Abstract
Calcium/calmodulin-stimulated protein kinase II (CaMKII) is a multi-functional kinase that controls a range of cellular functions, including proliferation, differentiation and apoptosis. The biological properties of CaMKII are regulated by multi-site phosphorylation. However, the role that CaMKII phosphorylation plays in cancer cell metastasis has not been examined. We demonstrate herein that CaMKII expression and phosphorylation at T286 is increased in breast cancer when compared to normal breast tissue, and that increased CAMK2 mRNA is associated with poor breast cancer patient prognosis (worse overall and distant metastasis free survival). Additionally, we show that overexpression of WT, T286D and T286V forms of CaMKII in MDA-MB-231 and MCF-7 breast cancer cells increases invasion, migration and anchorage independent growth, and that overexpression of the T286D phosphomimic leads to a further increase in the invasive, migratory and anchorage independent growth capacity of these cells. Pharmacological inhibition of CaMKII decreases MDA-MB-231 migration and invasion. Furthermore, we demonstrate that overexpression of T286D, but not WT or T286V-CaMKII, leads to phosphorylation of FAK, STAT5a, and Akt. These results demonstrate a novel function for phosphorylation of CaMKII at T286 in the control of breast cancer metastasis, offering a promising target for the development of therapeutics to prevent breast cancer metastasis.
Similar content being viewed by others
Introduction
Breast cancer is the second most commonly diagnosed cancer world-wide1. Despite improvements in survival rates, ~1/3 of patients will develop distant metastases2, and once breast cancer has metastasised, it is generally thought to be incurable. Recent work has demonstrated that calcium signaling is a controller of breast cancer cell metastasis3,4,5. However, the precise mechanisms involved remain to be fully elucidated.
The multifunctional serine (S)/threonine (T) protein kinase, calcium/calmodulin-stimulated protein kinase II (CaMKII), is one of the major calcium sensors in cells6. CaMKII has four isoforms (α, β, γ, δ), one or more of which are expressed in virtually every tissue. As such, CaMKII is involved in controlling a range of cellular processes, including synaptic plasticity and memory consolidation7,8, vascular smooth muscle polarization and migration9, cell proliferation10,11,12, fertilization13, and mammary gland lumen formation14. Additionally, recent evidence has implicated CaMKII in controlling cancer cell metastasis15. Decreasing CaMKII expression in osteosarcoma16 and prostate17 cancer cells inhibits motility and invasion.
The biological properties of CaMKII are controlled by multi-site phosphorylation and via targeting to specific subcellular microdomains18,19. When intracellular calcium levels rise, calcium binds to calmodulin, which activates CaMKII, and leads to phosphorylation of CaMKII at T286. Phosphorylation of CaMKII at T286 induces autonomous activation of CaMKII, and sustains CaMKII activity in the absence of an increase in calcium18. Phosphorylation of CaMKII at T286 has been implicated in a number of neuronal processes, and has been shown to be essential for the acquisition of fear and spatial learning7,20,21. However, the functions controlled by pT286-CaMKII in non-neuronal cells remain largely unexplored. Recently, CaMKII phosphorylation at T286 has been shown to be increased in a range of cancer types16,22,23, but little is known about the functions of this in cancer cells. Britschgi et al.24 demonstrated that phosphorylation of CaMKII at T286 contributes to the oncogenic effects of anoctamin-1 (ANO1) in breast cancer. This suggests that phosphorylation of CaMKII at T286 may control processes involved in breast cancer tumourigenesis and progression.
The present study investigated the function of expression and phosphorylation of CaMKII in breast cancer cell proliferation, anchorage-independent growth, migration and invasion. Furthermore, we demonstrate herein that CaMKII phosphorylation at T286 is a prognostic factor for breast cancer, and that increased CaMKII expression and T286 phosphorylation indicates poorer overall and distant metastasis free survival in breast cancer patients. Additionally, pharmacological inhibition of phosphorylation of CaMKII at T286 prevents breast cancer cell migration and invasion. Taken together, our data indicate that T286 phosphorylation of CaMKII controls breast cancer cell migration and invasion, and highlights the potential therapeutic implications of preventing CaMKII phosphorylation at T286 as a new treatment for controlling breast cancer cell metastasis.
Results
High CaMKII expression predicts poor breast cancer patient prognosis
CaMKII expression and phosphorylation at T286 was initially examined in a panel of normal and cancerous breast cell lines with varying levels of aggressiveness. Whilst the level of total CaMKII remained relatively unchanged in the panel of breast cell lines examined, breast cancer cells expressed significantly higher levels of pT286-CaMKII when compared to normal breast cell lines (Fig. 1A,B). Furthermore, breast cancer cells that exhibit a more invasive phenotype (MDA-MB-231 and SK-BR-3) possessed the highest proportions of pT286-CaMKII (Fig. 1A,B).
CaMKII phosphorylation at T286 is associated with more aggressive breast cancer, and high CAMK2 expression predicts worse overall and distant metastasis free survival in breast cancer patients.
(A) Total endogenous CaMKII and T286 phosphorylation was determined by western blot. GAPDH expression was used as a loading control. Blots are representative of three independent experiments. (B) The proportion of CaMKII phosphorylated at T286 was determined by normalising the level of T286 phosphorylation to total CaMKII expression. **denotes statistical significance p < 0.01, as determined by one-way ANOVA. (C) Kaplan-Meier curves showing the overall survival [OS] and (D) distant metastasis free survival [DMFS] in a publically available 1881-sample breast cancer data set25, with high (blue), mid (red) or low (grey) expression of the CAMK2 family in breast cancer tumours when assessing all tumour subtypes together. P values were computed by a likelihood-ratio test.
