Abstract
We have studied photoinduced reduction of absorption and emission in p3ht, a semiconducting polymer, and found that the rate of photodegradation (destruction of the constituent thiophene rings) does not correlate with the luminescence intensity and, correspondingly, does not depend on the excited state concentration. This conclusion rules out Purcell enhancement of radiative decay rate as a possible explanation of the recently discovered reduction of the p3ht photodegradation rate in the vicinity of metallic substrates and lamellar metal-dielectric metamaterials.
Similar content being viewed by others
Introduction
It is well known that spatially inhomogeneous dielectric environments, which can be as simple as interfaces between the two media or as complex as nanostructured composite materials metamaterials1,2,3, can control scores of physical phenomena, including the rates4,5,6,7, the spectra8,9, and the directionality10 of spontaneous emission, stimulated emission11, and Förster energy transfer12,13,14. Following the arguments of the Marcus theory15,16, which states that the rates of chemical reactions (determined by the reorganization (polarization) energies of surrounding molecules) critically depend on local dielectric permittivities at optical and microwave frequencies, one can further infer that nonlocal dielectric environments, e.g. the vicinity to metallic surfaces, can influence the rates of chemical reactions as well.
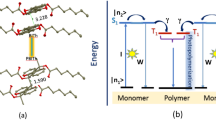
This hypothesis has been experimentally tested in our recent work17, in which we studied the effect of metallic and lamellar metal-dielectric substrates on photodegradation of the poly-3-hexylthiophene (p3ht) semiconducting polymer. (The structure of p3ht, composed of thiophene rings with attached alkyl chains, as well as its absorption and emission spectra are depicted in Fig. 1.) It has been found that under white light (halogen lamp) illumination, there are two known from the literature mechanisms that contribute to photodegradation of p3ht. In the first (radical chain) process, oxygen-based radicals attack the π-carbon atom of the alkyl side chain, by abstraction of hydrogen, leading to chain scission and photobleaching18,19. The corresponding action spectrum rises toward short, ultraviolet (UV), wavelengths. In the second process, singlet oxygen, whose formation is facilitated by photoexcitation of p3ht, attacks the polymer backbone, leading to photobleaching without influencing the side chains18,20. In the latter case, the action spectrum of photodegradation follows the absorption spectrum of the polymer18. Both mechanisms cause reduction of the p3ht absorption band. At the same time, random scission of the polymer backbone, which is characteristic of the singlet oxygen mechanism, causes formation of a large number of short polymer chains and a corresponding blue shift of the absorption spectrum21.
The major experimental result of ref. 17, which is most closely related to the present short communication, is as follows: The rates of photodegradation of the p3ht films deposited onto metallic or layered metal-dielectric substrates (with MgF2 spacer on top) are smaller than those in similar p3ht films deposited onto purely dielectric substrates. This result has been interpreted as an experimental evidence of inhibition of a chemical reaction by nonlocal dielectric environment17.
At the same time, one question of fundamental and practical importance has not been addressed in ref. 17. Thus, photodegradation of organic molecules often stems from a much higher reactivity of excited molecular states than the ground state22. Therefore, the vicinity to metallic surfaces and lamellar metal-dielectric metamaterials can shorten the decay time of the p3ht excited state (via increased density of photonic states6,23, leading to enhancement of the Purcell factor24) and, correspondingly, affects the rate of the photodegradation.
The purpose of the present work was to study the effect of the p3ht excited state concentration on the rate of its photodegradation and answer the question whether the enhanced density of photonic states and enhanced Purcell factor in the vicinity of metamaterials and metallic surfaces can explain the result of ref. 17 discussed above.
