Abstract
One of the main azole-resistance mechanisms in Candida pathogens is the upregulation of drug efflux pumps, which compromises the efficacy of azoles and results in treatment failure. The combination of azole-antifungal agents with efflux pump inhibitors represents a promising strategy to combat fungal infection. High-throughput screening of 150 extracts obtained from endolichenic fungal cultures led to the discovery that the extract of Phialocephala fortinii exhibits potent activity for the reversal of azole resistance. From P. fortinii cultures, a total of 15 quinone derivatives, comprising 11 new derivatives and 4 known compounds, were obtained. Among these compounds, palmarumycin P3 (3) and phialocephalarin B (8) specifically modulate the expression of MDR1 to inhibit the activity of drug efflux pumps and therefore reverse azole resistance. The present study revealed Mdr1 targeting as an alternative mechanism for the discovery of new agents to fight antifungal drug resistance.
Similar content being viewed by others
Introduction
Candida albicans is the most frequent human commensal opportunistic fungal pathogen, resulting in high morbidity and mortality, particularly in immunosuppressed patients1,2,3. Reflecting the widespread and prolonged usage of antibiotics, the emergence of pathogenic fungi with multidrug resistance (MDR) is also increasing, further complicating antifungal therapy4,5. Azole antifungal drugs are commonly used for fungal infections, but an increasing incidence of azole resistance is occurring in the clinic6. The mechanisms leading to azole resistance include alterations in the sterol biosynthetic pathway, increased expression of the ERG11 gene encoding the target enzyme of fluconazole (FLC), sterol 14α-demethylase (Erg11), mutations in the ERG11 gene resulting in the reduced affinity of Erg11 to FLC, and the overexpression of genes encoding membrane transport proteins, which pump FLC out of the cell7,8,9. Among these pumps, the overexpression of drug transporters is a principal mechanism utilized by Candida species to alleviate antibiotic stress through a reduction in the intracellular accumulation. In Candida species, 2 gene transporter families, the CDR genes of the ATP-binding cassette super family and the MDR genes of the major facilitators class, encode drug transporters10. In Candida albicans, Cdr1 and Cdr2 are ATP-binding cassette transporters that use energy derived from ATP hydrolysis to transport drugs outside the cells, while Mdr1, a major facilitator superfamily (MFS) protein, utilizes a proton gradient for drug extrusion9. Many FLC-resistant clinical C. albicans isolates constitutively overexpress MDR111,12,13,14,15. The inactivation of MDR1 in MDR1-overexpressing C. albicans isolates is an important pathway to increase the susceptibility of these microbes to FLC16.
The combination of azoles and other non-antifungal agents, such as specific inhibitors of efflux pumps, is a promising approach to manage resistant Candida infections17,18,19,20,21. Natural products are an important source for the discovery of active agents, reflecting the versatile structures of the products22,23,24. In previous studies, we focused on the development of diversified natural products with antifungal activities from bryophytes or endolichenic fungi25,26,27,28. Therefore, we developed both an Alamar Blue assay and an agar diffusion assay to screen a natural products library for hits that chemosensitize C. albicans to fluconazole (FLC) treatment.
In the present study, we examined the reversal of azole resistance in 150 extracts from endolichenic fungi cultures, leading to the discovery that the extract of Phialocephala fortinii displayed potent activity to reverse azole resistance. Isolation of P. fortinii metabolites afforded 15 quinone derivatives. Among the isolated compounds, palmarumycin P3 (3) and phialocephalarin B (8), which two representatives of the compounds obtained, could specifically modulate the expression of MDR1 to inhibit the activity of drug efflux pumps and therefore reverse azole resistance.
Results
HTSS for antifungal hits from a microbial natural product library
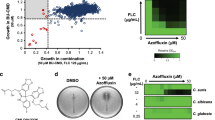
A library of 150 endolichenic fungi was isolated from collected lichens. We prepared microbial fermentation extracts of the endolichenic fungi and screened them to identify the hits using Alamar Blue or agar diffusion assays. These hits should show low antifungal activity by themselves and potent enhancement of the efficacy of FLC against azole-resistant C. albicans isolates. Among 150 culture extracts, P. fortinii culture demonstrated potent capability of reversal of azole resistance and low cell toxicity (Supplementary Results Fig. S1). Thus, P. fortinii was fermented at a large scale for subsequent analysis.
