Abstract
Nitrogen (N) deposition has been steadily increasing for decades, with consequences for soil respiration. However, we have a limited understanding of how soil respiration responds to N availability. Here, we investigated the soil respiration responses to low and high levels of N addition (0.4 mol N m−2 yr−1 vs 1.6 mol N m−2 yr−1) over a two-year period in a semiarid Leymus chinensis grassland in Inner Mongolia, China. Our results show that low-level N addition increased soil respiration, plant belowground biomass and soil microbial biomass carbon (MBC), while high-level N additions decreased them. Soil respiration was positively correlated with plant belowground biomass, MBC, soil temperature and soil moisture. Together plant belowground biomass and MBC explained 99.4% of variation in mean soil respiration, with plant belowground biomass explaining 63.4% of the variation and soil MBC explaining the remaining 36%. Finally, the temperature sensitivity of soil respiration was not influenced by N additions. Overall, our results suggest that low levels of N deposition may stimulate soil respiration, but large increases in N availability may decrease soil respiration, and that these responses are driven by the dissimilar responses of both plant belowground biomass and soil MBC.
Similar content being viewed by others
Introduction
Human activities such as fossil fuel combustion, land use change, and fertilizer production have significantly increased nitrogen (N) input to the biosphere1, which has greatly altered ecosystem structure and functions2, including the terrestrial carbon (C) cycle. Soil respiration plays a crucial role in regulating climate-C feedbacks, which is recognized as the second largest C efflux between the atmosphere and terrestrial ecosystems3,4. Predictions from global modeling studies suggest that even a small change in soil respiration has the potential to impact atmosphere CO2 accumulation and the global C budget5,6. It is therefore important to understand the responses of soil respiration to N increases, which may greatly affect the direction and extent of the C balance response.
Soil respiration consists of two components, root respiration and microbial respiration. Root respiration derives from plant roots, mycorrhizal fungi, and other associated microorganisms (rhizosphere microorganisms) that use C that has been recently fixed by plant photosynthesis. Microbial respiration derives from decomposition of plant residues and soil organic matter. Previous studies have shown that N addition impacts soil respiration by altering plant above- and belowground biomass7 and their ratios8, water availability9, litter quantity and quality10, soil microbial biomass11,12, and temperature sensitivity13. Among these factors, plant belowground biomass has been recognized as the leading impact factor of root respiration14, while soil microbial biomass has been recognized as the leading impact factor of microbial respiration11,12. Thus, investigating plant belowground biomass and microbial biomass C (MBC) may be very helpful in explaining the underlying mechanisms of how N addition affects soil respiration. This better understanding of the underlying mechanisms driving the soil respiration response to increased N availability could help improve predictions of how soil C cycling may respond to N increases in the future and aid optimization of carbon-nitrogen-climate interaction models.
Past investigation of the responses of soil respiration to experimental N addition have been inconsistent9,13,15,16, because N addition could either increase root biomass17 or decrease C allocation to belowground biomass8, as well as alter soil microorganisms18,19. Changes in the relative contribution of root respiration to soil respiration could also be important in interpreting different responses of soil respiration to different levels of N addition20. In general, short-term studies have found that soil respiration was stimulated by relatively low levels of N additions (not more than 10 g N m−2)21, particularly in N-limited ecosystems, such as the semiarid grasslands of the Loess Plateau, Inner Mongolia, and the alpine steppe in northern Tibet13,22,23. In contrast, high levels of N addition (more than 15 g N m−2) have less consistent effects on soil respiration. For example, soil respiration was reduced in response to a high level of N addition in a mature tropical forest in southern China16, but increased in a temperate forest at Harvard Forest and a semiarid grassland in northern China9,24. Thus, the levels of N addition may be a dominant driver of different responses of soil respiration to N addition.
Here, we conducted an experiment with low and high levels of N addition in a semiarid grassland in Inner Mongolia, which is a part of the largest contiguous grassland in the world. We hypothesize that 1) low-level N additions should increase soil respiration, while high-level of N additions should decrease soil respiration and 2) the response of belowground biomass and MBC to N addition should highly related with soil respiration.