Model
Let us start with the simple analytical model and consider the rate equations describing the concentrations of the molecular ground state n0 [cm−3] and the excited state n1 [cm−3]:

where P [W/cm2] is the pumping power (assumed to be monochromatic); S [cm2] is the pumped spot area; σ[cm2] is the absorption cross section; h [J·s] is the Planck’s constant; A [s−1] is the radiative decay rate; W [s−1] is the non-radiative decay rate; γ [s−1] is the rate of photodegradation, turning molecules (thiophene rings) into the reaction products; and N [cm−3] is the total concentration of the molecules. Correspondingly, the rate equation describing the overall reduction of N due to the chemical reaction reads:

At weak pumping used in our experiment, n1≪n0, N and n0 − n1 ≈ N. Furthermore, as the characteristic time of photodegradation (hours) is much longer than the excited state decay time (A + W + γ)−1 (nanoseconds), the time derivative in the first formula of Eq. (1) can be neglected to yield

(The second formula of Eq. (3) shows the abovementioned dependence of the excited state concentration n1 on the radiative decay rate A). Since the intensity of spontaneous emission I [cm−3.s−1] (the number of photons generated in unit volume per second) is proportional to the excited state concentration n1,

the slow time dependence n1(t) (at cw pumping) can be monitored by measuring the time dependence of the spontaneous emission I(t). Furthermore, by combining Eqs (2) and (4), one gets

Photoinduced luminescence quenching in p3ht is reportedly governed by several processes occurring at strongly different time scales21,25. Thus, the collisional quenching (due to encounter of the polymer excited states with the ground state oxygen) is nearly instantaneous; the light-assisted formation of a charge transfer complex between the polymer and oxygen26,27 occurs within minutes, and reduction of the luminescence intensity due to photo-oxidation of the polymer takes hours28. The products of the latter photo-oxidation act as efficient luminescence quenching centers. Correspondingly, a small loss in absorbance has been reported to lead to a substantial photoluminescence quenching21,25.
Therefore, the experimentally measured emission kinetics I(t) (at cw pumping) is expected to have a complex character. Substituting it to Eq. (5) and solving the equation numerically, one can obtain the calculated dependence Ncalc(t) (with γ/A being a fitting parameter), which can be compared with the experimental dependence Nexp(t). If the experimental and the calculated curves N(t) will match each other, then the change of the p3ht photodegradation rate in the vicinity of metal-dielectric substrates17 can be explained by the modified density of photonic states and enhanced Purcell enhancement factor. However, if the experimental and the calculated curves N(t) will not agree with each other, an alternative explanation for the experimental findings of ref. 17 will have to be sought.
Experimental samples
The material of choice in the present study was the same poly-3-hexylthiophene (p3ht) polymer, which was used in our recent work17. The preparation and characterization of the experimental samples – thin p3ht films deposited onto glass substrates – is discussed in Methods.
In order to study the effect of aging on the photodegradation of p3ht, some films were made from fresh solutions (prepared in the same day) while other films were made from the solutions, which were several days or several weeks old. Furthermore, some films were measured in the same day they were prepared, while other films were stored for several days before they were experimentally studied. (All solutions and films were stored in the dark, in an ambient room atmosphere at room temperature.)
Experimental Measurements
In our studies, we performed multiple repetitive photo-exposures and measurements of absorption and emission spectra of p3ht films. The only source of photo-exposure in these experiments was the probing light in the spectrophotometer, which was used to measure absorption, and the excitation light in the spectrofluorimeter, which was used to measure emission. The typical scan parameters are described in Methods. An absorption scan followed by an emission scan composed a cycle. The cycle number (termed Measurement Number) is shown on the horizontal axis in Fig. 2a,b, as discussed below.
(a) Photoinduced reduction of absorption (~Nexp(t), blue diamonds) and emission (Iexp(t), red squares) with increased number of photoexposure cycles. (b) Comparison of the experimentally measured photodegradation Nexp(t) (same as in Fig. 2a, blue diamonds) and the one calculated under the assumption that the rate of photodegradation is proportional to the excited state concentration Ncalc(t) (red squares).