Identification of single compounds as active components in the hit
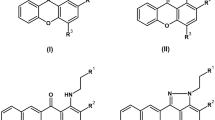
P. fortinii culture was extracted using EtOAc and repeatedly subjected to chromatography over silica gel, Sephadex LH-20, MPLC and further semi-preparative HPLC under bioassay-guided separation, generated fifteen quinone derivatives, including six spirobisnaphthalenes (1–6), four perylenequinones (7–10) and five naphthalenone (11–15). Among these derivatives, eleven compounds were novel compounds, indicated in a red colour (Fig. 1). To elucidate the structures of new compounds, including palmarumycin P1-P4 (1–4), phialocephalarin A-D (7–10), and juglanone C-E (11–13), HRESIMS, 1H and 13C NMR, and 2D NMR were performed. The spectra revealed that these compounds have similar structural features as members of the spirobisnaphthalenes, perylenequinones, and naphthalenones, respectively (Supplementary Results Tables S1–S3). By determining HMBC spectra, the planar structures of these compounds were unambiguously established. The absolute configurations of 2, 3, 4, and 7 were further determined based on a single-crystal X-ray diffraction analysis with Cu Kα radiation (Supplementary Results). The absolute configuration assignments of the other new compounds were determined through a comparison of the CD spectra (Supplementary Results Figs S8, 47, 55, 63, 71, 79, and 87). The known compounds were identified through a comparison of the spectroscopic data with previously reported data29,30,31.
All the pure compounds were assayed for the inhibition of C. albicans growth or the reversal of the azole resistance of clinical strain 24D, which displays relatively high transcriptional expression of MDR1 when incubated with FLC among our collected clinical isolates (unpublished data). The results showed that these compounds alone did not exert any inhibitory activity against the growth of C. albicans. However, spirobisnaphthalene and perylenequinone derivatives (1–10) conferred 64-fold or higher sensitivity on strain 24D to FLC (Fig. 2 and Supplementary Results Table S4). Because compounds 3 and 8 are the major constituents in the EtOAc extract, these compounds were selected for the subsequent studies.
(A,B) Growth inhibitory effects against C. albicans 24D under the indicated treatments were revealed using the Alamar blue assay. The cells were treated with the indicated drugs for 48 h, followed by further analysis using Alamar blue staining for 2 h in the dark, revealing pink supernatant when the cells proliferated (A). The growth percentage was measured using a spectrophotometer at 570 nm (B). (C) The growth inhibitory effect under the indicated treatments was revealed using the disk diffusion assay. For this assay, the individual test organism 24D (1 × 106 CFUs/ml) was plated on Mueller Hinton agar (MHA) medium supplemented with 2% glucose. Cellulose disks impregnated with FLC (2 μg), either compound (64 μg) or a combination of each agent (2 μg of FLC and 16 μg of compound) were placed onto MHA agar plates. Each plate was incubated at 30 °C for 48 h for the agar diffusion assay.
Inhibition of C. albicans multidrug resistance through the modulation of efflux pumps
Compounds 3 and 8 displayed azole-reversal effects together with FLC against several clinical azole-resistant strains, including 24D, 28I, CA10, CA406, CA417, and CA631 (Table 1). The Alamar Blue assay showed that the addition of 3 or 8 could facilitate inhibition of the growth of C. albicans strain 24D by 94.25 ± 1.04% and 93.77 ± 0.79%, respectively, through FLC, whereas FLC alone caused only a 9.11 ± 4.2% reduction in growth (Fig. 2A,B). The agar plate assay further confirmed the observed enhanced inhibitory action when FLC was applied together with compound 3 or 8 (Fig. 2C). The cytotoxicities of these two compounds were evaluated based on the IC50 values against the following normal cell lines: human umbilical vein endothelial cells (HUVEC), human bronchial epithelium (HBE) cells and non-neoplastic, immortalized human prostatic epithelial (RWPE-1) cells. The IC50 values of compounds 3 and 8 were much higher than the dose used in the combination treatment (Table 2), suggesting low toxicity in the application. Upregulation of drug efflux pumps has been reported as one of the most important factors, conferring azole resistance10,11. The efflux activity assay revealed that compounds 3 and 8 could facilitate the accumulation of Rh123 based on the flow cytometry analysis and CLSM observation, suggesting an inhibitory effect on the efflux pumps (Fig. 3).
