Abstract
Important evolutionary and ecological consequences arise from the ability of female turtles to store viable spermatozoa for an extended period. Although previous morphological studies have observed the localization of spermatozoa in Pelodiscus sinensis oviduct, no systematic study on the identification of genes that are involved in long-term sperm storage has been performed. In this study, the oviduct of P. sinensis at different phases (reproductive and hibernation seasons) was prepared for RNA-Seq and gene expression profiling. In total, 2,662 differentially expressed genes (DEGs) including 1,224 up- and 1,438 down-regulated genes were identified from two cDNA libraries. Functional enrichment analysis indicated that many genes were predominantly involved in the immune response, apoptosis pathway and regulation of autophagy. RT-qPCR, ELISA, western blot and IHC analyses showed that the expression profiles of mRNA and protein in selected DEGs were in consistent with results from RNA-Seq analysis. Remarkably, TUNEL analysis revealed the reduced number of apoptotic cells during sperm storage. IHC and TEM analyses found that autophagy occurred in the oviduct epithelial cells, where the spermatozoa were closely attached. The outcomes of this study provide fundamental insights into the complex sperm storage regulatory process and facilitate elucidating the mechanism of sperm storage in P. sinensis.
Similar content being viewed by others
Introduction
Sperm storage in the female reproductive tract is defined as the retention of viable spermatozoa for an extended period of time1. It is typically used by a wide variety of animal species, including mammals, insects, fish, birds and reptiles, whose copulation is consistently asynchronous with ovulation2. Some mammalians and birds have evolved the capacity to store viable spermatozoa for only a few days or even hours, whereas some fish and reptiles can store spermatozoa for more than one year3,4. In reptiles, long-term sperm storage contributes to appropriately synchronize copulation, fertilization and nesting, which ensures the survival of these species5. Moreover, the storage of spermatozoa enables the females to control paternity through the selective use of spermatozoa6. Therefore, the elucidation of the molecular mechanism underlying sperm storage is beneficial for this species.
Considerable studies have suggested that the intimate contact between spermatozoa and the surface of oviduct epithelial cells is necessary to prolong spermatozoa survival, which is especially important for stimulating the synthesis of new proteins in the oviduct cells7,8. Furthermore, the oviduct cells have distinct secretory functions, providing nourishment and protection for the stored spermatozoa9. In vitro, binding to the oviduct epithelium prolongs spermatozoa survival and extends the motile life span in some species10. In reptiles, sperm-storage tubules (SSTs) in the oviduct can prevent sperm degeneration and maintain the potential fertilizing capacity of spermatozoa over long periods11. Recent studies have suggested that the microvillus blebs (MvBs) on the apical tips of SST epithelial cells contribute to the sustained sperm storage12. Understanding the relationship of spermatozoa and oviduct is a prerequisite to elucidate the mechanism of prolonged sperm storage.
Just as viviparity is regarded as having evolved in reptiles more than 100 times, the mechanisms of sperm storage have evolved multiple times13. Studies have shown the presence of anti-apoptotic proteins at the site of sperm storage in oviduct giving rise to speculation that this could be a functional component of sperm storage mechanisms14,15. Furthermore, other studies have suggested that immunity is an important mechanism underlying sperm storage. Low levels of cytokines during the period of sperm storage are thought to facilitate spermatozoa survival in hens16. More recently, some reports have shown that the long-term storage of spermatozoa is associated with autophagy, whose primary function is to degrade long-lived proteins and recycle cellular components17,18. Comprehensive dissection of the molecular regulatory mechanism of oviduct sperm storage in vivo will require further studies, which will contribute to further studies on spermatozoa conservation in vitro.
The Chinese soft-shelled turtle (Pelodiscus sinensis) belongs to a commercially important aquaculture reptile with high edible and pharmaceutical value in Asian countries such as China, Japan and Korea19. Spermatogenesis, copulation and ovulation are seasonal and segregational in turtles5. Our earlier studies clearly provided structural and ultrastructural evidence for sperm storage in the oviduct of female P. sinensis, and spermatozoa could survive in the oviduct during the hibernation season (from November to the following April)9,20. Recently, the draft genome sequences of P. sinensis have been published21, providing a useful database for genomic and functional investigations on some important biological traits in Chinese soft-shelled turtle. Next-generation sequencing (NGS) technologies have been proven to be an efficient and accurate choice for measuring gene expression under diverse biological conditions22. In particular, NGS-based RNA sequencing (RNA-Seq) has been widely employed for global gene expression profiling in some reptile species, including tuatara (Sphenodon punctatus), green anole lizard (Anolis carolinensis), garter snake (Trimeresurus elegans) and painted turtle (Chrysemys picta)23,24,25,26. Nevertheless, there is still limited knowledge concerning global gene expression and the mechanism underlying sperm storage in the P. sinensis oviduct.
In this study, RNA-Seq and digital gene expression (DGE) tag profiling from the P. sinensis oviduct during reproductive (July) and hibernation (January) seasons were performed using the Illumina HiSeq 2500 platform. The objectives of this study were to comprehensively identify the differentially expressed genes (DEGs) related to sperm storage and dissect the molecular mechanism underlying long-term sperm storage in P. sinensis. The critical genes involved in the immune response, apoptosis and autophagy regulation were isolated and characterized during sperm storage. The differential expressions of several DEGs related to sperm storage were validated by RT-qPCR, and their protein levels were detected by ELISA, western blot and IHC analysis. The number of apoptotic cells was detected by TUNEL analysis in July and January. Furthermore, morphological evidence further confirmed the storage of spermatozoa in the P. sinensis oviduct. These results will enhance our understanding of sperm storage in female P. sinensis and help elucidate the underlying mechanism in turtles.
Results
Sperm storage in the oviduct of P. sinensis
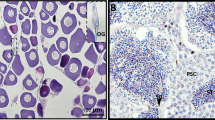
In P. sinensis, spermatogenesis and ovulation are out of phase with each other. After mating during the period from June to August, spermatozoa are stored in the oviduct from November to the following April (hibernation season)27. In this study, morphological analysis showed that spermatozoa were stored in the lumen (Fig. 1A,B) and SSTs (Fig. 1C,D) of the P. sinensis oviduct during the hibernation season (January). These spermatozoa were attached to the epithelial surface (Fig. 1B,D) and were primarily oriented with the heads toward epithelial cilia (Fig. 1E,F). Given our aims in global gene expression analysis during sperm storage, the oviduct of P. sinensis in July and January (of the following year) were chosen for RNA-Seq.
Distribution of spermatozoa in the oviduct of P. sinensis during the hibernation season (January), H&E staining and TEM.