Results and Discussion
Photo-exposure of p3ht films caused reduction of their absorption and emission. This is evident from Fig. 2a, depicting absorption and emission maxima plotted versus the number of photo-exposure cycles. The decay of absorption, ~Nexp(t), (by ≈5% after 20 photo-exposure cycles) was nearly linear. Over the same time, the emission intensity Iexp(t) decreased nearly twentyfold, and its decay was neither linear nor exponential, Fig. 2a. After substituting the experimental emission decay kinetics to Eq. 5 and performing numerical integration, we have obtained the calculated dependence Ncalc(t), which is compared to the experimental curve Nexp(t) in Fig. 2b. (Note that the dependence on t is equivalent to the dependence on the number of photoexposure cycles.) The constant γ/A was chosen to bring the calculated curve Ncalc(t) to the range of the experimental values Nexp(t) (it does not affect the shape of the curve).
The film, whose decay kinetics are shown in Fig. 2, was spin-coated using freshly made solution and experimentally studied in the same day. The kinetics in (i) the film made from a month-old solution and measured the same day, Fig. SI-1, and (ii) the film prepared from a fresh solution and measured three days later, Fig. SI-2, are shown in the Supplementary Information section. They are qualitatively similar to the ones in Fig. 2 in a sense that the shapes Ncalc(t) and Nexp(t) are wholly different. In two out of six samples studied, the absorption decay curves Nexp(t) were very slightly arched, Fig. SI-2. Yet, the experimental Nexp(t) and the calculated Ncalc(t) curves, qualitatively similar to those in Fig. 2b, were dramatically different from each other. We did not find any correlation between the behavior of the Nexp(t) and Nexp(t) curves and aging of the films or the solutions.
We, thus, have shown that the rate of photodegradation of p3ht does not depend on the luminescence intensity I and excited state concentration n1, contrary to the predictions of Eqs 4 and 5. This rules out Purcell enhancement of spontaneous emission as a possible explanation of the reduced photodegradation rate in the vicinity of metallic surfaces and lamellar hyperbolic metamaterials reported in our recent study17. This brings us back to the line of reasoning of ref. 17, suggesting that the explanation of the observed phenomenon should be sought in the arguments of the Marcus theory.
Summary
We have experimentally studied photo-induced reduction of absorption and emission in the p3ht semiconducting polymer. We have found that the rate of photodegradation of the polymer (destruction of thiophene rings and reduction of absorption) does not correlate with the luminescence intensity and, correspondingly, does not increase proportionally with the increase of the excited state concentration. This conclusion is seemingly surprising, because one of the two known mechanisms of p3ht degradation – singlet oxygen mechanism – is predicted to depend on the excited state concentration of the thiophene rings constituting the polymer25. At the same time, the efficiency of the singlet oxygen photodegradation mechanism in solid p3ht films is reportedly small in comparison with that of the UV light-triggered radical chain mechanism18,29, making the conclusion above plausible.
The experimental results of this study lead us to the conclusion of practical importance – that reduced photodegradation of p3ht in vicinity of metallic surfaces and lamellar hyperbolic metamaterials17 cannot be explained by the increased density of photonic states and the Purcell enhancement of the spontaneous emission decay rate24,30. Instead, as discussed below, the explanation for the latter phenomenon is to be sought in the arguments of the Marcus theory.
Recently, the reduction of both charge separation and charge recombination rates in the triphenylene: perylene diimide dyad {TriPh(donor): PerDi(acceptor)} has been observed in vicinity of metallic surfaces and lamellar metal-dielectric metamaterials31. The qualitatively similar effect (reduction of the recombination rate) has been reported in the p3ht:PC60BM polymer blend31. These two experiments show the generality of our recent finding17 (reduction of the rate of a chemical reaction in vicinity of metal). The results of ref. 31 have been explained in terms of the modified Marcus theory. In particular, it has been shown that interaction of molecular dipoles with their mirror images reduces the dipole energy the same way as modification of the local dielectric permittivity does, leading to the reduction of the reaction rates. We infer that the same explanation is applicable to the experimental result of our ref. 17 (re photodegradation of p3ht).