(A) The measurement of Rh123 efflux, induced through glucose in the FLC-resistant strain 24D, was assessed using flow cytometry detection. (B) The intracellular accumulation of Rh123 in C. albicans cells in response to treatment with compound 3 (16 μg/ml) or 8 (16 μg/ml) was revealed through CLSM observation.
Compounds 3 and 8 specifically modulate MDR1 expression in C. albicans
Most clinically drug-resistant isolates of C. albicans overexpress genes encoding Cdr1, Cdr2 or Mdr1 drug efflux pump proteins32,33,34. Here, we observed that compound 3 or 8 reduced the expression of MDR1 in azole-resistant strain 24D (Fig. 4A). However, these two compounds had less potent effects on the reduction of CDR1 or CDR2 expression (Fig. 4A). The transcriptional expression of MDR1 with time of exposure to compound 3 or 8 was monitored by using quantitative real-time PCR (qPCR). The results demonstrated that MDR1 expression was firstly induced by 3 or 8 during the initial 1 hour, and followed by a decrease within the next 5 hours in our test (Fig. 4B). The induced expression at the initial 1 hour probably acts as a feedback of compromised efflux pump function.
(A,B) The azole-resistant isolate (24D) was incubated with FLC for 3 h at 30 °C and subsequently cultured in RPMI 1640 medium containing the indicated agents. (A) After 3 h of incubation, the relative expression of CDR1, CDR2 and MDR1 genes was determined through qPCR and normalized to 18S. (B) At indicated time ranging from 0.5 to 6 h, the MDR1 expression of C. albicans in the presence of compound 3 or 8 was monitored by using qPCR. (C) C. tropicalis FLC-resistant isolate (NPC-T001) was treated with FLC (1 μg/mL), tested compound (8 μg/mL) or their combination. After 12 h of culture, RNA was extracted for MDR1 transcript analysis by using qPCR. The bars represent the means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
To confirm that MDR1 was the primary effector of compound 3 or 8, mutant strains deficient in efflux pumps, including DSY488 (cdrlΔ/Δ), DSY653 (cdr2Δ/Δ), DSY465 (mdrlΔ/Δ), and DSY659 (cdrlΔ/Δ, cdr2Δ/Δ), were examined for susceptibility to the combination treatment of FLC and compound 3 or 8. The results showed that the combination treatment displayed synergistic action against mutant strains lacking CDR1, CDR2, or both genes, while the synergistic index was lower in mutant strain deficient in MDR1 (Tables 3 and 4). We also measured the effect of the combination treatment on a CDR1 and CDR2-overexpressing strain (YEM15) and an MDR1-overexpressing strain (YEM13). We observed that compound 3 or 8 could reverse the drug resistance of YEM13 with a decrease in the minimal inhibitory concentration (MIC) of FLC from 64 to 2–4 μg/ml, whereas a high dose of compound 3 or 8 was required to sensitize YEM15 to FLC (Tables 3 and 4). These results implied that the regulation of MDR1 was probably the prime target of compounds 3 and 8.
Compounds 3 and 8 reverse the FLC resistance of C. tropicalis through a reduction in MDR1 expression
Compounds 3 and 8 could not only reverse the azole resistance of C. albicans strains but also sensitize the azole resistance of a C. tropicalis strain to FLC. We observed that compound 3 or 8 reduced the MIC of FLC against C. tropicalis strain NPC-T001 from 128 to 1 μg/ml when used at the concentration of 8 μg/ml. The qPCR assay revealed that compound 3 or 8 caused 5.54- or 3.73-fold reductions in MDR1 expression, respectively, compared with the control. When the strain was exposed to FLC, the expression of MDR1 increased 3.73-fold. However, the addition of compound 3 or 8 reduced the MDR1 expression in FLC-treated cells by 7.56 or 12.98-fold, respectively (Fig. 4C). These results implied that compounds 3 and 8 could be developed as MDR1 modulators to reverse the azole resistance in Candida species.