We next assessed whether CaMKII expression is associated with breast cancer patient outcome by examining CAMK2A, CAMK2B, CAMK2G and CAMK2D mRNA expression in a publically available 1881-sample breast cancer data set25. High CAMK2 mRNA expression was associated with significantly worse overall (Fig. 1C) and distant metastasis free survival (Fig. 1D) in breast cancer patients when all tumour subtypes were assessed together. These findings were confirmed in an additional 3,935 patient cohort26. Additionally, increased CAMK2 mRNA expression was associated with significantly worse distant metastasis free survival in luminal A (p = 0.016) and triple negative breast cancer (p = 0.006) subtypes in the additional 3,935 patient cohort, but not luminal B or Her-2 subtypes. Furthermore, when the CAMK2 genes were examined independently, high CAMK2A mRNA expression was associated with significantly worse overall (p = 0.01, p = 0.01007, p = 0.01) and distant metastasis free survival (p = 0.05, p = 0.02434, p = 0.01) in Luminal A, Luminal B, and triple negative breast cancer subtypes, respectively. High CAMK2B mRNA expression was associated with worse overall and distant metastasis free survival in estrogen receptor positive (ER) tumours (p = 0.00077 and p = 0.0341, respectively). Increased CAMK2G mRNA expression was associated with significantly worse overall and distant metastasis free survival in Luminal A (p = 0.0029, p = 0.000217, respectively) and ER positive (p = 0.04, p = 0.0239, respectively) tumours. By contrast, high CAM2D mRNA was not associated with significantly worse overall or distant metastasis free survival in the cohorts examined.
To examine the level of CaMKII phosphorylation at T286 in breast cancer tissues and to confirm that total CaMKII is overexpressed in breast cancer tissues at the protein level, CaMKII protein expression and phosphorylation at T286 was examined in 70 breast cancer, 40 matched normal breast, and 10 lymph node metastases patient samples by immunohistochemistry. Total and phosphorylated CaMKII expression was scored on a scale of 0–300, as previously described27. In contrast to that observed in the established breast cell lines (Fig. 1A,B), total CaMKII expression was significantly increased in primary breast cancer (Fig. 2B,G; p < 0.00001) and lymph node metastases (Fig. 2C,G; p < 0.00001) when compared to normal breast tissue (Fig. 2A). Furthermore, phosphorylation of CaMKII at T286 was also significantly increased in primary breast cancer (Fig. 2E,H; p < 0.001) and metastases (Fig. 2F,H; p < 0.001) when compared to the normal breast tissue (Fig. 2D). Total CaMKII expression (Fig. 2G; p < 0.01) and phosphorylation at T286 (Fig. 2H; p < 0.05) were further increased in lymph node metastases, when compared to the primary breast cancer samples, providing further evidence for a role of CaMKII in breast cancer cell metastases. Taken together, these data demonstrate that both CaMKII expression and phosphorylation at T286 are increased in breast cancer tissue, as well as lymph node metastases, and may be potentially useful biomarkers to predict patient outcome and likelihood of metastasis. However, the functions controlled by CaMKII in breast cancer cells remain largely unexplored.
CaMKII expression and phosphorylation at T286 is increased in primary breast cancer and lymph node metastases tissues.
(A,D) Normal breast, (B,E) primary breast cancer, and (C,F) lymph node metastases were examined for (A–C) total CaMKII and (D–F) pT286-CaMKII expression by immunohistochemistry. Staining was quantified and expressed as an H-score. (G) Quantification of total CaMKII and (H) pT286-CaMKII expression in 70 primary breast cancer, 40 matched normal breast, and 10 lymph node metastases cores. Photomicrographs are representative of each tissue type. *denotes statistical significance p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.00001, as determined by one-way ANOVA.
CaMKII promotes migration, invasion and anchorage independent growth of breast cancer cells
As increased CAMK2 mRNA expression predicts that breast cancer patients will have shorter overall and distant metastasis free survival (Fig. 1), and phosphorylation of CaMKII at T286 was significantly increased in breast cancer and lymph node metastases tissue (Fig. 2), we tested whether CaMKII overexpression or phosphorylation at T286 could alter processes known to be involved in breast cancer cell metastasis. To investigate the role of CaMKII on these cellular processes, a wild-type (WT) form of CaMKII, a T286D phosphomimic mutant form (T286 mutated to D), and a T286V phosphonull mutant form (T286 mutated to V) of CaMKII were transfected into the triple negative MDA-MB-231 line, and the luminal A MCF-7 breast cancer line, and effects on migration, invasion, proliferation, and anchorage independent growth determined. MDA-MB-231 inducibly transfected cells overexpressed FLAG-tagged CaMKII mutants following 24 and 48 h treatment with 2 μg/ml doxycycline, whereas non-induced mutant CaMKII and EV cell lines did not express detectable levels of CaMKII (Supplementary Figure S1A). Furthermore, there was no significant difference between the cell lines overexpressing the 3 CaMKII mutants (p > 0.9868). MCF-7 cells stably transfected with various mutant forms of FLAG-tagged CaMKII expressed ~10-fold greater levels of CaMKII compared to EV cells (Supplementary Figure S1B). Additionally, there was no significant difference between the cell lines overexpressing the 3 CaMKII mutants (p > 0.9928; Supplementary Figure S1B). Importantly, basal phosphorylation of CaMKIIα in these transfected cells was not altered (Supplementary Figure 1A,B).
We firstly investigated the effects of T286D phosphomimic mutation of CaMKII on the proliferative capacity of MDA-MB-231 and MCF-7 breast cancer cells. As we and others have previously shown11,16,22, overexpression of WT CaMKII significantly increases cell proliferation, when compared to EV control cells, as measured by Cell Titer Blue (Fig. 3A,B) and clonogenic assays (Fig. 3C,D). Additionally, we show that T286D phosphomimic mutation has no further effect on proliferation rates of breast cancer cells in vitro (Fig. 3).
CaMKIIα controls cellular proliferation in breast cancer cells.
(A) MDA-MB-231 cells inducibly expressing or (B) MCF-7 cells stably expressing empty vector (EV), wild-type (WT), T286D, or T286V CaMKII were generated. Cell viability was measured at 0, 24, 48, and 72 h post-CaMKII expression or plating via Cell Titre Blue Assay. *denotes statistical significant difference from EV control cells, as determined by one-way ANOVA (p < 0.05). n = 4. (C) MDA-MB-231 and (D) MCF-7 cells expressing CaMKII mutants were grown for 8 (MDA-MB-231) or 21 (MCF-7) days. After this time, colonies were stained with 0.5% crystal violet/10% methanol/PBS for 30 mins, and then counted. Photomicrographs are representative of three independent experiments, performed in triplicate. *denotes statistical significant difference p < 0.05, as determined by one-way ANOVA.