Results
Soil respiration was significantly affected by N addition (p < 0.05) during the experimental period (Fig. 1a). The low-level N addition treatment significantly increased soil respiration (p < 0.05), while the high-level N addition treatment significantly decreased soil respiration (p < 0.05). Additionally, soil respiration showed a clear seasonal pattern, with the highest rates in late June and the lowest in early May (Fig. 1a). Nitrogen addition strongly affected cumulative soil CO2 efflux through its effect on soil respiration (Fig. 1b). The cumulative soil CO2 efflux was 18.67% higher in low-level N addition treatment plots, but 11.44% lower in high-level N addition treatment plots than in control plots. The annual mean soil respiration rate was highest under the low-level N addition treatment at 6.04 μmol CO2 m−2 s−1, and lowest under the high-level N addition treatment at 4.56 μmol CO2 m−2 s−1, with an intermediate value under the control treatment at 5.13 μmol CO2 m−2 s−1 (Fig. 2a). Nitrogen addition had no significant effect on the temperature sensitivity of soil respiration (Q10 values, Fig. 2b). The highest value of temperature sensitivity occurred in the high-level N addition treatment at 2.94, and the lowest value occurred in the low-level N addition treatment at 2.18.
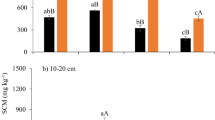
Plant belowground biomass was significantly affected by N addition (p < 0.05, Fig. 2c). Mean plant belowground biomass values were 787.97 g m−2, 887.55 g m−2, and 717.51 g m−2 in the control, low-level N addition treatment, and high-level N addition treatment plots, respectively. Plant belowground biomass was 12.64% higher in the low-level N addition treatment and 8.94% lower in the high-level N addition treatment than in the control treatment. Similarly, soil MBC increased significantly by low-level N addition treatment, while decreased significantly by high-level N addition (p < 0.05, Fig. 2d). Soil MBC was 6.67% higher in the low-level N addition treatment and 55.78% lower in the high-level N addition treatment than in the control treatment.
Mean soil respiration was significantly and positively correlated with both plant belowground biomass and MBC (R2 = 0.99, F2,6 = 504.9, p < 0.001; Fig. 3). Based on the relative importance analysis, plant belowground biomass explained 63.4% of the variance in mean soil respiration, while MBC explained the remaining 36.0%.
Both soil temperature and soil moisture showed strong seasonal variation during the experimental period (p < 0.05), with the highest value of soil temperature occurring in late July and the lowest value in early May (Fig. 4a,b). Mean soil temperature was 22.35 °C (range: 8.18 °C in May to 30.02 °C in July). Mean soil moisture was 13.22% (range: 6.29–24.11%) across all plots during the experiment. Nitrogen addition had no significant influence on either soil temperature or soil moisture (p > 0.05, Fig. 4a,b). Soil respiration was significantly and positively correlated with both soil moisture and soil temperature (R2 = 0.739, F2,168 = 238.0, p < 0.001; Fig. 4c,d). Based on the relative importance analysis, soil moisture explained 66.7% of the variance in soil respiration, while soil temperature explained the remaining 7.2%.
Discussion
In this study, the effects of N addition on soil respiration were divergent, i.e., low-level N additions increased soil respiration and cumulative soil CO2 efflux, while high-level N additions decreased them. Consistent with our study, low levels of N addition have been shown to increase soil respiration in several previous studies9,13,25,26, particularly in N-limited ecosystems like our research site. In contrast, the response to N addition levels where the supply of ammonium and nitrate are in excess of the total combined plant and microbial demand (i.e., N-saturation) has been shown to be much more complex. In a mature, N-saturated tropical forest in southern China, low-level N additions showed no significant effect on soil respiration, while high-level N additions significantly reduced soil respiration16. Similarly, in no-till, corn-based midwestern U.S. cropping systems, soil respiration was also significantly reduced with high levels of N addition (29.1 g N m−2)27. Further, in a temperate forest, soil respiration increased after high-level N additions (15 g N m−2) only in the first year of treatment, then was significantly reduced with high-level N additions thereafter24. These responses may have occurred because high-levels of N addition can shift an ecosystem from N-limitation to N-saturation, which should negatively affect soil respiration16. Thus, the effects of N addition on soil respiration appear to depend on nutrient limitation and the rate of N addition.
The divergent response of soil respiration on N addition in the semiarid grassland studied here appears to mainly be mediated by plant belowground biomass and soil microbial activity. In this experiment, both microbial biomass and plant belowground biomass were highly positively correlated with soil respiration, together explaining 99.4% variation of soil respiration, indicating that they are the dominant factors that control soil respiration. Consistent with our results, significant positive correlations between soil respiration and fine root biomass as well as between soil respiration and belowground net productivity were found in cottonwood and loblolly pine plantations and a semiarid grassland, respectively9,14. These responses are likely due to the alleviation of plant and soil microbial N limitation by low-level N additions28,29, resulting in the stimulation of soil microbial respiration30 and plant belowground biomass.