Methods
Experimental Samples
The material of choice in the present study was the same poly-3-hexylthiophene (p3ht) polymer (98% head to tail regioregular, Mn = 54,000–75000, from Sigma Aldrich), which was used in our recent work17. The polymer was dissolved in chloroform (ACS grade, from Fischer Scientific) to a concentration of 10–12 mg/ml. It was spin coated onto glass substrates at 500, 3000, then 7000 rpm for a total of 25s (using the Spin coater P6700 from Specialty Coating Systems) and dried in air. The thickness of the fabricated thin films (measured with the Bruker DekTakXT profilometer) ranged from 65 nm to 105 nm, depending on the concentration of p3ht in the solution.
Experimental Scan Parameters
The only source of photo-exposure in multiple repetitive measurements of absorption and emission spectra of p3ht films was the probing light in the spectrophotometer (Perkin Elmer Lambda 900) and the excitation light in the spectrofluorimeter (Horiba Scientific Fluorolog 3). The typical scan parameters were as follows. (i) Absorption measurements. Scan range: 320 nm–800 nm, increment: 2 nm, bandwidth (slit): 2 nm, integration time: 1.52 s, scan time: 6 min. (ii) Emission measurements. Excitation wavelength: 520 nm (absorption maximum of p3ht20), excitation bandwidth (slit): 14.7 nm, scan range: 560 nm–800 nm, increment: 5 nm, integration time: 10 s, scan time: 8 min. Approximately 30 seconds between the scans was needed for removing the sample from one instrument and setting it up for next the scan in the other instrument.
Additional Information
How to cite this article: Peters, V. N. et al. Study of the effect of excited state concentration on photodegradation of the p3ht polymer. Sci. Rep. 6, 33238; doi: 10.1038/srep33238 (2016).
References
Engheta, N. & Ziolkowski, R. W. In Electromagnetic Metamaterials: Physics and Engineering Exploration (eds Engheta, N. & Ziolkowski, R. W. ) Ch. 1, 5–41 (Wiley Publishing, 2006).
Noginov, M. A. In Tutorials in Metamaterials (eds Noginov, M. A. & Podolskiy, V. A. ) Ch. 5, 129–161 (CRC Press, 2011).
Cai, W. & Shalaev, V. In Optical Metamaterials: fundamentals and applications (eds Cai, W. & Shalaev, V. ) Ch. 1, 1–10 (Springer, 2010).
Drexhage, K. H. Influence of a dielectric interface on fluorescence decay time. J. of Luminescence 1, 2, 693–701 (1970).
Jacob, Z., Smolyaninov, I. & Narimanov, E. E. Broadband Purcell effect: Radiative decay engineering with metamaterials. Applied Physics Letters 100, 181105 (2012).
Noginov, M. A. et al. Controlling spontaneous emission with metamaterials. Optics Letters 35(11), 1863–1865 (2010).
Jacob, Z. et al. Engineering photonic density of states using metamaterials. Applied Physics B 100(1) 215–218 (2010).
Zia, R. & Karaveli, S. Spectral tuning by selective enhancement of electric and magnetic dipole emission. PRL 106(109), 193004 (2011).
Gu, L. et al. Blue shift of Spontaneous Emission in hyperbolic metamaterial. Scientific Reports 4, 4969 (2014).
Gu, L. et al. Angular distribution of emission from hyperbolic metamaterials. Sci. Rep. 4, 7327 (2014).
Kitur, J. et al. Stimulated emission of surface plasmons on top of metamaterials with hyperbolic dispersion. ACS Photonics 2(8), 1019–1024 (2015).
Andrew, P. & Barnes, W. L. Forster Energy Transfer in an Optical Microcavity. Science 290(5492), 785–788 (2000).
Andrew, P. & Barnes, W. L. Energy transfer across a metal film mediated by Surface Plasmon Polariton. Science 306(5698), 1002–1005 (2004).
Tumkur, T. U. et al. Control of Forster energy transfer in the vicinity of metallic surfaces and hyperbolic metamaterials. Faraday Discussions 178, 395–412 (2015).