We next observed the effects of CaMKII and T286D phosphomimic mutation on breast cancer cell migration. Both MDA-MB-231 (Fig. 4A) and MCF-7 cells (Fig. 4B) overexpressing WT-CaMKII migrated significantly more rapidly than empty vector (EV) control cells (p < 0.01 and p < 0.001, respectively), demonstrating that CaMKII can likely control breast cancer cell migration. Furthermore, MDA-MB-231 and MCF-7 cells overexpressing the T286D phosphomimic mutant form of CaMKII (Fig. 4A,B) migrated significantly more rapidly than the WT and T286V phosphonull forms of CaMKII, indicating that phosphorylation of CaMKII at T286 further increases the migratory capacity of breast cancer cells. As wound healing assays cannot separate migration from proliferation, a transwell migration assay was also performed, and T286D phosphomimic mutation was once again shown to significantly increase MDA-MB-231 (Fig. 4C) and MCF-7 (Fig. 4D) migration, when compared to expression of EV, WT and T286V phosphonull mutant forms of CaMKII. Taken together, this demonstrates that the increased rate of wound closure observed in the T286D phosphomimic mutant overexpressing breast cancer cells (Fig. 4A,B) was not due to increased proliferative capacity, but that T286D phosphomimic mutation of CaMKII increases the migratory capability of breast cancer cells, without altering proliferation rates.
T286D phosphomimic mutation of CaMKII enhances breast cancer cell migration.
(A) MDA-MB-231 and (B) MCF-7 cells expressing empty vector (EV), wild-type (WT), T286D, or T286V CaMKII were grown to confluence, and a wound was made by scratching with a pipette tip. The wounds were photographed hourly for 72 h to measure wound closure over time. Photomicrographs are representative of three independent experiments performed in triplicate. Wound widths are expressed as % of 0 h wound width. *denotes statistical significance from EV control cells (p < 0.05). ϕdenotes statistical significance compared to WT and T286V cells (p < 0.05), as determined by one-way ANOVA. n = 3. (C) MDA-MB-231 and (D) MCF-7 cells expressing CaMKII mutants were placed in the upper chamber of a Transwell, and allowed to migrate through the uncoated membrane (8 μm pore) for 4 h. n = 3. *denotes statistical significance p < 0.05, ***p < 0.001, ****p < 0.0001 as determined by one-way ANOVA.
Similarly, significantly greater numbers of MDA-MB-231 (Fig. 5A) and MCF-7 (Fig. 5B) cells overexpressing WT-CaMKII invaded through Matrigel plugs when compared to control EV cells (p < 0.05, for both), and phosphomimic mutation of T286 further enhanced invasion of both cell lines when compared to WT and T286V expressing cells (Fig. 5A,B; p < 0.001, for both). This is the first evidence identifying cellular functions controlled by pT286-CaMKII in cancer cells.
T286D phosphomimic mutation of CaMKII enhances breast cancer cell invasion.
(A) MDA-MB-231 and (B) MCF-7 cells expressing CaMKII mutants were examined for ability to invade through Matrigel plugs. n = 3. *denotes statistical significance p < 0.05, **p < 0.01, ***p < 0.001, as determined by one-way ANOVA.
The ability of cancer cells to grow in the absence of adhesion to extracellular matrix (ECM) is closely correlated with tumourigenicity in animal models28. WT-CaMKII overexpression significantly increased the ability of both breast cancer cell lines to grow in the absence of ECM (Fig. 6A,B; p < 0.01 for both). Furthermore, overexpression of the T286D phosphomimic form of CaMKII further significantly enhanced the ability of both the invasive MDA-MB-231 (Fig. 6A), and the non-invasive MCF-7 (Fig. 6B) breast cancer cells to grow in a semi-solid medium, when compared to the WT and T286V phosphonull forms of CaMKII. This indicates that phosphorylation of CaMKII at T286 enhances the tumourigenicity of breast cancer cells in vitro.
T286D phosphomimic mutation of CaMKII enhances anchorage independent growth.
(A) MDA-MB-231 and (B) MCF-7 cells expressing empty vector (EV), wild-type (WT), T286D, or T286V CaMKII were grown for 14 (MDA-MB-231) or 24 (MCF-7) days in soft agar. After this time, colonies were stained with 0.5% crystal violet/PBS overnight, and then colonies >50 μm were counted using an inverted microscope. Photomicrographs are representative of three independent experiments, performed in triplicate. Results are presented as the number of colonies. *denotes statistical significant difference p < 0.05, **p < 0.01, as determined by one-way ANOVA.
Pharmacological inhibition of CaMKII decreases breast cancer cell migration and invasion in vitro
Taken together, our data suggest that activation of CaMKII can enhance breast cancer cell motility, invasiveness and tumourigenicity. To investigate whether pharmacological inhibition could potentially decrease breast cancer cell motility and invasion in vitro, we inhibited CaMKII activity using two different pharmacological inhibitors with varying mechanisms of action. KN-93 prevents the activation of CaMKII by calcium/calmodulin, but does not inhibit CaMKII that is already autonomously active. However, KN-93 can also inhibit molecules unrelated to CaMKII, such as ion channels. CaMKII specific effects can be determined when the effects of KN-93 are compared to its inactive analogue, KN-92. Myristoylated autocamtide-2-related autoinhibitory peptide (myr-AIP), competes with substrates at the active site of CaMKII, and inhibits activity of CaMKII irrespective of whether CaMKII is autonomously active or not10. Both AIP and KN-93, but not KN-92, significantly decreased migration (Fig. 7A,B) and invasion (Fig. 7C) of MDA-MB-231 cells. These findings demonstrate that pharmacological inhibition of CaMKII can significantly inhibit migration and invasion of highly aggressive breast cancer cells in vitro.
Pharmacological inhibition of CaMKII activity prevents breast cancer cell migration and invasion.
