Zak, A. Fatty acids as biocompounds: their role in human metabolism, health and disease–a review. Part 1: classification, dietary sources and biological functions. Biomedical papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia 155, 117–130 (2011).
Burdge, G. C. & Calder, P. C. Introduction to fatty acids and lipids. World review of nutrition and dietetics 112, 1–16 (2015).
Murphy, R. A. et al. Loss of adipose tissue and plasma phospholipids: relationship to survival in advanced cancer patients. Clinical nutrition 29, 482–487 (2010).
Lagerstedt, S. A. et al. Quantitative determination of plasma c8–c26 total fatty acids for the biochemical diagnosis of nutritional and metabolic disorders. Molecular genetics and metabolism 73, 38–45 (2001).
Nakamura, M. T., Yudell, B. E. & Loor, J. J. Regulation of energy metabolism by long-chain fatty acids. Progress in lipid research 53, 124–144 (2014).
Harizi, H., Corcuff, J. B. & Gualde, N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends in molecular medicine 14, 461–469 (2008).
Zhang, Y. et al. Ammonia-treated N-(1-naphthyl) ethylenediamine dihydrochloride as a novel matrix for rapid quantitative and qualitative determination of serum free fatty acids by matrix-assisted laser desorption/ionization-Fourier transform ion cyclotron resonance mass spectrometry. Analytica chimica acta 794, 82–89 (2013).
Zhang, Y. et al. Serum unsaturated free Fatty acids: potential biomarkers for early detection and disease progression monitoring of non-small cell lung cancer. Journal of Cancer 5, 706–714 (2014).
Zhang, Y. et al. Serum Unsaturated Free Fatty Acids: A Potential Biomarker Panel for Differentiating Benign Thyroid Diseases from Thyroid Cancer. Journal of Cancer 6, 1276–1281 (2015).
Min, Q. et al. N-Doped Graphene: An Alternative Carbon-Based Matrix for Highly Efficient Detection of Small Molecules by Negative Ion MALDI-TOF MS. Analytical chemistry 86, 9122–9130 (2014).
Zerouga, M. et al. Phospholipid class as a determinant in docosahexaenoic acid’s effect on tumor cell viability. Anticancer research 16, 2863–2868 (1996).
Begin, M. E., Das, U. N., Ells, G. & Horrobin, D. F. Selective killing of human cancer cells by polyunsaturated fatty acids. Prostaglandins, leukotrienes, and medicine 19, 177–186 (1985).
Nash, S. H. et al. Association between Serum Phospholipid Fatty Acids and Intraprostatic Inflammation in the Placebo Arm of the Prostate Cancer Prevention Trial. Cancer prevention research 8, 590–596 (2015).
Cvetkovic, Z. et al. Abnormal fatty acid distribution of the serum phospholipids of patients with non-Hodgkin lymphoma. Annals of hematology 89, 775–782 (2010).
Murphy, R. A. et al. Aberrations in plasma phospholipid fatty acids in lung cancer patients. Lipids 47, 363–369 (2012).
Moser, A. B., Jones, D. S., Raymond, G. V. & Moser, H. W. Plasma and red blood cell fatty acids in peroxisomal disorders. Neurochemical research 24, 187–197 (1999).
Zuijdgeest-van Leeuwen, S. D. et al. Fatty acid composition of plasma lipids in patients with pancreatic, lung and oesophageal cancer in comparison with healthy subjects. Clinical nutrition 21, 225–230 (2002).
de Castro, J. et al. Platelet linoleic acid is a potential biomarker of advanced non-small cell lung cancer. Experimental and molecular pathology 87, 226–233 (2009).
de Castro, J. et al. Erythrocyte fatty acids as potential biomarkers in the diagnosis of advanced lung adenocarcinoma, lung squamous cell carcinoma, and small cell lung cancer. American journal of clinical pathology 142, 111–120 (2014).
Kim, Y. W. et al. MALDI-MS-based quantitative analysis for ketone containing homoserine lactones in Pseudomonas aeruginosa. Analytical chemistry 87, 858–863 (2015).