(A) Spermatozoa stored within the oviduct. (B) Spermatozoa were embedded among the cilia of the oviduct. (C) Spermatozoa stored in both the SST and lumen of the oviduct. (D) Spermatozoa were embedded among the cilia of SST. (E,F) Many spermatozoa were embedded among the cilia. Cilia (Ci), epithelium (*), gland cell (G), lumen (L), spermatozoa (Sp), secretory cell (SC), ciliated cell (CC). Scale bar = 50 μm (C), 20 μm (A), 10 μm (B,D), 2 μm (E) and 1 μm (F).
RNA-Seq and identification of DEGs
In this study, two cDNA libraries from the P. sinensis oviduct in July (FU_1) and January (FU_2) were constructed and sequenced using Illumina RNA-Seq technology. Approximately 42.5 and 44.1 million raw reads were obtained from the FU_1 and FU_2 libraries, respectively (Fig. 2). After removing the low-quality reads and adapter sequences, 40,987,980 and 42,517,640 clean reads were successfully obtained from the two libraries, among which 73.75% (30,229,347 in FU_1) and 69.85% (29,697,850 in FU_2) reads were mapped to the genome sequences of Chinese soft-shelled turtle (Table 1). To evaluate the gene expression levels, the FPKM method was used to calculate and normalize the read counts in the FU_1 and FU_2 libraries. The thresholds of |log2 (Fold change)| ≥ 1 and FDR ≤ 0.001 were used to determine DEGs. As a result, 2,662 DEGs were successfully identified, including 1,224 significantly up-regulated and 1,438 significantly down-regulated genes in the FU_2 library compared with the FU_1 library (Fig. 3).
Functional annotation and enrichment analysis
GO classification and enrichment analyses with P-value ≤ 0.05 were performed according to three main ontologies: biological process, cellular component and molecular function. A total of 247 and 51 significantly enriched GO terms were obtained for the up- and down-regulated DEGs, respectively. The top 30 most enriched GO terms are shown in Fig. 4. Among these enriched terms, the most up-regulated genes were involved in some functional categories such as ‘binding’, ‘cellular component’, ‘cell part’, ‘cell’ and ‘cellular process’ (Supplementary Table S1), while the down-regulated genes were predominantly related to ‘single-organism process’, ‘organelle’, ‘membrane-bounded organelle’, ‘cytoplasm’ and ‘catalytic activity’ (Supplementary Table S2).
Pathway enrichment analysis with a Q-value ≤ 0.05 was implemented to assess the activated biological pathways during sperm storage. As a result, these DEGs were significantly enriched in seven and six KEGG pathways for up- and down-regulated genes (Supplementary Table S3), respectively. Among the up-regulated DEGs, the major enriched pathways were related to ‘Regulation of autophagy’ (pss04140), ‘Intestinal immune network for IgA production’ (pss04672), ‘Ribosome’ (pss03010) and ‘RNA transport’ (pss03013). The significantly enriched pathways for down-regulated DEGs were predominantly involved in ‘Apoptosis’ (pss04210), ‘p53 signaling pathway’ (pss04115), ‘MAPK signaling pathway’ (pss04010) and ‘mTOR signaling pathway’ (pss04150).
Identification of critical DEGs involved in the sperm storage of P. sinensis
The RNA-Seq data and gene expression analysis showed several significantly DEGs involved in the immune response, apoptosis signalling pathway and autophagy regulation between reproductive and hibernation seasons (Supplementary Fig. S2). It is well known that the immunological response is a key component of the mechanism underlying sperm storage, and Toll-like receptors (TLRs) play important roles in innate immunity of the male reproductive tract28,29. In this study, DEGs were identified that were related to the Toll-like receptor (TLR) signalling pathway (pss04620) during sperm storage and included TLR2, TLR4, CTSK, TRAF3, IRAK2, TOLLIP and MyD88 (Table 2; Supplementary Fig. S2A). Among them, TRAF3, IRAK2 and TOLLIP were up-regulated, whereas TLR2, TLR4, CTSK and MyD88 were down-regulated. Moreover, several proinflammatory cytokines such as TNF, IL1B (IL1β) and IL18 were identified in the process of sperm storage and were significantly down-expressed during the hibernation season (Table 2; Supplementary Fig. S2A).
From the NGS-based RNA-Seq analysis, the apoptosis signalling pathway (pss04210) was identified as the most frequently used pathway related to sperm storage. As a result, several genes were found to be related to the pathway of apoptosis, such as MCL1 and BCL2L11 belonging to the Bcl-2 family, CASP6 and CASP9 from the Caspase family, and BAG4 and BAG5 encoding BAG1-related protein (Table 3; Supplementary Fig. S2B). Moreover, the p53 signalling pathway (pss04115) plays an important role in the induction of apoptosis30, and some differentially regulated genes were identified to be involved in this pathway (Supplementary Fig. S2C). Importantly, most of the p53 signalling-related genes were down-regulated, such as PERP, CytC, PIDD and SCOTIN (Table 3), indicating that the regulation of the expression of these genes may be crucial for modulating the network of sperm storage.
Furthermore, our results showed that many pathways were involved in the regulation of sperm storage, and the regulation of autophagy (pss04140) appears to be most significant in the pathway enrichment analysis. In this study, some autophagy-related genes such as ATG16L1, ATG14, ATG5 and ATG12 were found to be differentially expressed (Table 4; Supplementary Fig. S2D). In addition, MAP1LC3B (LC3B) encoding microtubule-associated protein 1 light chain 3 beta, and BNIP3, belonging to the pro-cell death member of the Bcl-2 family, were significantly up-regulated during the hibernation season.
Expression profile analysis by RT-qPCR
To validate the differential expression patterns of DEGs during sperm storage in P. sinensis, 17 genes were randomly selected and subjected to RT-qPCR analysis. These selected genes included 7 immune-response genes (including CTSK, IL1B, TLR2, MyD88, IL18, TNF and TLR4), 7 apoptosis-related genes (including BAG5, BAG4, BCL6, CytC, CASP9, BCL2L11 and MCL1) and 3 genes (including MAP1LC3B, ATG14 and BNIP3) related to autophagy regulation. Moreover, the relative expression levels of these genes determined by RT-qPCR analysis were compared with the read counts calculated by FPKM method. The results revealed that these gene expression patterns were in agreement with the transcript abundance changes from RNA-Seq (Fig. 5), indicating the highly accuracy and quality of DGE sequencing and expression analysis.
Expression validation of selected DEGs related to sperm storage in P. sinensis.
(A) The relative expression levels of DEGs by RT-qPCR (green bar) were compared with the transcript abundances from RNA-Seq (blue bar). (B) Heat map diagram of the expression patterns of DEGs in P. sinensis. The red and green colours indicate up- and down-regulated genes in the FU_2 library compared with those in the FU_1 library, respectively.