Confluent monolayers of parental MDA-MB-231 cells were treated with 20 μM KN-92 or KN-93, or 10 μM myr-AIP for 30 min, and a wound was made by scratching the monolayer with a pipette tip. (A) Wounds were photographed hourly for 72 h to measure wound closure over time. Photomicrographs are representative of three independent experiments performed in triplicate. Wound widths are expressed as % of 0h wound width. *denotes statistical significance from untreated cells, p < 0.05, as determined by one-way ANOVA. (B) Following this treatment, cells were placed in the upper chamber of a Transwell, and allowed to migrate through the uncoated membrane (8 μm pore) for 4 h. n = 3. **denotes statistical significance p < 0.01, ***p < 0.001, as determined by one-way ANOVA. (C) Parental MDA-MB-231 cells were treated with 40 μM KN-92 or KN-93, or 10 μM myr-AIP for 30 min, and the ability of cells to invade through Matrigel plugs was examined. *denotes statistical significance p < 0.05, **p < 0.01, ***p < 0.001, as determined by one-way ANOVA.
Overexpression of T286D-CaMKII leads to increased phosphorylation of FAK, STAT5a, and Akt
CaMKII phosphorylates a range of proteins involved in breast cancer cell metastasis; however, the proteins phosphorylated by pT286-CaMKII in breast cancer cells have not been investigated. We screened 44 proteins involved in breast cancer cell migration and invasion simultaneously using a Phosphoproteome Profiler Array, and confirmed expression/phosphorylation of 7 of the proteins that were significantly altered in cells overexpressing the T286D phosphomimic mutant form of CaMKII, compared to those overexpressing the WT and T286V phosphonull form, to identify proteins/pathways that were altered following T286 phosphorylation.
All CaMKII overexpressing breast cancer cells had increased levels of pERK1/2 and vimentin and decreased levels of E-cadherin (Fig. 8A–E), and MDA-MB-231 cells also possessed elevated levels of pFAK (Fig. 8C,D). Furthermore, MDA-MB-231 (Fig. 8C,D) and MCF-7 cells (Fig. 8C,E) overexpressing the T286D phosphomimic mutant form of CaMKII had significantly elevated levels of pFAK, pSTAT5a and pAkt, when compared to EV, WT and T286V phosphonull control cells. This indicates that T286 phosphorylation of CaMKII can lead to increased activation of FAK, STAT5a and Akt.
CaMKII overexpression alters the phosphorylation and expression of multiple proteins in breast cancer cells.
(A) Expression and phosphorylation of 44 proteins were examined by Proteome Profile Human Phospho-Kinase Array following inducible overexpression of empty vector (EV), wild-type (WT), T286D phosphomimic mutant, or T286V phosphonull mutant forms of CaMKII in MDA-MB-231 cells. (B) The relative expression and phosphorylation of these proteins were quantitated by densitometric analysis, and expression normalised to that observed in the EV control cells. (C) The expression and phosphorylation of 7 proteins found to be differentially expressed/phosphorylated following T286D phosphomimic mutant overexpression in the array were confirmed by Western blot in MDA-MB-231 and MCF-7 cells. Blots are representative of three independent experiments. The relative expression of these proteins were quantitated by densitometric analysis, and expression normalised to that observed in EV (D) MDA-MB-231 and (E) MCF-7 cells. *denotes statistical significance from EV cells, Φdenotes statistical significance from WT cells, φdenotes significance from T286V expressing cells.
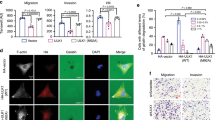
Overexpression off T286D-CaMKII may enhance the epithelial-mesenchymal transition in breast cancer cells
The epithelial-mesenchymal transition (EMT) allows epithelial cells to acquire characteristics of mesenchymal cells, such as enhanced motility and invasiveness. As such, EMT plays an important role in the development of metastasis. We next investigated the ability of T286D phosphomimic mutation of CaMKII to alter markers of EMT in breast cancer cells. We found that overexpression of CaMKII in both MDA-MB-231 and MCF-7 cells, significantly increased mesenchymal markers (e.g. vimentin) (Fig. 8C–E), and significantly decreased epithelial markers (e.g. E-cadherin and beta-catenin) (Fig. 8C–E) when compared to EV control cells. Furthermore, overexpression of the T286D phosphomimic mutant resulted in a further significant decrease in beta-catenin and E-cadherin when compared to WT and T286V mutant expressing cells (Fig. 8C–E). This indicates that CaMKII, and specifically pT286-CaMKII, may mediate breast cancer cell motility by initiating the EMT.
Discussion
Recent studies have demonstrated that CaMKII is involved in controlling osteosarcoma and gastric cancer cell invasion and migration16,22, and lung cancer tumourigenicity29, and we have previously implicated CaMKII in breast cancer cell proliferation11,30. However, the role of CaMKII phosphorylation in cancer cell invasion and migration has not previously been explored. Our data presented herein show that breast cancer cell invasion, migration and anchorage independent growth can be enhanced by phosphorylation of CaMKII at T286, and that if this phosphorylation is prevented using pharmacological inhibitors, this invasion and migration can be prevented. Furthermore, our data indicates that the cellular effects of pT286-CaMKII may be mediated by initiating the EMT, and by activation of FAK, STAT5a and Akt.
Increased CAMK2 mRNA was associated with significantly worse overall and distant metastasis free survival in the breast cancer patients examined (Fig. 1), indicating that high CAMK2 mRNA is a potentially novel biomarker for breast patient outcome. However, precisely how increased CAMK2 is producing these adverse effects is unknown.
Whilst there are four isoforms of CaMKII, overexpression of WT-CaMKIIα has previously been shown to control osteosarcoma16 and gastric22 invasion and migration. We also found that increased CAMK2A mRNA expression was associated with significantly worse distant metastasis free survival in Luminal A and triple negative breast cancer patients. Therefore, we utilised CaMKIIα and Luminal A and triple negative cell lines for our overexpression experiments performed in this study.
Daft et al.16 and Liu et al.22 have previously examined the role of CaMKIIα in osteosarcoma and gastric cancer cell metastasis, however no previous investigation of the function of CaMKII phosphorylation in these processes has been performed. Herein, we show that pT286-CaMKII is increased in primary breast cancers and their associated lymph node metastases, when compared to normal breast tissue (Fig. 2).
