Abstract
Air pollution can cause oxidative stress and adverse health effects such as asthma and other respiratory diseases, but the underlying chemical processes are not well characterized. Here we present chemical exposure-response relations between ambient concentrations of air pollutants and the production rates and concentrations of reactive oxygen species (ROS) in the epithelial lining fluid (ELF) of the human respiratory tract. In highly polluted environments, fine particulate matter (PM2.5) containing redox-active transition metals, quinones, and secondary organic aerosols can increase ROS concentrations in the ELF to levels characteristic for respiratory diseases. Ambient ozone readily saturates the ELF and can enhance oxidative stress by depleting antioxidants and surfactants. Chemical exposure-response relations provide a quantitative basis for assessing the relative importance of specific air pollutants in different regions of the world, showing that aerosol-induced epithelial ROS levels in polluted megacity air can be several orders of magnitude higher than in pristine rainforest air.
Similar content being viewed by others
Introduction
Anthropogenic air pollution leads to a massive increase of atmospheric aerosol and oxidant concentrations on local, regional, and global scales, posing a major threat to public health1,2. The concentrations of fine particulate matter in polluted urban air are several orders of magnitude higher than in pristine air (~10–1000 μg m−3 vs. ~1–10 μg m−3)2, and high pollutant levels can cause serious respiratory and cardiovascular diseases, leading to elevated mortality3,4,5,6. For example, mortality rates in the 90 largest U.S. cities were found to rise on average by 0.5% with each 10 μg m−3 increase in fine particulate matter7, and globally the annual number of premature deaths due to air pollution are estimated to exceed 3 million with an increasing trend8. Fine air particulate matter contains redox-active components like transition metals and quinones originating from gasoline and diesel motor exhaust, cigarette smoke, and other sources including secondary organic aerosol (SOA) formation in the atmosphere9,10,11. Upon inhalation and deposition in the human respiratory tract, such air pollutants can induce and sustain chemical reactions that produce reactive oxygen species (ROS; OH, O2−, HO2, O3, and H2O2) in the epithelial lining fluid (ELF) covering the airways12. The ELF contains a range of antioxidants and surfactants13 (Supplementary information, Supplementary Tables 1 and 3), and it extends from the nasal cavity to the pulmonary alveoli with a film thickness that decreases from several micrometers in the upper airways to dozens of nanometers in the lungs14.
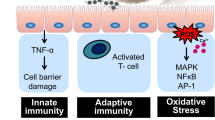
As illustrated in Fig. 1, the redox-active pollutants and ROS undergo a multitude of radical and redox reaction cycles in the ELF and the initial step is the transfer of electrons from antioxidants to transition metal ions or quinones forming reduced metal ions or semiquinones, respectively2,11,15. The redox-active transition metal ions and quinones are regenerated by reaction with O2 forming O2− radicals that are further converted into hydrogen peroxide, which is central to radical reaction cycles and oxidative stress in the respiratory tract16. OH radicals, the most reactive form of ROS, can be produced via Fenton-like reactions of H2O2 with iron or copper ions17 and can also be released upon interaction of SOA with water18,19. Numerous studies have shown that excess ROS can cause oxidative stress injuring cells and tissues in the respiratory tract2,16. Thus, characterizing the formation of ROS in the ELF is crucial for understanding how air pollution leads to adverse health effects like asthma, allergies and other respiratory diseases.
Interaction of air pollutants and reactive oxygen species (ROS) in the epithelial lining fluid (ELF) of the human respiratory tract.
ELF can be regarded as an interface between atmospheric and physiological chemistry, through which air pollution and environmental change can induce oxidative stress and adverse health effects. Atmospheric ozone and OH radicals react with surfactants and antioxidants (ascorbate, uric acid, reduced glutathione, α-tocopherol) forming secondary organic oxidants. Redox-active components of fine particulate matter, including quinones, iron and copper ions, can trigger and sustain catalytic reaction cycles generating ROS and oxidative stress.