In contrast to the positive effects of low-level N additions in our study, both microbial biomass and plant belowground biomass decreased consistently with high-level of N addition, leading to a similar decrease in soil respiration. N-induced decreases in soil respiration have previously been ascribed to decreased relative allocation of C to plant belowground biomass or reduced microbial activity and biomass with N additions, both of which are important factors affecting soil respiration16,24,31,32. In this study, plant belowground biomass contributed more to the variation of soil respiration than MBC. As plant belowground biomass contributes to root respiration and soil MBC contributes to microbial respiration, our results suggest that high-level N additions likely act to reduce both root respiration and microbial respiration, with slightly higher effects of root respiration than microbial respiration in driving the observed shift in total soil respiration. In contrast, a study in a drier grassland showed that microbial respiration responded more sensitively to N addition than root respiration13.
The effects of N addition on microbial and root respiration may differ with ecosystems and N conditions. Additionally, other factors such as soil pH30, soil dissolved organic carbon, aboveground biomass7, litter quantity and quality10, also impact the effects of N addition on soil respiration. Nitrogen saturation and decreasing soil pH are likely to occur with continuous exposure to high levels of N addition30,33, which may negatively affect plant growth due to magnesium limitation or aluminum toxicity and decreased microbial abundance, activity, and biomass, all ultimately leading to reduced soil respiration34,35,36,37,38. The response of soil respiration to such N additions should be closely related to the threshold point of the ecosystem30 beyond which ecosystem functions may shift in unpredicted ways; importantly this threshold point may differ across ecosystems2,39. Overall, plant belowground biomass and microbial biomass are dominant factors that affect soil respiration with N addition.
Soil respiration was significantly and positively related to soil temperature and soil moisture in this study, and they showed similar seasonal dynamics (Fig. 4). Consistent with our study, many previous studies have shown positive exponential or linear correlations between soil respiration and soil temperature4,22,40, and positive coorrelations between soil respiration and soil moisture9,40. The seasonal variation of soil respiration in our study appeared to be affected mainly by soil moisture, likely because soil moisture is the primary limiting factor for plant growth, net primary productivity, and microbial activity in the semiarid grassland of northern China41,42. However, not surprisingly, neither soil moisture nor soil temperature responded to the N addition treatments, and therefore did not affect the response of soil respiration to N additions. Therefore, soil moisture and temperature appear to be primarily important factors affecting the seasonal dynamics of soil respiration, but not the response to N availability.
In conclusion, N additions affected soil respiration divergently, with low-level N additions increasing soil respiration, while high-level N additions decreased soil respiration. Although soil temperature and soil moisture primarily explained the soil respiration dynamics, they were not affected by N additions and therefore did not drive the observed shifts in soil respiration under altered N conditions. Instead, the soil respiration response to N addition appeared to mirror changes in plant belowground biomass and MBC with the N treatments. Thus, these biotic shifts in plant belowground biomass and MBC may be the primary mechanism through which changes in soil respiration in response to N availability are manifest. Overall, low levels of N deposition may have weaker effects on C dynamics than previously believed. In contrast, high levels of N addition to natural systems, e.g., through agricultural runoff, will likely have large consequences for the C cycle and future research efforts should focus on these effects.
Material and Methods
Study sites and field sampling
The N addition experiment was conducted in a semiarid Leymus chinensis grassland (116°42′E, 43°38′N) near the Inner Mongolia Grassland Ecosystem Research Station (IMGERS) in China, which has been fenced since 1999 to prevent grazing by large animals. Mean annual precipitation at the site is approximately 350 mm, with the rainy season, during which most precipitation falls, occurring from June to August. Mean annual air temperature is −0.4 °C, and the average monthly temperature ranges from −23.0 °C in January to 17.9 °C in July from 1980–200330. The vegetation is dominated by the perennial rhizomatous grass Leymus chinensis and the perennial bunchgrass Stipa grandis.
The N enrichment experiment was initiated in May 2006. Nitrogen addition treatments were established in 6 × 8 m plots, which were separated by 1 m walkways. Three replicates of each N addition treatment were established: control (without N added), low N (LN; 5.6 g N m−2 yr−1 added), and high N (HN; 22.4 g N m−2 yr−1 added), resulting in 9 experimental plots overall. Nitrogen was added in the form of urea (CO(NH2)2), which was thoroughly mixed with sand and evenly spread across the plot surface in May 2006 and 2007, following a rainfall event each year. To ensure N was only the limiting nutrient, 1.55 g P m−2 yr−1 was added to the N addition treatment plots as KH2PO443.