Our findings are consistent with the previous studies examining the role of CaMKII in invasion and migration, and suggest that CaMKIIα can control breast cancer cell migration and invasion. CaMKII is a multifunctional kinase that has been shown to phosphorylate several proteins involved in invasion and migration, including FAK31, matrix metalloproteinase-9 (MMP-9)22, and stathmin32.
Our results suggest that it is not just CaMKII expression that is important for controlling cancer cell invasion and migration, but rather that phosphorylation of CaMKII at T286 can further enhance these processes (Figs 4 and 5), indicating that abundant autonomous activation of CaMKII may result in significantly worse outcome for breast cancer patients by increasing breast cancer cell motility and invasiveness. Additionally, we show that pharmacological inhibition of CaMKII activity using two distinct inhibitors (KN-93 and myr-AIP) prevents breast cancer cell migration and invasion (Fig. 7). Taken together, these findings indicate that CaMKII inhibitors may be useful for preventing breast cancer metastasis.
To investigate the molecular mechanisms underlying the pro-metastatic properties of pT286-CaMKII in breast cancer, over 44 proteins known to be important in cancer cell metastasis were examined. Increased levels of pFAK, pSTAT5a, and pAkt (Fig. 8) were noted in cells expressing high levels of the T286D phosphomimic mutant form of CaMKIIα. FAK is a well known promoter of tumour progression and metastasis33, and has previously been shown to be activated by CaMKII in murine fibroblast cells34. The Akt-mTOR signalling pathway is known to promote tumourigenesis and metastasis of breast cancer35, and CaMKII can activate Akt in vascular smooth muscle cells36. STAT5a was first identified as a “mammary gland factor”37, but its role in breast cancer progression has not been fully elucidated, as its activation has been shown to play a role in mammary tumour initiation38, and to maintain differentiation and suppress the EMT39. However, a role for active STAT5 in metastasis of other cancers has been established, and active STAT5a promotes prostate cancer invasion and migration40. Whilst CaMKII does not phosphorylate STAT5a at the site examined in this study, a FAK-mediated pathway can control STAT5 activation in leukaemia cells41. Furthermore, FAK42 and Akt43 are essential for inducing EMT in hepatocytes and cancer cells, respectively. Taken together, our data suggest that pT286-CaMKII may enhance breast cancer metastasis via a FAK and Akt-dependent mechanism.
Our findings have identified a new mechanism for controlling breast cancer cell metastasis, specifically phosphorylation of CaMKII at T286. Autonomously activated CaMKII enhances breast cancer metastasis, and pharmacological inhibition of CaMKII activity prevents breast cancer cell invasion and migration in vitro. These data provide evidence that CaMKII activation is a novel target for the treatment of breast cancer metastasis.
Materials and Methods
Cell Lines and Generation of Mutant CaMKII Expressing Cells
MCF-7 (ATCC HTB-22), SKBR-3 (ATCC HTB-30), and T47D (ATCC HTB-133) cells were purchased from the ATCC (Manassas, VA, USA) and maintained in RPMI-1640, supplemented with 2 mM glutamine and 10% heat-inactivated fetal calf serum (FCS; Sigma-Aldrich, Castle Hill, NSW, Australia). MDA-MB-231 (ATCC HTB-26) and 184A1 (ATCC CRL-8798) were purchased from the ATCC and maintained in DMEM, supplemented with 15 mM HEPES, 2 mM glutamine, and 10% FCS. Human Mammary Epithelial Cells (HMEC) were purchased from Life Technologies, and maintained in HMEC basal serum free medium supplemented with HMEC Supplement and 0.05 mg/ml bovine pituitary extract. All cell culture reagents were purchased from Life Technologies (Mulgrave, VIC, Australia) unless otherwise noted.
MDA-MB-231 cells inducibly expressing FLAG-tagged-CaMKIIα mutants (empty vector [EV], wild-type [WT], T286D phosphomimic, T286V phosphonull), and MCF-7 cells stably expressing CaMKIIα-FLAG-tagged mutants were generated as previously described11,30.
CaMKII Inhibitors
2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine [KN-93], and 2-[N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine, phosphate [KN-92] (Calbiochem, Kilsyth, VIC, Australia) were dissolved in dimethyl sulphoxide (DMSO), and myristoylated-AIP [autocamtide-2-related inhibitory peptide; myristoyl-Lys-Lys-Ala-Leu-Arg-Arg-Gln-Glu-Ala-Val-Asp-Ala-Leu] (Biomol, Hamburg, Germany) was dissolved in distilled water. Stock solutions were stored at −20 °C.
Bioinformatics Analysis of CAMK2 Expression
Bioinformatic analysis of the four genes encoding CaMKII (CAMK2A, CAMK2B, CAMK2G, CAMK2D) were assessed individually and as a group using data from the gene expression based outcome for breast cancer online algorithm (GOBO)25. GOBO is a web based analysis tool that utilises 11 publically available Affymetrics U133A gene expression data curated from 1,881 breast cancer patients with associated stage, grade, nodal status and intrinsic molecular classification25. Association of outcome was investigated for the total patient cohort, irrespective of subset, with relapse free survival, distant metastasis free survival, or overall survival as end points, and no time-dependent censoring.
Retrospective Kaplan–Meier relapse free survival analyses of 3,935 human patients with invasive breast cancer were performed using an updated version of the previous Kaplan-Meier plotter database26. Patients were divided into two groups according to the median mRNA expression levels of the four genes encoding CaMKII. Each percentile of expression between the lower and upper quartiles was computed, and the best performing threshold was used as cutoff for the Kaplan–Meier analyses.