The production rate and concentration of ROS induced by air pollutants in the ELF, however, have hardly been quantified so far2,12,14. For this purpose we have developed a kinetic multi-layer model of surface and bulk chemistry in the epithelial lining fluid (KM-SUB-ELF), which explicitly treats mass transport and chemical reactions involving air pollutants, ROS, antioxidants and surfactants as detailed in the supplement (Supplementary Fig. 1. & Supplementary Table 1)20. It can reproduce experimental data available on the formation and concentrations of H2O215 and OH17 in surrogate ELF containing quinones, iron and copper ions (Supplementary Fig. 2). Characteristic concentration levels of air pollutants in the ELF can be derived from ambient concentrations, breathing rates, and deposition rates in the respiratory tract as detailed in the supplement15. With this approach and the KM-SUB-ELF model, we obtained chemical exposure-response relations between the production rates and concentrations of ROS in the ELF and the ambient concentrations and composition of fine particles with diameters <2.5 μm (PM2.5) characteristic for a wide range of geographic locations with different levels of air pollution (Supplementary Tables 4–6).
Figure 2A shows ROS production rates calculated for each location and each redox-active component plotted against the ambient concentration of PM2.5. The data points fall into corridors outlining the relative importance of different chemical components for ROS production in the ELF. The highest ROS production rates are caused by copper and iron ions followed by quinones and SOA. As illustrated in Fig. 2B, the far most abundant form of ROS in the ELF are H2O2 molecules, which are three to four orders of magnitude more abundant than the O2− radicals initially produced from molecular oxygen, six to seven times more abundant than the HO2 radicals formed by proton transfer to O2−, and approximately ten orders of magnitude more abundant than the OH radicals formed by Fenton-like reactions of H2O2 (Fig. 1). The wide range of concentration levels reflects the vastly different chemical reactivities and lifetimes of the different ROS species. Due to its relatively low reactivity and decomposition rates, H2O2 is a reservoir species with a chemical lifetime of several hours, whereas the lifetime of OH radicals is less than a microsecond due to their rapid reactions with antioxidants.
Chemical exposure-response relations for reactive oxygen species (ROS) produced in the human respiratory tract upon inhalation of fine particulate matter (PM2.5).
It is shown as a function of PM2.5 concentrations with redox-active components as observed at various geographic locations around the world (Supplementary Tables 4–7). (A) ROS production rates induced by copper (Cu), iron (Fe), secondary organic aerosol (SOA), and quinones. (B) Characteristic concentration levels of different types of ROS and (C) total ROS concentration in the epithelial lining fluid after two hours of inhalation and deposition of ambient PM2.5. In panel (C), the green-striped horizontal bar indicates the ROS level characteristic for healthy humans (~100 nmol L−1), and the gray envelope represents the range of aerosol-induced ROS concentrations obtained with the approximate upper and lower limit mass fractions of redox-active components typically observed in ambient PM2.5. Total water-soluble fractions of iron and copper can range from ~5–25% and ~20–60%, respectively, in a wide range of different environments, which are represented by the error bars. (D) Fractional change of ROS concentrations upon removal of 50% of redox-active components from PM2.5 calculated for selected geographic locations with different PM2.5 concentration levels and composition (Supplementary Table 7).
The total concentration of ROS generated by redox-active components of PM2.5 deposited in the ELF ranges from ~10 nmol L−1 under clean conditions up to almost ~250 nmol L−1 under highly polluted conditions as shown in Fig. 2C. The data points are a subset of the data points in Fig. 2A,B, representing the locations for which we could obtain measurement data or well-founded estimates for the characteristic concentration of each of the redox-active components (Supplementary Tables 4–7). The upper and lower bounds of the grey-shaded envelope around the data points are constrained by the approximate upper and lower limit mass fractions and water-soluble fractions of redox-active components typically observed in ambient PM2.5 (see Supplementary section 2).
The green-striped horizontal bar in Fig. 2C indicates ROS concentration levels characteristic for the ELF or bronchoalveolar lavage of healthy humans, respectively, which are around ~100 nmol L−121,22. Compared to this reference level, the ROS concentrations generated by redox-active particulate matter inhaled from pristine marine or rainforest air (PM2.5 ≪ 10 μg m−3) are much lower and appear negligible with regard to airway oxidative stress (ROS < 50 nmol L−1). In moderately polluted air (PM2.5 ≈ 10–50 μg m−3), the particle-generated ROS concentrations can be of similar magnitude or higher than the physiological background level (ROS ≈ 50–200 nmol L−1) and may thus significantly contribute to oxidative stress depending on aerosol concentration and chemical composition. In heavily polluted air (PM2.5 >≈ 50 μg m−3), the particle-generated ROS concentrations are as high as the ROS concentrations observed in the bronchoalveolar lavage of patients with acute inflammatory diseases in respiratory tract (ROS ≈ 100–250 nmol L−121,22). The pathologically high ROS concentrations calculated for the ELF in airways exposed to high ambient aerosol concentrations are consistent with epidemiology-based air quality standards and regulations of the World Health Organization (WHO) and various national environmental protection agencies aiming at PM2.5 concentrations less than ~20–40 μg m−3 averaged over one day and less than ~10–20 μg m−3 averaged over one year3,4,5.