Soil respiration
Three PVC collars (5 cm in height and 11 cm in diameter) were inserted into the soil in each plot, with the bottom of the collars buried 3 cm below the soil surface. Soil respiration was measured approximately once per week from 1st May to 23rd September in 2007 with an LI-6400 portable photosynthesis system attached to a soil CO2 flux chamber (991 cm3 in total volume; LI-COR 6400-09 TC, LI-COR Inc., Lincoln, NE, USA). Soil respiration measurements were made on the third day after a rainfall event, to avoid the confounding effects of precipitation on soil respiration to the extent possible. The measurements were conducted between 8:00 am and 12:00 pm, and each measurement was completed within 2 min.
Soil moisture and soil temperature
Discrete soil temperature and volumetric soil moisture measurements were made concurrently with soil respiration measurements. Soil temperature was measured using a thermocouple probe connected to a LI-6400. The probe was inserted into the soil to a depth of 5 cm in a location adjacent to the PVC collar. Volumetric soil moisture was determined at a range of depths from 0–10 cm using a TRIME TDR probe (IMKO, Ettlingen, Germany) in the same location where soil respiration was measured.
Plant belowground biomass
Plant belowground biomass was estimated from 1 m deep soil cores collected on 28th August 2007 with a soil auger (7 cm inner diameter). Three soil cores were taken from each plot and sufficiently mixed to create a homogenous sample. To ensure there was no clay sticking to the roots, the roots were washed three times over a 0.5-mm sieve. All biomass samples were oven-dried to a constant weight at 85 °C.
Soil microbial biomass carbon
Microbial biomass C (MBC) was determined using the fumigation extraction technique44. Soil samples were collected from each plot after the plant biomass harvest. Each sample comprised five soil cores (3 cm in diameter and 10 cm in depth) and were placed in individual plastic bags and then immediately stored at 4 °C. Soil samples from single sampling cores were divided into paired subsamples of 10 g each. One subsample was immediately extracted with 20 ml 0.5 M K2SO4 for 60 min on a rotary shaker at 150 rpm. The second subsample was fumigated under chloroform vapor for 24 h in a desiccator. followed by ten vacuum/release purge cycles. and then extracted as described above. Extracts were filtered using a 0.2 lm syringe filter and analyzed for total organic carbon (TOC) using a TOC analyzer (Dimatec Analysentechnik GmbH, Essen, Germany). MBC was calculated as the difference in TOC between the two subsamples.
Statistical analysis
Statistical significance was defined at the 95% confidence level (alpha = 0.05). Repeated measures ANOVAs were performed to examine the effects of N additions on soil respiration, soil temperature and soil moisture, and standard ANOVAs were performed to examine the effects of N additions on plant belowground biomass and MBC. Cumulative soil CO2 (g CO2 m−2) efflux was estimated as the sum of efflux during the days between sampling dates.
A multiple regression was used to examine the effects of soil temperature and soil moisture on soil respiration with the following linear equation:

where R is the soil respiration, ST is the soil respiration, SM is the soil moisture and a, b and c are coefficients. Q10 values were obtained as follows:

where coefficient a was from Eq. (1)45.
Two linear multiple regressions were performed to determine the mechanisms underlying the soil respiration responses to N additions. First, mean soil respiration was regressed against plant belowground biomass and MBC. Second, the time series data was used to examine the relationships between soil respiration and soil temperature and soil moisture. The strengths of the relationships within each regression were assessed by comparing the R2 contribution of each regressor averaged over all possible orderings of the regressors in the model using the relaimpo package in R. Regressions incorporating non-linear terms were also tested, but rejected in favor of the models described above based on AIC.
All ANOVAs were performed using SPSS 19.0 for Windows (USA) and all regressions were performed using R v. 3.2.446.
Additional Information
How to cite this article: Zhu, C. et al. Divergent Effects of Nitrogen Addition on Soil Respiration in a Semiarid Grassland. Sci. Rep. 6, 33541; doi: 10.1038/srep33541 (2016).
References
Galloway, J. N. et al. Nitrogen cycles: Past, present, and future. Biogeochemistry 70, 153–226 (2004).
Bai, Y. et al. Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: Evidence from inner Mongolia Grasslands. Global Change Biol 16, 358–372 (2010).