Tissue microarray
Tissue microarrays (TMAs) were purchased from SuperBioChip Laboratories (Seoul, South Korea), and consisted of 70 breast cancer cores, 10 matched lymph node cores, and 40 matched normal breast tissue samples. The tissues were stained for CaMKII expression and phosphorylation at T286 using a rabbit monoclonal antibody against total CaMKII (1:85; Abcam, Cambridge, MA, USA) or pT286-CaMKII (1:100; Abcam), using the Ventana Discovery Ultra automated system (Ventana Medical Systems Inc., Tucson, AZ, USA). The slides were dewaxed by heating at 69 °C for 24 mins, and antigen retrieval was performed using a high pH buffer at 99 °C for 32 min. Endogenous peroxidase activity was blocked with Inhibitor CM (Ventana Medical Systems) for 8 mins, and then primary antibody was added for 1 h at 35 °C. The samples were then incubated with Discovery OmniMap anti-rabbit HRP for 32 mins (Ventana Medical Systems). The slides were developed with 3,3′-diaminobenzidine tetrahydrochloride substrate and counterstained with haematoxylin (Ventana Medical Systems). Negative controls were included by using non-immune rabbit sera and omitting the primary antibody incubation step. Slides were scanned using an Aperio Scanscope (Leica Biosystems, North Ryde, NSW, Australia), and the H-score calculated. This method assigns an IHC H-score to each patient on a continuous scale of 0–300, based on the percentage of cells at different staining intensities, which was determined using HALO software (Indica Labs, Corrales, NM). The H-score was calculated as follows:
1 × (% of lightly stained cells) + 2 × (% of intermediate stained cells) + 3 × (% of darkly stained cells)27.
Soft Agar Assay
Anchorage independent growth of transfected MDA-MB-231 and MCF-7 cells was measured by plating 10,000 cells in semisolid culture media (1.2% methylcellulose; Sigma-Aldrich) supplemented with 20% FCS (and 2 μg/ml doxycycline for MDA-MB-231 cells), on a layer of 0.7% agar in growth medium. Fresh growth medium was added weekly. At the end of 14–24 days incubation, colonies were stained with 0.05% crystal violet/PBS solution overnight at room temperature and colonies >50 μm were counted. Assays were plated in triplicate, and three independent experiments were performed.
Tumourigenic Assay
Tumourigenicity of transfected MDA-MB-231 and MCF-7 cells was measured by plating 1000 cells in 6-well plates in growth medium (with 2 μg/ml doxycycline for MDA-MB-231 cells). At the end of 8–21 days incubation, colonies were stained with crystal violet for 30 min and counted. Assays were plated in triplicate, and three independent experiments were performed.
Cell Titre Blue Assay
Transfected MDA-MB-231 and MCF-7 cells were seeded (1 × 104/well). At various times post plating (stable MCF-7 cells) or post-CaMKII induction (2 μg/ml doxycycline for the MDA-MB-231 cells), proliferation was assessed using the Cell Titer-Blue Cell Viability Assay (Promega, Alexandria, NSW, Australia), as per manufacturer’s instructions. Assays were plated in quadruplicate, and three independent experiments were performed.
Scratch Migration Assay
Scratch wound migration assays were conducted using transfected MCF-7 and MDA-MB-231 cells, and parental MDA-MB-231 cells following treatment with CaMKII inhibitors. Parental MDA-MB-231 confluent cell monolayers in 24-well plates were preincubated for 30 mins with 20 μM KN-92 or KN-93, or 10 μM myr-AIP. Confluent monolayers of transfected MDA-MB-231 (pre-treated with 2 μg/ml doxycycline for 24 h) and MCF-7 cells in 24-well plates were scratched with a P200 tip. Scratched monolayers were washed twice with sterile phosphate buffered saline (PBS). Medium was replaced with serum free media (with 2 μg/ml doxycycline for MDA-MB-231 cells), and wounds were photographed hourly for 72 h using a humidified, automated live cell microscope at 37 °C/5% CO2 (Carl Zeiss, North Ryde, NSW, Australia). Cell migration was analysed using AxioVision v4.9.1 software (Carl Zeiss) to measure the size of the wound, by averaging three individual measurements of wound size for each wound at each time point. Results from three independent experiments with three replicates per experiment were pooled, and data were expressed as percentage of the wound width (compared to 0 h).
Transwell Migration Assay
The migratory properties of transfected MCF-7 and MDA-MB-231 cells, and parental MDA-MB-231 cells treated with CaMKII inhibitors were investigated using a Cultrex BME Cell Invasion Assay Kit (R&D Systems, Gymea, NSW, Australia), as per the manufacturer’s instructions. Parental MDA-MB-231 cells were serum starved for 24 h, and then pretreated for 30 min with 20 μM KN-92 or KN-93, or 10 μM myr-AIP prior to plating. MDA-MB-231 cells inducibly expressing CaMKII were pre-treated with 2 μg/ml doxycycline (in serum free media) for 24 h to induce CaMKII expression prior to plating. MCF-7 cells were serum starved for 24 h prior to plating. Transwell chambers (8 μM pore) were left uncoated. Cells (5 × 104 cells/chamber in serum free media) were added to the top chambers, and 10% FCS was added to the lower chambers. The cells were allowed to migrate for 4 h, after which time, medium was removed from the top and bottom chambers. Migrated cells on the underside of the chamber were detected by incubating with Calcein-AM dissolved in 1 x Cell Dissociation Buffer (final concentration: 0.8 μM) for 1 h. Fluorescence of the samples was determined at λexcitation 485 nm and λemission 520 nm using an ELISA plate reader (FLUOStar Optima; BMG Labtech, Mornington, VIC, Australia). The number of cells that migrated through the BME coat were calculated using a standard curve, per manufacturer’s instructions. Results from three independent experiments with three replicates per experiment were pooled.
Transwell Invasion Assay
The invasive properties of transfected MCF-7 and MDA-MB-231 cells, and parental MDA-MB-231 cells treated with CaMKII inhibitors were investigated as described above, with the following modification. Prior to the addition of cells, transwell chambers (8 μM pore) were coated with 1 x basement membrane extract (BME) solution in coating buffer overnight at 37o C, before excess buffer was removed.