For selected geographic locations covering a wide range of PM2.5 concentration levels, Fig. 2D shows how the particle-generated ROS concentrations in the ELF would change in response to reducing the concentration of individual or multiple redox-active components by 50%. At all locations strongly influenced by anthropogenic air pollution, the reduction of copper would have the largest effect and reduce the total ROS concentration by ~20%, while the reduction of SOA would decrease total ROS by only ~5% (Supplementary Table 7). Our results are consistent with recent experimental studies emphasizing the high potential of copper to cause oxidative stress and adverse health effects23,24.
The reduction of iron would decrease ROS by ~10% in moderately polluted air (PM2.5 ≈ 10–50 μg m−3). For extremely polluted air (PM2.5 > 100 μg m−3), on the other hand, we found that particle-generated ROS levels would even increase upon removal of iron. This is because iron is much more efficient in catalyzing decomposition of H2O2 to OH radicals (Fenton reaction), while copper and iron are both contributing in similar ways to ROS production. Indeed iron dominates the production of OH radicals (Supplementary Figs 4 and 5), and the reduction of iron leads to a much stronger decrease of OH concentrations (up to ~40%) than the reduction of copper (Supplementary Fig. 6). Thus, reducing iron may still be important for decreasing oxidative stress caused by OH radicals.
Sensitivity studies indicate that Cu and Fe are the redox-active aerosol components most important for ROS production upon inhalation of PM2.5 in polluted regions even if the soluble fractions of the metals are assumed to be low (Supplementary Fig. 3). Thus, we suggest that the emission of copper- and iron-containing particles should be considered as a major target of air pollution control with regard to oxidative stress in the human respiratory tract. Automobile brake and tire wear are major sources of Cu and Fe in polluted air25, and reducing these emissions might help to reducing oxidative stress and the adverse health effects related to air particulate matter and road traffic especially in densely populated regions. Correlations with inflammatory and oxidative stress parameters have also been reported for other water-soluble metal ions such as Zn and As26,27. Both the concentrations and the soluble fractions of metal ions in PM2.5 should thus be more widely monitored to gather comprehensive input for epidemiological studies assessing the reasons and mechanisms of aerosol health effects and oxidative stress in the respiratory system. Due to air exchange, the concentrations and composition of PM2.5 in indoor air tends to be similar to outdoor air28,29. During periods of activity such as cooking, cleaning and smoking, however, indoor concentrations of PM2.5 can be much higher30, and metal ions can also be released from indoor sources like vacuum cleaners, blenders, food processors, and laser printers29. Thus, the inhalation of metal-containing particles is likely also relevant for the health effects of indoor air pollution.
Our findings demonstrate that the complex radical and redox reaction cycles outlined above (Fig. 1) can lead to non-linear changes of ROS concentrations in the ELF in response to concentration changes or removal of individual redox-active components of PM2.5. Thus, we suggest and intend to further advance and apply numerical and experimental techniques for the determination of chemical exposure-response relations for the design of efficient control strategies against adverse health effects of aerosols from different sources.
The understanding and characterization of non-linear interactions also play an important role in controlling the formation and effects of gaseous atmospheric photooxidants that may contribute to oxidative stress in the respiratory tract. Our model results show that OH radicals inhaled with ambient air are rapidly scavenged by surfactants and antioxidants, whereas the ELF is readily saturated with ozone approaching Henry’s law equilibrium concentrations (Supplementary Fig. 7). Figure 2B shows the relative abundance of O3 compared to other ROS species, indicating that the O3 concentration is higher than O2− concentrations but less than H2O2 concentrations. Nevertheless, elevated ozone can contribute to oxidative stress by depleting antioxidants and surfactants in the ELF: As shown in Fig. 3, an increase of ozone from typical background concentration levels (~30 ppb) to summer smog conditions (>≈100 ppb) reduces the chemical half-life from days to hours for antioxidants and from hours to minutes for surfactants, which may be comparable or shorter than the physiological replenishment rates31. In contrast, the H2O2-dominated ROS concentrations generated upon inhalation of PM2.5 do not substantially reduce the chemical half-life and concentration of antioxidants and surfactants (Supplementary Fig. 8). Thus, polluted air combining high PM2.5 and high ozone concentrations is expected to cause particularly high oxidative stress with high levels of ROS and low levels of antioxidant and surfactant concentrations in the ELF. Moreover, some of the surfactant ozonation products may act as transduction molecules to trigger the release of endogenous mediators of inflammation32, and the ozonolysis of antioxidants may lead to formation of long-lived reactive oxygen intermediates33 including the harmful ascorbate ozonide, especially at the low pH levels characteristic for patients with respiratory diseases34,35,36 (Supplementary Figs 9 and 10).