Circulating TNFα, IL1β and IL18 concentrations in oviduct tissue
To further detect the protein levels of three immune response-related genes such as TNFα, IL1β and IL18, the concentrations of circulating TNFα, IL1β and IL18 in the oviduct of P. sinensis were detected during the reproductive and hibernation seasons using ELISA in this study. The results showed that the circulating TNFα, IL1β and IL18 concentrations were significantly lower (P < 0.05) in January than in July (Fig. 6), which is consistent with their down-expression patterns by DGE analysis. These results implied that the decreased immune reaction may be in favor of the long-term sperm storage in female P. sinensis.
Evaluation of cell apoptosis by TUNEL
The effect of stresses imposed by seasonal change on P. sinensis oviduct apoptosis was revealed by in situ 3’-OH end tailing of fragmented DNA. In July, many TUNEL-positive cells were observed (Fig. 7A). Most of the TUNEL-positive cells were oviduct epithelial cells and gland cells (Fig. 7A). In January, the number of apoptotic cells was substantial decreased (Fig. 7B), which is consistent with the down-regulated enriched pathways of apoptosis and p53 signaling. No labelling was seen in negative control sections (Fig. 7C). These results suggested that the number of apoptotic cells were decreased during the sperm storage in female P. sinensis.
Representative photographs of TUNEL staining in July (A), January (B), negative control (C) of the oviduct, and the counting of the TUNEL positive cells (D).
Epithelium (*), gland cell (G), lumen (L), TUNEL positive cells (↑). Scale bar = 20 μm. The values (*) obtained in July were significantly higher (P < 0.05) than those obtained in January.
Expression and localization of autophagy protein LC3B in the P. sinensis oviduct
Western blot was performed to validate the ratio of LC3B-II/LC3B-I in the oviduct of P. sinensis, with β-actin as an internal control. As shown in Fig. 8A, protein bands immunopositive for the LCB3-I and LCB3-II forms were clearly evident in each sample. Compared with July, the ratio of LC3B-II/LC3B-I was significantly increased (P < 0.05) in January, indicating that the autophagy level was much higher in the period of sperm storage.
LC3B protein expression in the P. sinensis oviduct during the reproductive (July) and hibernation (January) seasons.
(A) Western blot analysis of LC3B protein expression. The histogram represents densitometric analysis of the immunoblots. (B) Immunohistochemical localization of LC3B. (a) July, (b) January, (c) negative control and (d) the quantification of LC3B positive cells. Epithelium (*), gland (G), lumen (L), LC3B positive cells (↑). Scale bar = 20 μm. The values (*) obtained in January were significantly higher (P < 0.05) than those obtained in July.
Immunohistochemistry (IHC) also showed an LC3B-positive reaction in the oviduct (Fig. 8B). Immunostaining was primarily observed in the cytoplasm of oviduct epithelial cells (including ciliated cells and secretory cells), whereas no staining was detected in the negative control sections (Fig. 8Bc). The results showed that autophagy was found in the oviduct epithelial cells where the spermatozoa intimately contact.
Numerous autophagosomes were observed by TEM within oviduct epithelial cells during the hibernation season
To determine whether autophagy occurs in the oviduct of P. sinensis, we observed the ultrastructural morphology of the oviduct by TEM in January and July. In January, double- and multi-membrane-bound autophagic vesicles containing undigested cytoplasmic material were observed within the oviduct epithelial cells (Fig. 9), typical features of autophagosomes immediately prior to lysosomal fusion. In addition, these cells included ciliated cells (Fig. 9B,C) and secretory cells (Fig. 9A). Moreover, in July, there are no typical autophagosomes were observed in the oviduct epithelial cells (Fig. 9E,F). These results are consistent with those of LC3B immune staining (Fig. 8B).
TEM photograph of oviduct epithelial cells in January (A–D) and July (E,F).
(A,F) secretory cells. (B–D) ciliated cells. (E) secretory and ciliated cells. Autophagosome (white arrow), secretory cell (SC), ciliated cell (CC), cilia (Ci), secretory granules (SG), mitochondria (Mi), nucleus (N). Scale bar = 5 μm (E), 2 μm (B,C,F), 1 μm (A) and 200 nm (D).
Discussion
The female reproductive tract plays a major role in the stringent selection of spermatozoa, regulation of spermatozoa motility and survival of spermatozoa31. Recent studies have elucidated that the interaction between spermatozoa and oviduct epithelial cells could prolong the life span of stored spermatozoa in P. sinensis20,27. The evolved specialized SSTs can maintain spermatozoa viability for a long duration in some female reptiles, including turtles9,11. In the current study, the stored spermatozoa were found in both the lumen and SSTs of the P. sinensis oviduct during the hibernation season (January), suggesting that the oviduct might provide a suitable microenvironment to maintain spermatozoa for long periods in P. sinensis. Although morphological evidence has clearly confirmed the localization of spermatozoa in the female reproductive tract, the molecular mechanisms underlying long-term sperm storage in the oviduct of turtles remain obscure. In this study, two P. sinensis oviduct cDNA libraries during the reproductive and hibernation seasons were constructed and sequenced using RNA-Seq technology, respectively. A list of DEGs related to the immune response, apoptosis and autophagy signalling pathways were identified and comprehensively profiled. To our knowledge, this is the first research on the large-scale identification and profiling of sperm storage-related genes in P. sinensis.
Immune-response genes related to prolonged sperm storage
Spermatozoa and seminal proteins are antigenic to the female immune system32. However, the stored spermatozoa in the oviduct for long-time survival seem to be tolerated by the female. Increasing studies across diverse taxa have discussed the relationship between the immunological response in the reproductive tract and sperm storage33,34. Previous studies in insects have shown that a reduction in immune function was necessary for the prolonged sperm storage in females28, whereas the quality of stored spermatozoa decreased following an immune insult35. Mcnamara et al.36 suggested that a significant immune challenge resulted in a reduction in the viability of stored spermatozoa in female Teleogryllus oceanicus. Atikuzzaman et al.37 reported that the spermatozoa surviving in the SSTs of hens require the differential expressions of specific genes, and most immune-reactive genes were down-regulated. In the present study, many genes implicated in the TLR signalling pathway were significantly differentially expressed during the hibernation season compared with the reproductive season. The TLR signalling pathway is responsible for modulating the innate immune responses in vertebrates against parasites, bacteria and viruses38. The TLR signal pathway cascade could be activated as soon as specific ligand bind to TLRs, inducing the release of inflammatory cytokines and chemokines39. The overexpression of the TLR2, 4 genes in human spermatozoa reduced the motility of spermatozoa and impaired the potential for fertilization significantly40. Our previous study demonstrated the distribution of TLR2, 4 in the oviduct of P. sinensis and inferred the possible role of TLR2, 4 in sperm storage41. In this study, the expressions of several transcripts encoding TLR2, TLR4, CTSK and MyD88 were significantly decreased during the hibernation season, suggesting that TLR cascades contributed to the tolerance of the stored spermatozoa in response to immune reaction in female P. sinensis.