Western Blotting
Stably transfected MCF-7 cells or inducibly transfected MDA-MB-231 cells that had been treated with 2 μg/ml doxycycline for 24–48 h were lysed as previously described44. Cell lysates (10–20 μg) were separated using 10% SDS-polyacrylamide gel electrophoresis (PAGE), and then transferred to nitrocellulose membranes44. The primary antibodies used were as follows: GAPDH (1:2,000; BioVision, Milpitas, CA, USA), FAK (1:1,000; Abcam), pY397-FAK (1:1,000; Abcam), ERK1/2 (1:1,000; Abcam), pT204/T285-ERK (1:1,000; Abcam), Akt (1:1,000; Abcam), pS473-Akt (1:1,000; Abcam), E-cadherin (1:1,000; Abcam), β-catenin (1:4,000; Abcam), pY694-STAT5a (1:1,000; GeneTex, Redfern, NSW, Australia), STAT5a (1:1,000; GeneTex), vimentin (1:1,000; Abcam). Blots were scanned with a Fujifilm LAS-3000 Imaging System and analysed with MultiGauge Software (Fujifilm, Brookvale, NSW, Australia).
Proteome Profiler Human Phospho-Kinase Array
The simultaneous phosphorylation of 44 proteins in transfected cells were detected using the Proteome Profiler Human Phospho-Kinase Array Kit (R&D systems, Abingdon, UK), as per manufacturer’s instructions.
Data Analysis
All statistical analyses were conducted using GraphPad Prism software V6.0 (GraphPad, San Diego, CA, USA). Comparisons between mutants were made using one-way analysis of variance (ANOVA), with a Bonferonni post-test. The Kaplan-Meier survival analysis was calculated using the Cox proportional hazard model. All data is presented as the mean ± standard error of the mean (SEM) for the number of replicates (n).
Additional Information
How to cite this article: Chi, M. et al. Phosphorylation of calcium/calmodulin-stimulated protein kinase II at T286 enhances invasion and migration of human breast cancer cells. Sci. Rep. 6, 33132; doi: 10.1038/srep33132 (2016).
References
Ferlay, J. et al. In IARC CancerBase No. 11 (Lyon, 2013).
Kennecke, H. et al. Metastatic behaviour of breast cancer subtypes. J Clin Oncol 28, 3271–3277 (2010).
Cross, B. M., Breitwieser, G. E., Reinhardt, T. A. & Rao, R. Cellular calcium dynamics in lactation and breast cancer: from physiology to pathology. Am J Physiol Cell Physiol 306, C515–C526 (2014).
Stewart, T. A., Yapa, K. T. & Monteith, G. R. Altered calcium signaling in cancer cells. Biochim Biophys Acta 10.1016/j.bbamem.2014.08.016 (Epub ahead of print) (2014).
Chen, Y. F., Chen, Y. T., Chiu, W. T. & Shen, M. R. Remodeling of calcium signaling in tumor progression. J Biomed Sci 20, 23 (2013).
Skelding, K. A. & Rostas, J. A. The role of molecular regulation and targeting in regulating calcium/calmodulin stimulated protein kinases. Adv Exp Med Biol 740, 703–730 (2012).
Giese, K. P., Fedorov, N. B., Filipkowski, R. K. & Silva, A. J. Autophosphorylation of Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science 279, 870–873 (1998).
Miller, S. et al. Disruption of dendritic translation of CaMKIIalpha impairs stabilization of synaptic plasticity and memory consolidation. Neuron 36, 507–519 (2002).
Mercure, M. Z., Ginnan, R. & Singer, H. A. CaM kinase II delta2-dependent regulation of vascular smooth muscle cell polarization and migration. Am J Physiol Cell Physiol 294, C1465–C1475 (2008).
Skelding, K. A., Rostas, J. A. & Verrills, N. M. Controlling the cell cycle: The role of calcium/calmodulin-stimulated protein kinases I and II. Cell Cycle 10, 631–639 (2011).
Hoffman, A. et al. Dephosphorylation of CaMKII at T253 controls the metaphase-anaphase transition. Cell Signal 26, 748–756 (2014).
Yuan, K., Chung, L. W. K., Siegal, G. P. & Zayzafoon, M. alpha-CaMKII controls the growth of human osteosarcoma by regulating cell cycle progression. Lab Invest 87, 938–950 (2007).
Jones, K. T. Intracellular calcium in the fertilization and development of mammalian eggs. Clin Exp Pharmacol Physiol 34, 1084–1089 (2007).
Nguyen, T., Chen, C. J. & Shively, J. E. Phosphorylation of CEACAM1 molecule by calmodulin kinase IID in a three-dimensional model of mammary gland lumen formation. J Biol Chem 289, 2934–2945 (2014).
Wang, Y.-Y., Zhao, R. & Zhe, H. The emerging role of CaMKII in cancer. OncoTarget 6, 11725–11734 (2015).
Daft, P. G. et al. Alpha-CaMKII plays a critical role in determining the aggressive behavior of human osteosarcoma. Mol Cancer Res 11, 349–359 (2013).
Wang, Q. et al. A novel role for Wnt/Ca2+ signaling in actin cytoskeleton remodeling and cell motility in prostate cancer. Plos one 5, e10456 (2010).
Skelding, K. A. & Rostas, J. A. P. Regulation of CaMKII in vivo: the importance of targeting and the intracellular microenvironment. Neurochem Res 34, 1792–1804 (2009).
Abdul Majeed, A. B. B. et al. CaMKII kinase activity, targeting and control of cellular functions: effect of single and double phosphorylation of CaMKIIalpha. Calcium Signaling 1, 36–51 (2014).
Cho, Y. H., Giese, K. P., Tanila, H., Silva, A. J. & Eichenbaum, H. Abnormal hippocampal spatial representations in alphaCaMKIIT286A and CREBalphaDelta mice. Science 279, 867–869 (1998).
Irvine, E. E., Vernon, J. & Giese, K. P. AlphaCaMKII autophosphorylation contributes to rapid learning but its not necessary for memory. Nat Neurosci 8, 411–412 (2005).
Liu, Z., Han, G., Cao, Y., Wang, Y. & Gong, H. Calcium/calmodulin-dependent protein kinase II enhances metastasis of human gastric cancer by upregulating nuclear factor-kappaB and Akt-mediated matrix metalloproteinase-9 production. Mol Med Rep 10, 2459–2464 (2014).