Hibbard, K. A., Law, B. E., Reichstein, M. & Sulzman, J. An analysis of soil respiration across northern hemisphere temperate ecosystems. Biogeochemistry 73, 29–70 (2005).
Luo, Y., Wan, S., Hui, D. & Wallace, L. L. Acclimatization of soil respiration to warming in a tall grass prairie. Nature 413, 622–625 (2001).
Cox, P. M., Betts, R. A., Jones, C. D., Spall, S. A. & Totterdell, I. J. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408, 184–187 (2000).
Cramer, W. et al. Global response of terrestrial ecosystem structure and function to CO2 and climate change: Results from six dynamic global vegetation models. Global Change Biol 7, 357–373 (2001).
Cleveland, C. C. & Townsend, A. R. Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc Natl Acad Sci 103, 10316–10321 (2006).
Giardina, C., Binkley, D., Ryan, M., Fownes, J. & Senock, R. Belowground carbon cycling in a humid tropical forest decreases with fertilization. Oecologia 139, 545–550 (2004).
Xu, W. & Wan, S. Water- and plant-mediated responses of soil respiration to topography, fire, and nitrogen fertilization in a semiarid grassland in northern China. Soil Biol Biochem 40, 679–687 (2008).
Knorr, M., Frey, S. D. & Curtis, P. S. Nitrogen additions and litter decomposition: A meta-analysis. Ecology 86, 3252–3257 (2005).
Allison, S. D., Czimczik, C. I. & Treseder, K. K. Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Global Change Biology 14, 1156–1168 (2008).
Li, Y., Liu, Y., Wu, S., Niu, L. & Tian, Y. Microbial properties explain temporal variation in soil respiration in a grassland subjected to nitrogen addition. Scientific Reports 5 (2015).
Zhang, C. et al. Effects of simulated nitrogen deposition on soil respiration components and their temperature sensitivities in a semiarid grassland. Soil Biol Biochem 75, 113–123 (2014).
Lee, K.-H. & Jose, S. Soil respiration, fine root production, and microbial biomass in cottonwood and loblolly pine plantations along a nitrogen fertilization gradient. Forest Ecol Manag 185, 263–273 (2003).
Harpole, W. S., Potts, D. L. & Suding, K. N. Ecosystem responses to water and nitrogen amendment in a California grassland. Global Change Biol 13, 2341–2348 (2007).
Mo, J. et al. Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Global Change Biol 14, 403–412 (2008).
Cleveland, C. C. & Townsend, A. R. Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc Natl Acad Sci 103, 10316–10321 (2006).
Margesin, R., Hämmerle, M. & Tscherko, D. Microbial Activity and Community Composition during Bioremediation of Diesel-Oil-Contaminated Soil: Effects of Hydrocarbon Concentration, Fertilizers, and Incubation Time. Microbial Ecology 53, 259–269 (2007).
Allison, S. D. et al. Low levels of nitrogen addition stimulate decomposition by boreal forest fungi. Soil Biol Biochem 41, 293–302 (2009).
Yan, L., Chen, S., Huang, J. & Lin, G. Differential responses of auto‐and heterotrophic soil respiration to water and nitrogen addition in a semiarid temperate steppe. Global Change Biol 16, 2345–2357 (2010).
Lü, C. & Tian, H. Spatial and temporal patterns of nitrogen deposition in China: Synthesis of observational data. J Geophys Res: Atmos 112, n/a-n/a (2007).
Han, Y., Zhang, Z., Wang, C., Jiang, F. & Xia, J. Effects of mowing and nitrogen addition on soil respiration in three patches in an oldfield grassland in Inner Mongolia. J Plant Ecol 5, 219–228 (2012).
Sun, J., Cheng, G. & Fan, J. Soil respiration in response to a short-term nitrogen addition in an alpine steppe of Northern Tibet. Pol J Ecol 61, 655–663 (2013).
Bowden, R. D., Davidson, E., Savage, K., Arabia, C. & Steudler, P. Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the Harvard Forest. Forest Ecol Manag 196, 43–56 (2004).
Carreiro, M. M., Sinsabaugh, R. L., Repert, D. A. & Parkhurst, D. F. Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition Ecology 81, 2359–2365 (2000).
Qi, Y. et al. Differential responses of short-term soil respiration dynamics to the experimental addition of nitrogen and water in the temperate semi-arid steppe of Inner Mongolia, China. J Environ Sci 26, 834–845 (2014).
Grandy, A. S. et al. Soil respiration and litter decomposition responses to nitrogen fertilization rate in no-till corn systems. Agric Ecosyst Environ 179, 35–40 (2013).