Moreover, the other immune response, including TNF, IL1β and IL18, were found to be down-expressed during the hibernation season. TNF, as an inflammatory cytokine, is mainly produced by macrophages and monocytes during acute inflammation42. A previous study revealed that TNF has cytotoxic activity to cause germ cell apoptosis in mammalian cells43, inferring that the down-expression of TNF may be responsible for the stored spermatozoa in the oviduct of P. sinensis. Another inflammatory mediator, IL1β, could enhance the immune response induced by various stimuli, including mitogens, cytokines and microbial products44. Das et al.45 reported that the changes in mRNA expression of IL1β and TNF-related molecules were significant for spermatozoa survivability in hens, and the decrease in IL1β may permit spermatozoa to survive in SSTs. IL18, also known as IFN-inducing factor, is involved in inflammation, ischemic tissue injury and T-cell-mediated immunity46,47. The increased levels of IL1β and IL18 were correlated with the rate of spermatozoa with abnormal morphology in humans48. In this study, the down-expression of TNF, IL1β and IL18 during the hibernation season was validated by RT-qPCR and ELISA analyses. These results indicated that these immune-related genes may modulate the immune response in P. sinensis to tolerate the presence of allogeneic spermatozoa in the oviduct for lengthy periods.
Down-regulation of apoptosis genes may be in favour of long-term sperm storage
Apoptosis is a physiological programmed cell death process that plays an essential role in the process of gamete maturation and embryogenesis49. Studies have indicated that apoptosis, as the regulatory mechanism of germ cell death, is implicated in long-term sperm storage and plays vital roles in the removal of spermatozoa without viability from female reproductive tracts50,51. Previous study showed that the oviduct cells can secrete several apoptosis-related proteins to lumen, and these proteins could interact with the stored spermatozoa during the period of sperm storage52. Urhausen et al.15 reported that the expression of apoptosis-related proteins was investigated in the dog oviduct, suggesting that the control of apoptosis may be a major functional component of the mechanism underlying sperm storage prior to fertilization. Furthermore, in female Scotophilus heathi, the interplay between pro- and anti-apoptotic factors was shown to hold the key to prolonged sperm storage53. Recently, our direct evidence revealed that the anti-apoptosis might enable the oviduct to maintain the storage of spermatozoa in P. sinensis14. These findings suggest that the apoptosis of oviduct cells is closely related to the long-term spermatozoa survival in females.
In the present study, using TUNEL, which is a routine and normal method to detect apoptosis54, more apoptotic cells were detected in July than in January (Fig. 7). Moreover, some up-regulated anti-apoptosis genes and down-regulated pro-apoptosis genes were identified during sperm storage by functional annotation and expression analysis, indicating that these gene expression alterations might contribute to long-term sperm storage in P. sinensis. Among these up-regulated anti-apoptosis genes, BCL6 encodes a Kruppel-type zinc finger transcriptional repressor, and its overexpression was found to obviously inhibit spermatozoa apoptosis in response to various stressors55. MCL1, another member of the anti-apoptotic Bcl-2 family, is an early-response gene in the apoptotic signalling cascade and exerts its function in delaying apoptosis under apoptosis-inducing conditions56. The functions in anti-apoptotic activities were also shared by BAG4 and BAG5, which belong to the Bcl-2-associated athanogene (BAG)-family. Thus, the up-expression of these anti-apoptosis genes, including BCL6, MCL1, BAG4 and BAG5, may repress the apoptotic effect in the oviduct and thereby protect the resident spermatozoa in female P. sinensis. Moreover, in this study, the participation of down-regulated pro-apoptosis genes such as BCL2L11, was also found during sperm storage. BCL2L11 (also known as BIM) could promote the release of apoptogenic proteins and inactivate the anti-apoptosis BCL-2 proteins to trigger apoptosis57. These findings indicated that the down-regulated pro-apoptosis genes negatively control the initiation of apoptosis in P. sinensis.
It has been extensively shown that the p53 signalling pathway could induce DNA damage-triggered apoptosis in various cell types30. The effects of oxidative stress are particularly important during sperm storage either by cooling or in vivo, and the reactive oxygen species (ROS) was suggested as a main factor in the inhibition of spermatozoa longevity58,59. Previous studies have demonstrated that the decreased levels of ROS could suppress the release of CytC from mitochondria to the cytosol, promoting the increase in spermatozoa longevity in the female reproductive system for diverse organisms58,59. The CytC protein could bind to Apaf-1 and, in turn, activate CASP9, triggering apoptosis60. Moreover, the ROS-CytC-Caspase axis in the p53 pathway was suggested to play key roles in apoptosis61. Here we found that the two components of the p53-dependent apoptosis pathway, CytC and CASP9, were down-regulated during the hibernation season. Taken together, these findings implied that the oviduct contributing to prolong sperm storage in P. sinensis might suppress oxidative stress-induced apoptosis through the ROS-CytC-Caspase model in the p53 pathway.
Regulation of autophagy involved in sperm storage
Autophagy is a highly conserved catabolic process that is primarily responsible for the nonspecific degradation of redundant and recyclable cellular components62. In the process, portions of the cytoplasm, damaged proteins and organelles are sequestered in double- or multi-membrane structures called autophagosomes, which deliver material to lysosomes for digestion63. Recently, increasing reports have focused on the involvement of autophagy in spermatozoa. In Caenorhabditis elegans, fertilizing spermatozoa trigger the recruitment of autophagosomes and subsequent paternal mitochondria degradation to prevent paternal mitochondrial DNA transmission64. The expression of autophagy-related proteins in stallion spermatozoa suggested that autophagy is the main strategy for the survival of cooled stored spermatozoa17. Moreover, a series of autophagy-related genes (ATGs) was implicated in the regulation of autophagy. For instance, ATG14 is a critical element of autophagic initiation and can interact with phosphatidylinositol 3-phosphate in the bilayer membrane during autophagosome formation65. The overexpression of ATG14 has been demonstrated to enhance autophagic activity in yeast and mammals63,66. In addition, BNIP3, as a pro-cell death member of the Bcl-2 family, was found to inhibit the mTOR (mechanistic target of rapamycin) pathway and promote autophagy67. The knockdown of BNIP3 was shown to inhibit autophagy and promote the necrotic cell death of tumour cells68. In this study, the up-expression of ATG14 and BNIP3 was detected in the process of sperm storage, implying that these genes might promote autophagy during the hibernation season and thereby contribute to prolong the life span of stored spermatozoa in P. sinensis.