Discussion
MDR in Candida, resulting from the overexpression of efflux pumps, is a major obstacle in antifungal chemotherapy. Identifying selective, low-toxicity inhibitors/modulators of MDR might be a promising strategy to combat this problem. Compounds from natural products represent one of the most diverse and novel chemical scaffolds suitable for the development of new inhibitors/modulators35,36,37,38. Many researchers have recognized the value of screening for new modulators from natural sources, as natural extracts are typically low in toxicity and well tolerated in the human body. In the present study, HTSS was applied to identify the chemosensitizers from 150 endolichenic fungal extracts. Six spirobisnaphthalene derivatives and four perylenequinone derivatives with eight novel structures were isolated from the fungal extracts and demonstrated to harbour the ability to reverse azole resistance. Compounds 3 and 8, as the two major constituents of the spirobisnaphthalene and perylenequinone derivatives, were selected to investigate the mode of action. Compounds 3 and 8 inhibited the activity of efflux pumps, and could elevate the intracellular content of FLC when applied for treatment against azole-resistant strains. We subsequently utilized efflux pump-deficient strains and quantitative real-time PCR to verify that compounds 3 and 8 primarily affect the transcriptional levels of MDR1 and have a less potent effect on the expression of CDR1 and CDR2.
Overexpression of the multidrug efflux pump Mdr1 increased fluconazole resistance in C. albicans10. The upregulation of MDR1 is controlled through the transcription factors Mrr1 and Cap139. Gain-of-function mutations in Mrr1 or Cap1 render the transcription factors hyperactive and result in constitutive MDR1 overexpression39,40. Mrr1 contains multiple activation and inhibitory domains, which regulate MDR1 expression41. A previous study showed that the transcription factor Mcm1 was required for hyperactive Mrr1, causing MDR1 overexpression42. To date, the direct mutual interaction between MDR1 and transcription factors has not been elucidated. It is highly likely that compound 3 or 8 interferes with the interaction between the transcription of MDR1 and the transcription factors of Mrr1 and Cap1, although further evidence is needed.
Several efforts have focused on discovering selective inhibitors or modulators to overcome MDR in cancer chemotherapy over the years43,44,45,46. However, little effort has been made to investigate antifungal actions, particularly in clinical application. The results of the present study indicate that quinone derivatives are MDR1 modulators that can reverse azole resistance in Candida species. Compounds 3 and 8, originating from natural products, represent more selective and potent chemosensitizers to improve FLC in treating fungal infections.
Methods
Strains and growth conditions
The fungus Phialocephala fortinii used in the present study was isolated from the lichen Pamelia sp., collected in Mount Qingliang, Zhejiang Province, China. The fungus was identified using nuclear 18S rDNA sequences (GenBank: AB208110), assigned the accession no. 4537d and deposited in the lichen laboratory in the College of Life Sciences, Shandong Normal University, Jinan. The C. albicans isolates used in the present study are shown in Supplementary Results Table S5. C. albicans was propagated in yeast-peptone dextrose (YPD) medium in an orbital shaker at 30 °C and assayed in RPMI 1640 medium. The normal cell lines HUVEC, HBE and RWPE-1 were cultured as previously described27,47,48,49.
Crude extract preparation
A total of 150 endolichenic fungal species were provided from Professor Zuntian Zhao in Shandong Normal University. The individual colonies of each strain were streaked onto potato dextrose agar (PDA) plates. After 10 days of culture at 28 °C, the organisms were scraped and extracted using EtOAc for HTCC.
HTSS for antifungal hits using Alamar Blue and agar diffusion assays
The drug susceptibility test for screening antifungal hits was performed using Alamar Blue and disk diffusion assays as previously described, with slight modifications50,51. For the Alamar Blue assay, C. albicans isolates were cultured in YPD medium (1% yeast extract, 2% bacto peptone and 2% dextrose) at 30 °C with rotational shaking at 200 rpm. Overnight cultured cells were collected, washed and diluted to a cell density of 1 × 103 CFUs/ml in RPMI 1640 medium. Aliquots of 100 μl of the fungal suspension with 16, 32, and 64 μg/ml of the solubilized drugs containing 4 μg/ml FLC were added to the wells of 96-well flat-bottomed microtitration plates. After incubation for 48 h, 10 μl of Alamar blue was added to the wells, the subsequent colour change was photographed after 2 h of incubation in the dark, and the absorbance at 570 nm was measured using a spectrophotometer.