LeBauer, D. S. & Treseder, K. K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89, 371–379 (2008).
Xia, J. & Wan, S. Global response patterns of terrestrial plant species to nitrogen addition. New Phytol 179, 428–439 (2008).
Wei, C. et al. Nitrogen deposition weakens plant–microbe interactions in grassland ecosystems. Global Change Biol 19, 3688–3697 (2013).
Haynes, B. E. & Gower, S. T. Belowground carbon allocation in unfertilized and fertilized red pine plantations in northern Wisconsin. Tree Physiol 15, 317–325 (1995).
Treseder, K. K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol Lett 11, 1111–1120 (2008).
Guo, J. et al. Significant acidification in major Chinese croplands. Science 327, 1008–1010 (2010).
Aber, J. et al. Nitrogen saturation in temperate forest ecosystems. BioScience, 921–934 (1998).
Lucas, R. W. et al. A meta-analysis of the effects of nitrogen additions on base cations: Implications for plants, soils, and streams. Forest Ecol Manag 262, 95–104 (2011).
Ramirez, K. S., Craine, J. M. & Fierer, N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Global Change Biol 18, 1918–1927 (2012).
Söderström, B., Bååth, E. & Lundgren, B. Decrease in soil microbial activity and biomasses owing to nitrogen amendments. Can J Microbiol 29, 1500–1506 (1983).
Fierer, N. & Jackson, R. B. The diversity and biogeography of soil bacterial communities. PNAS 103, 626–631 (2006).
Fenn, M. E. et al. Nitrogen critical loads and management alternatives for N-impacted ecosystems in California. J Environ Manage 91, 2404–2423 (2010).
Deng, Q. et al. Responses of soil respiration to elevated carbon dioxide and nitrogen addition in young subtropical forest ecosystems in China. Biogeosciences 7, 315–328 (2010).
Liu, W., Xu, W., Han, Y., Wang, C. & Wan, S. Responses of microbial biomass and respiration of soil to topography, burning, and nitrogen fertilization in a temperate steppe. Biol Fertil Soils 44, 259–268 (2007).
Chen, Z. & Wang, S. P. Typical steppe ecosystem of China. (Science, 2000).
Yu, Q. et al. Linking stoichiometric homoeostasis with ecosystem structure, functioning and stability. Ecol Lett 13, 1390–1399 (2010).
Brookes, P. C., Landman, A., Pruden, G. & Jenkinson, D. S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17, 837–842 (1985).
Davidson, E. A., Janssens, I. A. & Luo, Y. On the variability of respiration in terrestrial ecosystems: moving beyond Q10 . Global Change Biol 12, 154–164 (2006).
R. Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria (2016).
Acknowledgements
We gratefully appreciate the Inner Mongolia Grassland Ecosystem Research Station (IMGERS) for providing the experimental sites and elemental analysis. This research was supported by Strategic Priority Research Program of the Chinese Academy of Sciences (XDB15010403), National Natural Science Foundation of China (31270476, 41320104002, 41203052).
Author information
Authors and Affiliations
Contributions
Q.Y. and Z.W.S. conceived and designed the research. C.Z. and H.H.W. carried out the field experiments. Y.P.M. and K.J.L.P. analyzed the data. C.Z. and Y.P.M. wrote the first draft, all the authors contributed to the revision of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhu, C., Ma, Y., Wu, H. et al. Divergent Effects of Nitrogen Addition on Soil Respiration in a Semiarid Grassland. Sci Rep 6, 33541 (2016). https://doi.org/10.1038/srep33541
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep33541
This article is cited by
-
Low Rather Than High Level Nitrogen Additions Accelerate Carbon Release Process and Inhibit Recalcitrant Carbon Allocation via Stirring Soil Enzymatic Activities in Plateau Meadows
Journal of Soil Science and Plant Nutrition (2024)
-
Nitrogen addition reduced carbon mineralization of aggregates in forest soils but enhanced in paddy soils in South China
Ecological Processes (2021)
-
Drivers of soil respiration in response to nitrogen addition in a Mediterranean mountain forest
Biogeochemistry (2021)
-
Effects of weeding and fertilization on soil biology and biochemical processes and tree growth in a mixed stand of Dalbergia odorifera and Santalum album
Journal of Forestry Research (2021)
-
Effects of nitrogen addition on root respiration of trees and understory herbs at different temperatures in Pinus tabulaeformis forest
Plant and Soil (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.