Furthermore, NGS-based expression analysis and RT-qPCR detected the increased transcript abundance of the LC3B gene, and western blot analysis further revealed the high ratio of LC3B-II/LC3B-I in the P. sinensis oviduct during the hibernation season. The microtubule-associated protein 1 light chain-3β (LC3B) exists in two forms: LC3B-I and LC3B-II. The unprocessed form of LC3B is cleaved by ATG4 into a cytosolic form, known as LC3B-I63. Upon induction of autophagy, LC3B-I is processed to LC3B-II, which is inserted into both the inner and outer membranes of the growing autophagic vesicle69. The conversion from LC3B-I to LC3B-II is a cellular readout of autophagy levels70. Therefore, our observations indicated that autophagy was increased during the sperm storage period in P. sinensis. Moreover, IHC showed that LC3B-positive cells were mainly located along the oviduct epithelium, in which ciliated cells and secretory cells are distributed. Furthermore, under TEM, autophagosomes containing undigested cytoplasmic material were observed and distributed within the ciliated cells and secretory cells, indicating that autophagy can occur in the oviduct epithelium when spermatozoa are stored during the hibernation season. It is well known that autophagy serves as an alternative energy source to sustain cellular function under stress71. A sufficient energy source at the site of sperm storage is essential for the prolonged survival of spermatozoa72. Furthermore, H&E staining and TEM analysis demonstrated that the spermatozoa were closely attached to the oviduct epithelium during sperm storage (Fig. 1). Hence, it is reasonably inferred that autophagy could provide available energy for the oviduct epithelial cells to support the stored spermatozoa in the female P. sinensis.
Methods
Animals
In this study, any work involving experimental animals was conducted according to the guidelines of the Animal Research Institute Committee of Nanjing Agriculture University, China. The experimental protocol and study were approved by the Science and Technology Agency of Jiangsu Province. The approval ID is SYXK (SU) 2010–0005. All efforts were made to minimize the animal’s suffering. Ten adult female P. sinensis in sex mature stage, from Yangcheng Lake in Suzhou (31°N, 120°E), southeastern China, were used in this study. The animals were anesthetized by sodium pentobarbital (20 mg/kg) administered intraperitoneally and were subsequently sacrificed through cervical dislocation. They were slaughtered in July (reproductive season) and January (hibernation season) with five turtles at each time. The oviduct of P. sinensis can be divided into five segments: infundibulum, magnum, isthmus, uterus, and vagina. The spermatozoa were predominantly stored in the anterior parts of the oviduct (including the vagina, uterus and isthmus)27. Therefore, the anterior parts of the oviduct were used for analysis in this study. One portion of the oviduct samples of each turtle was collected and immediately fixed for light and transmission electron microscopy (TEM), respectively. The other portion of the oviduct samples was ground in liquid nitrogen immediately and stored at −80 °C for gene expression analysis.
Haematoxylin-eosin (H&E) staining
The samples were embedded in paraffin after fixation in neutral-buffered formalin for 48 h, and serially sectioned (6 μm). The sections were stained with haematoxylin and counter-stained with eosin (haematoxylin for 1 min and 1% eosin for 10 sec) for observation under a light microscope (BX53; Olympus; Tokyo, Japan).
Transmission electron microscopy (TEM)
The tissues was cut into small sections (1 mm3) and immersed in a mixture of 2.5% glutaraldehyde fixative in phosphate-buffered saline (PBS) (4 °C, pH 7.4, 0.1 M) for 24 h, followed by post-fixation in similarly buffered 1% osmium tetroxide for 1 h at room temperature. Subsequently, the samples were dehydrated in ascending concentrations of ethyl alcohol, infiltrated with a propylene oxide-Araldite mixture and then embedded in Araldite. Ultrathin sections (50 nm thickness) were stained with uranyl acetate and lead citrate. The sections were examined and photographed using JEM-1200EX TEM.
RNA isolation, library construction and sequencing
Equal amounts of oviduct samples from five turtles in July and January were pooled for cDNA library construction. Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol and was incubated with 20 U/ml DNaseI (Sigma-Aldrich, St. Louis, MO, USA) for 15 min at 37 °C to remove genomic DNA contamination. Next, the RNA quality and quantity were examined using a NanoPhotometer spectrophotometer (IMPLEN GmbH, Munich, Germany) and an Agilent 2100 Bioanalyzer (Agilent Technologies, CA, USA). The RNA integrity number (RIN) of all samples was greater than 8.0. Two cDNA libraries were prepared using the NEBNext Ultra RNA Library Prep Kit for Illumina (NEB, USA) following the manufacturer’s recommendations. Briefly, mRNAs were enriched using magnetic beads with Oligo (dT) (Life Technologies, CA, USA) and then were fragmented into small pieces with NEBNext first-strand synthesis reaction buffer under an elevated temperature. First-strand cDNA was synthesized with random hexamer primers using M-MuLV Reverse Transcriptase Buffer (New England BioLabs, Whitby, ON). Second-strand cDNA synthesis was subsequently performed using DNA Polymerase I and RNase H. Thereafter, the cDNA was purified using the Qiaquick PCR extraction kit (Qiagen, Valencia, CA, USA) and subjected to end reparation and poly (A) tail addition. Suitable fragments were enriched by PCR amplification. The two libraries were sequenced using an Illumina HiSeq 2500 platform. The sequence data were deposited in NCBI Sequence Read Archive (SRA, http://www.ncbi.nlm.nih.gov/Traces/sra/) with accession numbers of SRX1675543 (FU_1) and SRX1672963 (FU_2).
Analysis of sequencing data
Raw data were filtered to remove both the reads containing adapter and ploy-N and the low-quality reads (Q < 20) generating the clean data. For sequence mapping, clean reads were aligned to the Chinese soft-shelled turtle genome sequence21 using TopHat v2.0.9, allowing no more than two base mismatches. The read counts of each gene were summarized by HTSeq v0.6.1 and adjusted using the edgeR program package through one scaling normalized factor. The DEGSeq R package (1.12.0) was used to detect DEGs. The expression of each gene was normalized by the reads per kilobase per million mapped reads (RPKM) among different samples. We judged the significant differences in gene expression using the threshold value of |log2 (Fold change)| ≥ 1 with a False Discovery Rate (FDR) ≤ 0.001 and P-value ≤ 0.005. The fold change was calculated as follows: Fold change = RPKM in FU_2/RPKM in FU_1.