For the agar diffusion assay, overnight cultures were diluted using PBS to 1 × 107 CFUs/ml. Aliquots of 100 μl of yeast suspension were spread onto Mueller Hinton agar (MHA) medium supplemented with 2% glucose. To examine the antifungal activity of each combination of partner drugs and FLC, cellulose disks impregnated with 16, 32, and 64 μg of the solubilized drugs and 4 μg of FLC and the control disk impregnated with the corresponding solvent were placed onto YPD agar plates. After 48 h of incubation at 30 °C, the horizontal and vertical diameters of the growth inhibition areas were recorded.
Isolation of active compounds
The endolichenic fungus P. fortinii was cultivated in three 500-ml Erlenmeyer flasks, each of which contained 100 ml of potato dextrose broth (PDB), at 25 °C on a rotary shaker (120 rpm) for 7 days to prepare the seed culture. Large-scale fermentation was performed in twenty 500-ml Erlenmeyer flasks, each of which contained 80 g of autoclaved rice, and subsequently these cultures were inoculated with the spore inoculum (15 ml) and cultured for 50 days at room temperature. The culture was subsequently extracted three times using EtOAc (6 L), and the organic solvent was evaporated under reduced pressure to generate a crude extract (72.2 g). The EtOAc extract was repeatedly subjected to chromatography over silica gel, Sephadex LH-20, MPLC and further semi-preparative HPLC to afford fifteen compounds. Silica gel (200–300 mesh; Qingdao Haiyang Chemical Co. Ltd., Qingdao, P. R. China) and Sephadex LH-20 gel (25–100 mm; Pharmacia Biotech, Denmark) were used for column chromatography (CC). MPLC was performed on a Leisure EZ Purifier apparatus equipped with a UV-VIS dual wavelength detector (210 and 254 nm) (Leisure Science Corporation) and an ODS column (30 × 130 mm). HPLC was performed on an Agilent 1100 G1310A isopump equipped with a G1322A degasser, a G1314A VWD detector (210 nm), and a ZORBAX SB-C18 5 mm column (9.4 × 250 mm).
Elucidation of the chemical structures
Optical rotations were obtained using an Anton paar MCP 200 polarimeter. The UV data were recorded on a UV-2450 spectrophotometer (Shimadzu, Japan). The CD spectra were obtained on a Chirascan spectropolarimeter. The IR spectra were measured on a Nicolet iN 10 Micro FTIR spectrometer. The NMR spectra were recorded on a Bruker Avance DRX-600 spectrometer at 600 (1H) and 150 (13C) MHz, with TMS as an internal standard. HRESIMS was performed on a Finnigan LC-QDECA mass spectrometer, and x-ray crystallographic analyses were conducted on a Bruker D8 venture or Bruker APEX DUO diffractometer, employing APEX II CCD using Cu Kα radiation.
Minimum inhibitory concentration determination
The minimum inhibitory concentrations (MIC80) of compounds against Candida species were determined through broth microdilution according to CLSI M27-A3 guidelines52. A susceptibility test of the efflux pump mutant strains was also conducted using the procedures for MIC determination.
Cytotoxicity detection through the MTT assay
A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazoliumbromide (MTT, Sigma) colorimetric assay was used to assess the proliferation and cytotoxicity against HUVEC, HBE and RWPE-1 cells in the presence of compound 3 or 847,48,49,53. The cells (1 × 104 per well) were seeded onto 96-well plates and incubated at 37 °C in a 5% CO2 incubator. After incubation of 24 h, the cells were treated with vehicle or desired concentrations of compounds 3 and 8 for an additional 24 h. After treatment for 24 h, the cells were incubated with MTT for an additional 4 h in the dark. The cell growth response to the chemicals was detected after measuring the light absorbance at 570 nm using a plate reader (Bio-Rad Laboratories, Richmond, CA). The IC50 values were calculated based on the percentage of viable cells.
The interaction of the tested compounds with FLC against Candida species
To assess the nature of the in vitro interactions between the tested agents and FLC against C. albicans and C. tropicalis strains, an FICI model was used to characterize the interactions between the tested agents and FLC through analysis of the data obtained from broth microdilution checkerboard assays. The FICI model is described as Σ FIC = FICA + FICB = MICAB/MICA + MICBA/MICB, where MICA and MICB are the MICs of drugs A and B when used alone, and MICAB and MICBA are the concentrations of drugs A and B in the iso-effective combinations, respectively. According to the results calculated from each dataset, synergy corresponds to a FICI value of ≤0.5, while antagonism reflects a FICI value of >4; otherwise, indifference is concluded54.