Gu, Y. et al. CaMKII gamma, a critical regulator of CML stem/progenitor cells, is a target of the natural product berbamine. Blood 120, 4829–4839 (2012).
Britschgi, A. et al. Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. Proc Natl Acad Sci USA 110, E1026–E1034 (2013).
Ringner, M., Fredlun, E., Hakkinen, J., Borg, A. & Staaf, J. GOBO: gene expression-based outcome for breast cancer online. PLoS One 6, e17911 (2011).
Gyorffy, B. et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1809 patients. Breast Cancer Res Treat 123, 725–731 (2010).
Pirker, R. et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer. Lancet Oncol 13, 33–42 (2012).
Freedman, V. H. & Shin, S. Cellular tumorigenicity in nude mice: correlation with cell growth in semisolid medium. Cell 3, 355–359 (1974).
Chai, S. et al. Ca2+/calmodulin-dependent protein kinase IIgamma enhances stem-like traits and tumorigenicity of lung cancer cells. Oncotarget doi: 10.18632/oncotarget.3866 (2016).
Skelding, K. A. et al. Regulation of CaMKII by phospho-Thr253 or phospho-Thr286 sensitive targeting alters cellular function. Cell Signal 22, 759–769 (2010).
Lu, K. K., Armstrong, S. E., Ginnan, R. & Singer, H. A. Adhesion-dependent activation of CaMKII and regulation of ERK activation in vascular smooth muscle. Am J Physiol Cell Physiol 289, C1343–C1350 (2005).
Li, N. et al. Siva1 suppresses epithelial-mesenchymal transition and metastasis of tumor cells by inhibiting stathmin and stabilizing microtubules. Proc Natl Acad Sci USA 108, 12851–12856 (2011).
Sulzmaier, F. J., Jean, C. & Schlaepfer, D. D. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer 14, 598–610 (2014).
Fan, R. S., Jacamo, R. O., Jiang, X., Sinnett-Smith, J. & Rozengurt, E. G protein-coupled receptor activation rapidly stimulates focal adhesion kinase phosphorylation at Ser-843. Mediation by Ca2+, calmodulin, and Ca2+/calmodulin-dependent kinase II. J Biol Chem 280, 2412–2420 (2005).
Li, X. et al. LIF promotes tumorigenesis and metastasis of breast cancer through the AKT-mTOR pathway. Oncotarget 5, 788–801 (2014).
Li, F. & Malik, K. U. Angiotensin II-induced Akt activation is mediated by metabolites of arachionic acid generated by CaMKII-stimulated Ca2(+)-dependent phospholipase A2. Am J Physiol Heart Circ Physiol 288, H2306–H2316 (2005).
Wakao, H., Gouilleux, F. & Groner, B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family an confers the prolactin response. EMBO J 13, 2182–2191 (1994).
Humphreys, R. C. & Hennighausen, L. Signal transducer and activator of transcription 5a influences mammary epithelial cell survival an tumorigenesis. Cell Growth Diff 10, 685–694 (1999).
Sultan, A. S., Brim, H. & Sherif, Z. A. Co-overexpression of Janus kinase 2 and signal transducer and activator of transcription 5a promotes differentiation of mammary cancer cells through revetrsal of epithelial-mesenchymal transition. Cancer Sci 99, 272–279 (2008).
Gu, L. et al. Stat5 promotes metastatic behavior of human prostate cancer cells in vitro and in vivo. Endocr Relat Cancer 17, 481–493 (2010).
Chatterjee, A. et al. Regulation of Stat5 by FAK and PAK1 in oncogenic FLT3- and KIT-driven leukemogenesis. Cell Rep 9, 1333–1348 (2014).
Cicchini, C. et al. TGFbeta-induced EMT requires focal adhesion kinase (FAK) signaling. Exp Cell Res 314, 143–152 (2008).
Larue, L. & Bellacosa, A. Epithelial-mesenchymal transition in development and cancer: role of phosphadtidylinositol 3′ kinase/AKT pathways. Oncogene 24, 7443–7454 (2005).
Skelding, K. A., Spratt, N. J., Fluechter, L., Dickson, P. W. & Rostas, J. A. alpha CaMKII is differentially regulated in brain regions that exhibit differing sensitivities to ischemia and excitotoxicity. J Cereb Blood Flow Metab 32, 2181–2192 (2012).
Acknowledgements
This work was supported by research funds from the Hunter Medical Research Institute, the Hunter Translational Cancer Research Unit, and the University of Newcastle. KAS was also supported by the Cure Cancer Australia Foundation, Cancer Australia and Tour de Cure. JSB was funded by the MM Sawyer Trust through the Hunter Medical Research Institute (HMRI). The authors would like to thank Dr. Rick Thorne, Mr. Clay Winterford, Mrs. Helen Carpenter, Ms. Batoul Al-Mudafer, Ms. Eden Alawie, and Mr. Aaran Ketheson for their technical assistance on various aspects of this project.
Author information
Authors and Affiliations
Contributions
K.A.S. conceived the study and wrote the first draft. M.C. performed the experiments with the assistance of H.E., J.G., J.M., A.H., E.A.P., H.J. and J.S.B. All authors were involved in the editing of subsequent drafts of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chi, M., Evans, H., Gilchrist, J. et al. Phosphorylation of calcium/calmodulin-stimulated protein kinase II at T286 enhances invasion and migration of human breast cancer cells. Sci Rep 6, 33132 (2016). https://doi.org/10.1038/srep33132
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep33132
This article is cited by
-
Gene expression signatures of individual ductal carcinoma in situ lesions identify processes and biomarkers associated with progression towards invasive ductal carcinoma
Nature Communications (2022)
-
The dysregulated expression and functional effect of CaMK2 in cancer
Cancer Cell International (2021)
-
Identification of oxytocin-related lncRNAs and assessment of their expression in breast cancer
Scientific Reports (2021)
-
CAMK2A supported tumor initiating cells of lung adenocarcinoma by upregulating SOX2 through EZH2 phosphorylation
Cell Death & Disease (2020)
-
TRPV6 calcium channel directs homeostasis of the mammary epithelial sheets and controls epithelial mesenchymal transition
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.