Enrichment analysis of differentially expressed genes
Gene ontology (GO) enrichment analysis of DEGs was performed using they GOseq R package, in which gene length bias was corrected. GO terms with a corrected P-value ≤ 0.05 were considered significantly enriched by DEGs. Pathway analysis was mainly based on the Kyoto Encyclopaedia of Genes and Genomes (KEGG, http://www.kegg.jp/kegg/pathway.html) database73. The KOBAS software was applied to test the statistical enrichment of differential expression genes in KEGG pathways.
RT-qPCR validation
The RNA extractions from P. sinensis oviduct in July and January were used for RT-qPCR analysis. Primers for RT-qPCR were designed using Beacon Designer 7.0 software (Premier Biosoft International, USA) and are listed in Supplementary Table S5. Total RNA samples were reverse transcribed into cDNAs using the SuperScript First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). RT-qPCR was performed according to previous reports20. The relative gene expression levels were calculated using the 2−ΔΔCT method with β-actin gene as the internal control. Three biological replicates were performed for each gene.
Enzyme-linked immunosorbent assay (ELISA)
Oviduct tissues were collected, and total proteins were extracted using a protein extraction kit (Thermo Fisher Scientific, Rockford, USA). Protein concentrations were measured using the Bradford method (BioRad Laboratories, CA, USA). TNFα, IL1β and IL18 in oviduct tissue homogenates were detected by ELISA using commercially available kits (KA0191, KA0356, KA0561; Abnova, Walnut, CA, USA). The pooled oviduct tissue homogenates were serially diluted and tested against a standard curve. Diluted samples running parallel to the standard curve indicated the validity of this assay in P. sinensis. The ELISA was performed according to the manufacturer’s instructions. All of the samples and standards were measured in duplicate.
TdT-mediated dUTP-biotin nick end labelling assay (TUNEL)
Oviduct sections were treated with 20 mg/mL proteinase K (Sigma-Aldrich, St. Louis, MO, USA) for 20 min at room temperature. Next, they were heated (60 °C) for 30 min in 0.1 M citrate buffer (pH 6.0), allowed to cool for 15 min, exposed to 3% H2O2 in water (to quench endogenous peroxidase activity) for 15 min, and rinsed thrice in distilled water (5 min/rinse). Staining was performed using a commercially available apoptosis detection kit (ApopTag Peroxidase In Situ Apoptosis Detection Kit, Millipore, Billerica, MA) following the manufacturer’s instructions. The TUNEL reaction mixture was incubated on the slides for 60 min at 37 °C. A positive signal was detected using diaminobenzidine (Vectorlabs, CA, USA). The slides were counterstained with haematoxylin before being mounted in neutral balsam (Sigma-Aldrich, St. Louis, MO, USA). Negative controls were processed in an identical manner, except that the TdT enzyme was replaced with the same volume of distilled water. From each specimen, three sections were initially examined under light microscopy at low magnification (×100). Five fields per section were randomly examined at a higher magnification (×400). Two investigators examined the samples microscopically in a blinded fashion. The percentage of the TUNEL positive cells was used to determine the apoptosis rate74.
Western blot
Western blot was performed as described previously20 using equal amounts of protein (40 μg/lane) from the oviduct. Anti-LC3B antibody (ab48394, Abcam Inc., Cambridge, MA, USA) was used at a concentration of 2 μg/ml, and protein band detection was performed using an ECL detection system (Vazyme Biotech, China). The validity of the anti-LC3B antibody in P. sinensis was determined by negative and positive control analysis (Supplementary Fig. S1).
Immunohistochemistry (IHC)
Immunohistochemistry staining was performed according to a previously described protocol14. Briefly, the sections were deparaffinized in xylene, cleared in an ethanol series, blocked with 3% H2O2 in methanol, and heated for 30 min in sodium citrate buffer (pH 6.0). The primary anti-LC3B antibody (ab48394, Abcam Inc., Cambridge, MA, USA) at a 1:200 dilution was incubated with the sections overnight at 4 °C. Negative controls were incubated with a non-specific anti-rabbit IgG. Biotinylated secondary antibody and Vector ABC reagent (Vector Laboratories, Burlingame, CA) were subsequently added according to the manufacturer’s instructions. After washing, the sections were incubated with FAST DAB Peroxidase Substrate (Sigma-Aldrich, St. Louis, MO, USA) and counterstained with haematoxylin for 30 sec. The slides were analysed under a light microscope and photographed. The LC3B positive cells were automatically counted at ten randomly selected fields per sample by using the Image-Pro Plus (IPP) software, version 6.0 (Media Cybernetics, Bethesda, MD, USA)75.
Statistical analysis
The data were expressed as the means ± SEM and were analyzed using SPSS software version 14.0 using an independent t-test. P-values less than 0.05 were considered to be statistically significant.
Additional Information
How to cite this article: Liu, T. et al. Global analysis of differential gene expression related to long-term sperm storage in oviduct of Chinese Soft-Shelled Turtle Pelodiscus sinensis. Sci. Rep. 6, 33296; doi: 10.1038/srep33296 (2016).
References
Holt, W. V. Mechanisms of sperm storage in the female reproductive tract: an interspecies comparison. Reprod. Domest. Anim. 46, 68–74 (2011).
Orr, T. J. & Marlene, Z. Sperm storage. Curr. Biol. 22, R8–R10 (2012).
Murphy, R. W., Berry, K. H., Edwards, T. & McLuckie, A. M. A genetic assessment of the recovery units for the Mojave population of the desert tortoise, Gopherus agassizii. Chelonian Conserv. Bi. 6, 229–251 (2007).
Phillips, K. P., Jorgensen, T. H., Jolliffe, K. G. & Richardson, D. S. Potential inter-season sperm storage by a female hawksbill turtle. Mar. Turt. Newsl. 140, 13–14 (2014).
Pearse, D. & Avise, J. Turtle mating systems: behavior, sperm storage, and genetic paternity. J. Hered. 92, 206–211 (2001).
Wedell, N., Gage, M. J. G. & Parker, G. A. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 17, 313–320 (2002).
Siegel, D. S. & Sever, D. M. Sperm aggregations in female Agkistrodon piscivorus (Reptilia: Squamata): a histological and ultrastructural investigation. J. Morphol. 269, 189–206 (2008).
Fazeli, A., Affara, N. A., Hubank, M. & Holt, W. V. Sperm-induced modification of the oviductal gene expression profile after natural insemination in mice. Biol. Reprod. 71, 60–65 (2004).
Han, X. et al. Ultrastructure of anterior uterus of the oviduct and the stored sperm in female Soft-Shelled Turtle, Trionyx sinensis. Anat. Rec. 291, 335–351 (2008).