Transport Assays
Transport assays were conducted by monitoring rhodamine 123 (Rh123) accumulation. The accumulation of Rh123 in azole-resistant cells was measured using flow cytometry (Becton-Dickinson Immunocytometry Systems) and observed using confocal microscopy. Briefly, overnight-cultured cells were collected, washed and resuspended in YPD medium. After incubation at 30 °C for 4 h with shaking, the cells were pelleted, washed and incubated with 5 μM Rh123 at 30 °C for 30 min in PBS, and subsequently the cells were incubated for an additional 30 min in the absence or presence of the tested compounds. The cells were subsequently harvested, and 10,000 cells were analysed in the acquisition. The analysis was performed using CellQuest software (Becton Dickinson Immunocytometry Systems).
qPCR analysis
The transcriptional expression of CDR1, CDR2 and MDR1 in the tested C. albicans or C. tropicalis isolates was measured using qPCR as previously reported55. The primers used are shown in Supplementary Results Table S6. 18S rRNA served as the internal control in C. albicans, while ACT1 was used as an internal control in C. tropicalis. The transcript levels of the detected genes were calculated using the formula 2−ΔΔCT.
Statistical analysis
The experimental data were statistically analysed using Student’s t-test. The asterisks indicate critical levels of significance (*p < 0.05, **p < 0.01, and ***p < 0.001).
Additional Information
How to cite this article: Xie, F. et al. Quinone derivatives isolated from the endolichenic fungus Phialocephala fortinii are Mdr1 modulators that combat azole resistance in Candida albicans. Sci. Rep. 6, 33687; doi: 10.1038/srep33687 (2016).
References
Goodman, J. L. et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N. Engl. J. Med. 326, 845–851 (1992).
US Public Health Service, Infectious Diseases Society of America, & Prevention of Opportunistic Infections Working Group. 2001 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. Infect Dis Obstet Gynecol 10, 3 (2002).
Ascioglu, S. et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34, 7–14 (2002).
Kontoyiannis, D. P. & Lewis, R. E. Antifungal drug resistance of pathogenic fungi. Lancet 359, 1135–1144 (2002).
Pfaller, M. A. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am. J. Med. 125, S3–S13 (2012).
Arendrup, M. Update on antifungal resistance in Aspergillus and Candida . Clin. Microbiol. Infect. 20, 42–48 (2014).
Kanafani, Z. A. & Perfect, J. R. Resistance to antifungal agents: mechanisms and clinical impact. Clin. Infect. Dis. 46, 120–128 (2008).
Spampinato, C. & Leonardi, D. Candida infections, causes, targets, and resistance mechanisms: traditional and alternative antifungal agents. Biomed Res Int 2013 (2013).
Sanglard, D. & Odds, F. C. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect Dis 2, 73–85 (2002).
Cannon, R. et al. Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 22, 291–321 (2009).
Albertson, G. D., Niimi, M., Cannon, R. D. & Jenkinson, H. F. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob. Agents Chemother. 40, 2835–2841 (1996).
Sanglard, D. et al. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39, 2378–2386 (1995).
White, T. C. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 41, 1482–1487 (1997).
Lopez-Ribot, J. L. et al. Distinct Patterns of Gene Expression Associated with Development of Fluconazole Resistance in Serial Candida albicans Isolates from Human Immunodeficiency Virus-Infected Patients with Oropharyngeal Candidiasis. Antimicrob. Agents Chemother. 42, 2932–2937 (1998).
Perea, S. et al. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 45, 2676–2684 (2001).
Wirsching, S., Michel, S. & Morschhäuser, J. Targeted gene disruption in Candida albicans wild‐type strains: the role of the MDR1 gene in fluconazole resistance of clinical Candida albicans isolates. Mol. Microbiol. 36, 856–865 (2000).
Liu, S. et al. Combination of fluconazole with non-antifungal agents: a promising approach to cope with resistant Candida albicans infections and insight into new antifungal agent discovery. Int. J. Antimicrob. Agents 43, 395–402 (2014).
Monk, B. C. & Goffeau, A. Outwitting multidrug resistance to antifungals. Science 321, 367–369 (2008).