Zhu, Y. et al. Graphene and graphene oxide: synthesis, properties, and applications. Advanced materials 22, 3906–3924 (2010).
Liu, Y., Liu, J., Deng, C. & Zhang, X. Graphene and graphene oxide: two ideal choices for the enrichment and ionization of long-chain fatty acids free from matrix-assisted laser desorption/ionization matrix interference. Rapid communications in mass spectrometry: RCM 25, 3223–3234 (2011).
Ichihara, K. & Fukubayashi, Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. Journal of lipid research 51, 635–640 (2010).
Salm, P., Taylor, P. J. & Kostner, K. Simultaneous quantification of total eicosapentaenoic acid, docosahexaenoic acid and arachidonic acid in plasma by high-performance liquid chromatography-tandem mass spectrometry. Biomedical chromatography: BMC 25, 652–659 (2011).
Shen, F. et al. Age-related distributions of nine fasting plasma free fatty acids in a population of Chinese adults. Clinica chimica acta; international journal of clinical chemistry 415, 81–87 (2013).
Guo, S., Wang, Y., Zhou, D. & Li, Z. Significantly increased monounsaturated lipids relative to polyunsaturated lipids in six types of cancer microenvironment are observed by mass spectrometry imaging. Scientific reports 4, 5959 (2014).
Menendez, J. A. & Lupu, R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nature reviews. Cancer 7, 763–777 (2007).
He, M., Guo, S. & Li, Z. In situ characterizing membrane lipid phenotype of breast cancer cells using mass spectrometry profiling. Scientific reports 5, 11298 (2015).
Rao, Y. P. & Lokesh, B. R. Modulatory effects of alpha-linolenic acid on generation of reactive oxygen species in elaidic acid enriched peritoneal macrophages in rats. Indian journal of experimental biology 52, 860–869 (2014).
Murphy, R. A. et al. Skeletal muscle depletion is associated with reduced plasma (n-3) fatty acids in non-small cell lung cancer patients. The Journal of nutrition 140, 1602–1606 (2010).
Quehenberger, O. & Dennis, E. A. The human plasma lipidome. The New England journal of medicine 365, 1812–1823 (2011).
Zhao, X. et al. Serum metabolomics study of polycystic ovary syndrome based on liquid chromatography-mass spectrometry. Journal of proteome research 13, 1101–1111 (2014).
Conway, E. M. et al. Macrophages, Inflammation, and Lung Cancer. American journal of respiratory and critical care medicine (2015).
Huang, Q. et al. Metabolic characterization of hepatocellular carcinoma using nontargeted tissue metabolomics. Cancer research 73, 4992–5002 (2013).
Taketo, M. M. & Sonoshita, M. Phospolipase A2 and apoptosis. Biochimica et biophysica acta 1585, 72–76 (2002).
Patterson, E. et al. Health implications of high dietary omega-6 polyunsaturated Fatty acids. Journal of nutrition and metabolism 2012, 539426 (2012).
Das, U. N. Essential fatty acids: biochemistry, physiology and pathology. Biotechnology journal 1, 420–439 (2006).
Hara, A. & Radin, N. S. Lipid extraction of tissues with a low-toxicity solvent. Analytical biochemistry 90, 420–426 (1978).
Acknowledgements
This study was funded by the National Natural Science Foundation of China (grant No. 91542101) to Z. Li.
Author information
Authors and Affiliations
Contributions
Z.L. and J.R. provided the original concept for the research and designed the study. J.R. performed the experiments with the assistance of Dan Zhang, Y.L., R.Z., S.G., Dan Zhou, M.Z. and Y.X. L.Q. and H.F. collected clinical information on participants. J.R. and Z.L. discussed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ren, J., Zhang, D., Liu, Y. et al. Simultaneous Quantification of Serum Nonesterified and Esterified Fatty Acids as Potential Biomarkers to Differentiate Benign Lung Diseases from Lung Cancer. Sci Rep 6, 34201 (2016). https://doi.org/10.1038/srep34201
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep34201
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.