Apichela, S., Jiménez-Díaz, M., Roldan-Olarte, M., Valz-Gianinet, J. & Miceli, D. In vivo and in vitro sperm interaction with oviductal epithelial cells of llama. Reprod. Domest. Anim. 44, 943–951 (2009).
Sarkar, S., Sarkar, N. & Maiti, B. Oviductal sperm storage structure and their changes during the seasonal (dissociated) reproductive cycle in the soft-shelled turtle Lissemys punctata punctata. J. Exp. Zool. A Comp. Exp. Biol. 295, 83–91 (2003).
Bakst, M. R. & Bauchan, G. Apical blebs on sperm storage tubule epithelial cell microvilli: Their release and interaction with resident sperm in the turkey hen oviduct. Theriogenology 83, 1438–1444 (2015).
Girling, J. E. The reptilian oviduct: a review of structure and function and directions for future research. J. Exp. Zool. 293, 141–170 (2002).
Le, Y. et al. B-cell lymphoma-2 localization in the female reproductive tract of the Chinese Soft-Shelled Turtle, Pelodiscus Sinensis and its relationship with sperm storage. Anat. Rec. 298, 2011–2017 (2015).
Urhausen, C. et al. Apoptosis in the uterotubal junction and oviductal isthmus during the estrous cycle of the bitch. Anat. Rec. 294, 342–348 (2011).
Das, S. C., Isobe, N. & Yoshimura, Y. Changes in the expression of interleukin-1β and lipopolysaccharide-induced TNF factor in the oviduct of laying hens in response to artificial insemination. Reproduction 137, 527–536 (2009).
Bolaños, J. G. et al. Autophagy and apoptosis have a role in the survival or death of stallion spermatozoa during conservation in refrigeration. PloS one 7, e30688 (2012).
Bolaños, J. G. et al. During cooled storage the extender influences processed autophagy marker light chain 3 (LC3B) of stallion spermatozoa. Anim. Reprod. Sci. 145, 40–46 (2014).
Ullah, S. et al. Identification and characterization of telocytes in the uterus of the oviduct in the Chinese soft-shelled turtle, Pelodiscus sinensis: TEM evidence. J. Cell. Mol. Med. 18, 2385–2392 (2014).
Liu, T. et al. Androgen-related sperm storage in oviduct of Chinese Soft-Shelled Turtle in vivo during annual cycle. Sci. Rep. 6, 20456 (2016).
Wang, Z. et al. The draft genomes of soft-shell turtle and green sea turtle yield insights into the development and evolution of the turtle-specific body plan. Nat. Genet. 45, 701–706 (2013).
Wang, Z., Gerstein, M. & Snyder, M. RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10, 57–63 (2009).
Miller, H. C., Biggs, P. J., Voelckel, C. & Nelson, N. J. De novo sequence assembly and characterisation of a partial transcriptome for an evolutionarily distinct reptile, the tuatara (Sphenodon punctatus). BMC genomics 13, 439 (2012).
Eckalbar, W. L. et al. Genome reannotation of the lizard Anolis carolinensis based on 14 adult and embryonic deep transcriptomes. BMC genomics 14, 49 (2013).
Schwartz, T. S. et al. A garter snake transcriptome: pyrosequencing, de novo assembly, and sex-specific differences. BMC genomics 11, 694 (2010).
Shaffer, H. B. et al. The western painted turtle genome, a model for the evolution of extreme physiological adaptations in a slowly evolving lineage. Genome. Biol. 14, R28 (2013).
Chen, S. et al. Sperm storage and spermatozoa interaction with epithelial cells in oviduct of Chinese soft-shelled turtle, Pelodiscus sinensis. Ecol. Evol. 5, 3023–3030 (2015).
Baer, B., Armitage, S. A. & Boomsma, J. J. Sperm storage induces an immunity cost in ants. Nature 441, 872–875 (2006).
Palladino, M. A., Johnson, T. A., Gupta, R., Chapman, J. L. & Ojha, P. Members of the Toll-like receptor family of innate immunity pattern-recognition receptors are abundant in the male rat reproductive tract. Biol. Reprod. 76, 958–964 (2007).
Clarke, A. R. et al. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature 362, 849–852 (1993).
Holt, W. V. & Lloyd, R. E. Sperm storage in the vertebrate female reproductive tract: how does it work so well? Theriogenology 73, 713–722 (2010).
Das, S. C., Naoki, I. & Yukinori, Y. Mechanism of prolonged sperm storage and sperm survivability in hen oviduct: a review. Am. J. Reprod. Immunol. 60, 477–481 (2008).
Palladino, M. A., Savarese, M. A., Chapman, J. L., Mary-Katherine, D. & Daniel, P. Localization of Toll-like receptors on epididymal epithelial cells and spermatozoa. Am. J. Reprod. Immunol. 60, 541–555 (2009).
Zandieh, Z. et al. Evaluation of immunological interaction between spermatozoa and fallopian tube epithelial cells. Andrologia 47, 1120–1130 (2015).
Km, R. P. F. Immune activation decreases sperm viability in both sexes and influences female sperm storage. P. Roy. Soc. B-biol. Sci. 279, 3577–3583 (2012).
Mcnamara, K. B., Lieshout, E. V. & Simmons, L. W. Females suffer a reduction in the viability of stored sperm following an immune challenge. J. Evol. Biol. 27, 133–140 (2013).
Atikuzzaman, M., Mehta, B. R., Fogelholm, J., Wright, D. & Rodriguezmartinez, H. Mating induces the expression of immune- and pH-regulatory genes in the utero-vaginal junction containing mucosal sperm-storage tubuli of hens. Pharmacoepidem. Dr. S 14, 659–664 (2015).
Khan, K. N., Michio, K., Akira, F., Masahiro, N. & Hideaki, M. Toll-like receptor system and endometriosis. J. Obstet. Gynaecol. Re. 39, 1281–1292 (2013).
Vaure, C. & Liu, Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immuno. 5, 316 (2014).
Youko, F. et al. Toll-like receptors (TLR) 2 and 4 on human sperm recognize bacterial endotoxins and mediate apoptosis. Hum. Reprod. 26, 2799–2806 (2011).
Li, Q. et al. Expression of TLR2/4 in the sperm-storing oviduct of the Chinese soft-shelled turtle Pelodiscus sinensis during hibernation season. Ecol. Evol. 5, 4466–4479 (2015).
Idriss, H. T. & Naismith, J. H. TNF? and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Techniq. 50, 184–195 (2000).
Grataroli, R. et al. Characterization of tumour necrosis factor-α-related apoptosis-inducing ligand and its receptors in the adult human testis. Mol. Hum. Reprod. 10, 123–128 (2004).