Spitzer, M. et al. Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol. Syst. Biol. 7, 499 (2011).
Schuetzer-Muehlbauer, M., Willinger, B., Egner, R., Ecker, G. & Kuchler, K. Reversal of antifungal resistance mediated by ABC efflux pumps from Candida albicans functionally expressed in yeast. Int. J. Antimicrob. Agents 22, 291–300 (2003).
Holmes, A. R. et al. The monoamine oxidase a inhibitor clorgyline is a broad-spectrum inhibitor of fungal ABC and MFS transporter efflux pump activities which reverses the azole resistance of Candida albicans and Candida glabrata clinical isolates. Antimicrob. Agents Chemother. 56, 1508–1515 (2012).
Harvey, A. L. Natural products in drug discovery. Drug Discov. Today 13, 894–901 (2008).
Paterson, I. & Anderson, E. A. The renaissance of natural products as drug candidates. Science 310, 451 (2005).
Koehn, F. E. & Carter, G. T. The evolving role of natural products in drug discovery. Nat Rev Drug Discov 4, 206–220 (2005).
Zhang, L. et al. Bisbibenzyls, a new type of antifungal agent, inhibit morphogenesis switch and biofilm formation through upregulation of DPP3 in Candida albicans . PloS ONE 6, e28953 (2011).
Chang, W., Li, Y., Zhang, L., Cheng, A. & Lou, H. Retigeric acid B attenuates the virulence of Candida albicans via inhibiting adenylyl cyclase activity targeted by enhanced farnesol production. PloS ONE 7, e41624 (2012).
Chang, W. et al. Lichen endophyte derived pyridoxatin inactivates Candida growth by interfering with ergosterol biosynthesis. BBA-General Subjects 1850, 1762–1771 (2015).
Li, Y. et al. Diorcinol D Exerts Fungicidal Action against Candida albicans through Cytoplasm Membrane Destruction and ROS Accumulation. PloS ONE 10, e0128693 (2015).
Sakemi, S. et al. CJ-12,371 and CJ-12,372, two novel DNA gyrase inhibitors. Fermentation, isolation, structural elucidation and biological activities. J. Antibiot. 48, 134–142 (1995).
Kokubun, T., Veitch, N. C., Bridge, P. D. & Simmonds, M. S. Dihydroisocoumarins and a tetralone from Cytospora eucalypticola . Phytochemistry 62, 779–782 (2003).
Zhu, Y. et al. Screening and isolation of antinematodal metabolites against Bursaphelenchus xylophilus produced by fungi. Ann. Microbiol. 58, 375–380 (2008).
Holmes, A. R. et al. ABC transporter Cdr1p contributes more than Cdr2p does to fluconazole efflux in fluconazole-resistant Candida albicans clinical isolates. Antimicrob. Agents Chemother. 52, 3851–3862 (2008).
Chen, L. et al. Overexpression of CDR1 and CDR2 genes plays an important role in fluconazole resistance in Candida albicans with G487T and T916C mutations. J. Int. Med. Res. 38, 536–545 (2010).
Dunkel, N., Blaß, J., Rogers, P. D. & Morschhäuser, J. Mutations in the multi-drug resistance regulator MRR1, followed by loss of heterozygosity, are the main cause of MDR1 overexpression in fluconazole-resistant Candida albicans strains. Mol. Microbiol. 69, 827–840 (2008).
Newman, D. J., Cragg, G. M. & Snader, K. M. Natural products as sources of new drugs over the period 1981-2002. J. Nat. Prod. 66, 1022–1037 (2003).
Arif, T. et al. Natural products–antifungal agents derived from plants. J Asian Nat Prod Res 11, 621–638 (2009).
Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 75, 311–335 (2012).
Cragg, G. M. & Newman, D. J. Natural products: a continuing source of novel drug leads. BBA-General Subjects 1830, 3670–3695 (2013).
Schubert, S. et al. Regulation of efflux pump expression and drug resistance by the transcription factors Mrr1, Upc2, and Cap1 in Candida albicans . Antimicrob. Agents Chemother. 55, 2212–2223 (2011).
Morschhäuser, J. et al. The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans . PLoS Pathog 3, e164 (2007).