Marcus, R. A. On the theory of oxidation reduction reactions involving electron transfer I. Journal of Chemical Physics 24(5), 966–978 (1956).
Marcus, R. A. Electrostatic free energy and other properties of states having nonequilibrium polarization. I*. Journal of Chemical Physics 24(5), 979–989 (1956).
Peters, V. N. et al. Control of a chemical reaction (photodegradation of the p3ht polymer) with nonlocal dielectric environments. Scientific Reports 5, 14620, 10.1038/srep14620 (2015).
Hintz, H. et al. Wavelength-dependent pathways of poly-3-hexylthiophene photo-oxidation. Chemistry of Materials 24, 2739–2743 (2012).
Manceau, M. et al. The mechanism of photo- and thermooxidation of poly(3-hexylthiophene) (P3HT) reconsidered. Polymer Degradation and Stability 94, 898–907 (2009).
Abdou, Mohamed S. A. & Holdcraft, Steven . Solid-state photochemistry of π-conjugated poly (3-alkylthiophenes). Can. J. Chem. 73, 1893–1901 (1995).
Hintz, Holger et al. Photodegradation of P3HT - A Systematic Study of Environmental Factors. Chemistry of Materials 23, 145–154 (2011).
Klessinger, Martin & Michl, Josef . In Excited States and Photochemistry of Organic Molecules. Ch. 7, 361–485 (VCH Publishers, 1995).
Pantell, R. H. & Puthoff, H. E. Fundamentals of Quantum Electronics John Wiley & Sons, New York Ch. 6 pp. 159–190 (1969).
Purcell, E. M. Spontaneous emission probabilities at radio frequencies. Phys. Rev. 69, 681 (1946).
Hintz, H. Photo-oxidation kinetics of poly-3-hexylthiophene thin films. Dissertation der Mathematisch-Naturwissenschaftlichen Fakultät der Eberhard Karls Universität Tübingen zur Erlangung des Grades eines Doktors der Naturwissenschaften (Dr. rer. nat.) vorgelegt von Holger Hintz aus Remagen, Tübingen (2011).
Abdou, M. S. et al. Interaction of Oxygen with Conjugated polymers: charge transfer complex formation with poly(3-alkylthiophenes). J. Am. Chem. Soc. 119(19), 4518–4524 (1997).
Egelhaaf, H.-J. et al. Influence of oxygen doping on the photoconductivity of π-conjugated molecules. Synthetic Metals 84, 897–898 (1997).
Lüer, L., Egelhaaf, H.-J. & Oelkrug, D. (Photo) conductivity of conjugated oligomer films: mobile charge carrier formation by oxygen. Optical Materials 9, 454–460 (1998).
Abdou, M. S. A. & Holdcroft, S. Mechanisms of photodegradation of poly (3-alkylthiophenes) in solution. Macromolecules 26, 2954–2962 (1993).
Alekseyev, L. V. & Narimanov, E. E. In Tutorials in Metamaterials (eds Noginov, M. A. & Podolsky, V. A. ) Ch. 7, 209–224 (CRC Press, 2011).
Lee, K. J. et al. Charge-transfer dynamics control by manipulating dielectric constants with hyperbolic metamaterial structures as solvent analogues. arXiv:1510.08574 cond-mat.mes-hall (2015).
Acknowledgements
The authors cordially thank N. Kotov and M. Brongersma for useful discussions. The work was partly supported by the Air Force Office of Scientific Research (AFOSR)(FA9550-14-1-022); National Science Foundation (NSF) (DMR 1205457 and DGE 0966188).
Author information
Authors and Affiliations
Contributions
V.N.P. conducted most of the experiments, calculations and the data analysis. R.A. and D.A.P. assisted with the experiments. M.A.N. designed the experiment. V.N.P. and M.A.N. wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Peters, V., Alexander, R., Peters, D. et al. Study of the effect of excited state concentration on photodegradation of the p3ht polymer. Sci Rep 6, 33238 (2016). https://doi.org/10.1038/srep33238
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep33238
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.