Tabona, P. et al. Homogeneous Escherichia coli chaperonin 60 induces IL-1 beta and IL-6 gene expression in human monocytes by a mechanism independent of protein conformation. J. Immunol. 161, 1414–1421 (1998).
Das, S. C., Naoki, I. & Yukinori, Y. Changes in the expression of interleukin-1beta and lipopolysaccharide-induced TNF factor in the oviduct of laying hens in response to artificial insemination. Reproduction 137, 527–536 (2009).
Akira, S. The role of IL-18 in innate immunity. Curr. Opin. Immunol. 12, 59–63 (2000).
Nakanishi, K., Yoshimoto, T., Tsutsui, H. & Okamura, H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. J. Neurosci. 12, 53–72 (2001).
Dziadecki, W., Celińska, A., Fracki, S., Bablok, L. & Barcz, E. Interleukin 1β and interleukin 18 and their connection with leukocytospermia in human semen. Centr. Eur. J. Immunol. 35, 157–161 (2010).
Brill, A., Torchinsky, A., Carp, H. & Toder, V. The role of apoptosis in normal and abnormal embryonic development. J. Assist. Reprod. Gen. 16, 512–519 (1999).
Aitken, R. J., Findlay, J. K., Hutt, K. J. & Kerr, J. B. Apoptosis in the germ line. Reproduction 141, 139–150 (2011).
Oosterhuis, G. J. E. et al. Measuring apoptosis in human spermatozoa: a biological assay for semen quality? Fertil. Steril. 74, 245–250 (2000).
Holt, W. V. Does apoptosis hold the key to long-term sperm storage mechanisms in vivo? Mol. Reprod. Dev. 7, 464–465 (2011).
Roy, V. K. & Krishna, A. Sperm storage in the female reproductive tract of Scotophilus heathii: Role of androgen. Mol. Reprod. Dev. 78, 477–487 (2011).
Labat-Moleur, F. et al. TUNEL apoptotic cell detection in tissue sections: critical evaluation and improvement. J. Histochem. Cytochem. 46, 327–334 (1998).
Kojima, S. et al. Testicular germ cell apoptosis in Bcl6-deficient mice. Development 128, 57–65 (2001).
Townsend, K. J., Trusty, J. L., Traupman, M. A. & Eastman, A. Craig RW. Expression of the antiapoptotic MCL1 gene product is regulated by a mitogen activated protein kinase-mediated pathway triggered through microtubule disruption and protein kinase C. Oncogene 17, 1223–1234 (1998).
Luo, S. & Rubinsztein, D. C. BCL2L11/BIM. Autophagy 9, 104–105 (2013).
Ball, B. A. Oxidative stress, osmotic stress and apoptosis: Impacts on sperm function and preservation in the horse. Anim. Reprod. Sci. 107, 257–267 (2008).
Collins, A., Williams, V. & Evans, J. Sperm storage and antioxidative enzyme expression in the honey bee, Apis mellifera. Insect. Mol. Biol. 13, 141–146 (2004).
Jiang, X. & Wang, X. Cytochrome C-mediated apoptosis. Annu. Rev. Biochem. 73, 87–106 (2004).
Yang, M. et al. Transcriptome analysis of human OXR1 depleted cells reveals its role in regulating the p53 signaling pathway. Sci. Rep. 5, 17409 (2015).
Li, Y., Lindsey, S. & Lenardo, M. J. The selectivity of autophagy and its role in cell death and survival. Opt. Lett. 4, 567–573 (2008).
Klionsky, D. J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 12, 1–222 (2016).
Rawi, S. A. & Galy, V. Postfertilization Autophagy of Sperm Organelles Prevents Paternal Mitochondrial DNA Transmission. Science 334, 1144–1147 (2011).
Zhong, Y. Q. et al. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat. Cell. Biol. 11, 468–476 (2009).
Xiong, X., Tao, R., Depinho, R. A. & Dong, X. C. The autophagy related gene 14 (Atg14) is regulated by forkhead box o transcription factors and circadian rhythms and plays a critical role in hepatic autophagy and lipid metabolism. J. Biol. Chem. 287, 39107–39114 (2012).
Gallo, S. et al. Agonist antibodies activating the Met receptor protect cardiomyoblasts from cobalt chloride-induced apoptosis and autophagy. Cell Death Dis. 5, e1185 (2014).
Tracy, K. et al. BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol. Cell. Biol. 27, 6229–6242 (2007).
Glick, D., Barth, S. & Macleod, K. F. Autophagy: cellular and molecular mechanisms. J. Pathol. 221, 3–12 (2010).
Vernon, P. J. & Daolin, T. Eat-me: autophagy, phagocytosis, and reactive oxygen species signaling. Antioxid. Redox. Signal. 18, 515–517 (2013).
Swampillai, A. L., Salomoni, P. & Short, S. C. The role of autophagy in clinical practice. Clin. Oncol-Uk. 24, 387–395 (2012).
Roy, V. K. & Krishna, A. Changes in glucose and carnitine levels and their transporters in utero-tubal junction in relation to sperm storage in the vespertilionid bat, Scotophilus heathi. J. Exp. Zool. Part. A 319, 56–67 (2013).
Kanehisa, M. et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 36, D480–D484 (2008).
Zhang, Y. et al. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit. Care 6, 1–9 (2010).
Zhou, K. L. et al. Stimulation of autophagy promotes functional recovery in diabetic rats with spinal cord injury. Sci. Rep. 5, 17130 (2015).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant number: 31272521), and Priority Academic Program for Development of Jiangsu Higher Education Institutions, PR-China.
Author information
Authors and Affiliations
Contributions
T.L. designed the experiments and drafted the manuscript. P.Y., H.C., Y.H., Y.L., Y.W., N.A. and X.C. participated in the design of the study and performed the data analysis. Q.C. conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Liu, T., Yang, P., Chen, H. et al. Global analysis of differential gene expression related to long-term sperm storage in oviduct of Chinese Soft-Shelled Turtle Pelodiscus sinensis. Sci Rep 6, 33296 (2016). https://doi.org/10.1038/srep33296
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep33296
This article is cited by
-
Genome-wide identification and comparison of differentially expressed profiles of miRNAs and lncRNAs with associated ceRNA networks in the gonads of Chinese soft-shelled turtle, Pelodiscus sinensis
BMC Genomics (2020)
-
Role of genome-wide mRNA-seq profiling in understanding the long-term sperm maintenance in the storage tubules of laying hens
Tropical Animal Health and Production (2019)
-
Transcriptome profile analysis reveals cardiotoxicity of maduramicin in primary chicken myocardial cells
Archives of Toxicology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.