Schubert, S., Popp, C., Rogers, P. D. & Morschhäuser, J. Functional dissection of a Candida albicans zinc cluster transcription factor, the multidrug resistance regulator Mrr1. Eukaryotic Cell 10, 1110–1121 (2011).
Mogavero, S., Tavanti, A., Senesi, S., Rogers, P. D. & Morschhäuser, J. Differential requirement of the transcription factor Mcm1 for activation of the Candida albicans multidrug efflux pump MDR1 by its regulators Mrr1 and Cap1. Antimicrob. Agents Chemother. 55, 2061–2066 (2011).
Wu, C.-P., Ohnuma, S. & Ambudkar, S. V. Discovering natural product modulators to overcome multidrug resistance in cancer chemotherapy. Curr Pharm Biotechnol 12, 609 (2011).
Thomas, H. & Coley, H. M. Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glycoprotein. Cancer control 10, 159–159 (2003).
Takara, K., Sakaeda, T. & Okumura, K. An update on overcoming MDR1-mediated multidrug resistance in cancer chemotherapy. Curr. Pharm. Des. 12, 273–286 (2006).
Huang, M., Jin, J., Sun, H. & Liu, G. T. Reversal of P-glycoprotein-mediated multidrug resistance of cancer cells by five schizandrins isolated from the Chinese herb Fructus Schizandrae . Cancer. Chemoth. Pharm. 62, 1015–1026 (2008).
Wu, C.-Y. et al. PCSK9 siRNA inhibits HUVEC apoptosis induced by ox-LDL via Bcl/Bax–caspase9–caspase3 pathway. Mol. Cell. Biochem. 359, 347–358 (2012).
Soria, J.-C. et al. Lack of PTEN expression in non-small cell lung cancer could be related to promoter methylation. Clin. Cancer Res. 8, 1178–1184 (2002).
Fernández-Martínez, A. B. et al. Vasoactive intestinal peptide (VIP) induces malignant transformation of the human prostate epithelial cell line RWPE-1. Cancer Lett. 299, 11–21 (2010).
Zhang, L. et al. High-throughput synergy screening identifies microbial metabolites as combination agents for the treatment of fungal infections. Proc. Natl. Acad. Sci. USA 104, 4606–4611 (2007).
Marchetti, O., Moreillon, P., Glauser, M. P., Bille, J. & Sanglard, D. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans . Antimicrob. Agents Chemother. 44, 2373–2381 (2000).
Clinical and Laboratory Standards Institute, Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, CLSI document M27-A33rd ed., CLSI, Wayne, PA (2008).
Spitzer, M. S. et al. Antiproliferative and cytotoxic properties of bevacizumab on different ocular cells. Br J Ophthalmol 90, 1316–1321 (2006).
Li, Y. et al. Synergistic and drug-resistant reversing effects of diorcinol D combined with fluconazole against Candida albicans . FEMS Yeast Res. 15 (2015).
Chang, W. et al. Retigeric acid B enhances the efficacy of azoles combating the virulence and biofilm formation of Candida albicans . Biol. Pharm. Bull. 35, 1794–1801 (2012).
Acknowledgements
We thank Professor Kim Lewis at Northeastern University for providing the efflux pump-related strains and Professor Qingguo Qi in Shandong University of China for donating the clinical strains. We are also grateful to Professor Judith Berman at the University of Minnesota for providing the plasmid to construct GFP-tagged strains. The normal cell lines were kindly provided by Professor Huiqing Yuan at Shandong University. This work was financially supported by the National Natural Science Foundation (nos. 81273383 and 81402804) and the China Postdoctoral Science Foundation (2014M551925).
Author information
Authors and Affiliations
Contributions
H.L., W.C. and F.X. designed the experiments and wrote the manuscript. F.X. and W.L. isolated the compounds and determined their structures. M.Z., H.S. and S.Z. bioscreened the compounds. W.C., M.Z. and Y.L. performed microscopic, real-time PCR and flow cytometry analysis. W.C., F.X. and H.L. analysed the data. W.C., F.X., M.Z. and H.L. prepared all the figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Xie, F., Chang, W., Zhang, M. et al. Quinone derivatives isolated from the endolichenic fungus Phialocephala fortinii are Mdr1 modulators that combat azole resistance in Candida albicans. Sci Rep 6, 33687 (2016). https://doi.org/10.1038/srep33687
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep33687
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.