In the course of the Anthropocene37, the average mixing ratios of ozone in continental background air have increased by factors of 2–4 from around 10–20 ppb from the beginning of the 19th century to 30–40 ppb in the 21st century1,2. In urban areas, ozone often reaches ~80–100 ppb during summer and can exceed 200 ppb during smog periods1,38. As ozone essentially saturates the ELF according to Henry’s law, an increase of ambient ozone translates into an almost proportional increase of ozone concentration in the ELF depleting antioxidants and surfactants in the respiratory tract. Thus, the global increase of both ozone and fine particulate matter in the air of megacities and other densely populated regions around the world constitutes a major threat to public health in the Anthropocene2.
The exposure-response relations determined in this study represent a chemical baseline for the primary chemical production of exogenous ROS and oxidative stress upon inhalation and deposition of redox-active air pollutants in the human respiratory tract. On top of this baseline, air pollutants can cause secondary production of endogenous ROS via biological interactions and responses of the human immune system, including the activation of macrophages, mitochondria and ROS-producing enzymes like NADPH-oxidase (“ROS-induced ROS”)16,39,40 or infections and microbial growth induced by biological and nutrient-rich particles41. Pollution-generated exogenous ROS may also form damage associated molecular patterns and trigger immune reactions leading to acute or chronic inflammation, e.g., through the toll-like receptor radical cycle42. These and other feedback loops may enhance or dampen the oxidative stress caused by the primary production of ROS from redox-active particulate matter, ozone and other atmospheric oxidants like nitrogen oxides. Further experimental investigations and model studies will be required to fully unravel and quantify the adverse health effects of air pollution. The modeling approach and exposure-response relations presented here provide a basis for such investigations and can help to identify key species and processes, such as the cycling of copper ions, to be addressed in further studies and in the advancement of target-specific regional air quality control strategies.
Methods
KM-SUB-ELF is based on the kinetic multi-layer model for aerosol surface and bulk chemistry (KM-SUB)20. The model treats the following processes explicitly: gas-phase diffusion, adsorption and desorption from the surface, bulk diffusion as well as chemical reactions at the surface and in the bulk. The ELF is split into different layers: a sorption layer, a surfactant layer, a near surface bulk layer and a number of bulk layers. The temporal evolution and concentration profile of the various reactants and products can be simulated by solving a set of ordinary differential equations, which describe mass balance of each species by mass transport fluxes and rates of chemical production and loss. The model includes reactions of O3 and OH with antioxidants and surfactants, reactions involving quinones, Fenton chemistry of iron ions, Fenton-like chemistry involving copper ions, and HOx chemistry. Please see the supplementary information for detailed chemistry and kinetic parameters as well as determination of ROS concentrations and production rates in the ELF.
Additional Information
How to cite this article: Lakey, P. S. J. et al. Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract. Sci. Rep. 6, 32916; doi: 10.1038/srep32916 (2016).
References
Monks, P. S. et al. Atmospheric composition change - global and regional air quality. Atmos. Environ. 43, 5268–5350 (2009).
Pöschl, U. & Shiraiwa, M. Multiphase Chemistry at the Atmosphere–Biosphere Interface Influencing Climate and Public Health in the Anthropocene. Chem. Rev. 115, 4440–4475 (2015).
Lim, S. S. et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2224–2260 (2012).
Pope, C. A. & Dockery, D. W. Health effects of fine particulate air pollution: lines that connect. J. Air Waste Manag. Assoc. 56, 709–742 (2006).
Brunekreef, B. & Holgate, S. T. Air pollution and health. Lancet 360, 1233–1242 (2002).
Rich, D. Q. et al. The triggering of myocardial infarction by fine particles is enhanced when particles are enriched in secondary species. Environ. Sci. Tech. 47, 9414–9423 (2013).
Kaiser, J. Evidence Mounts That Tiny Particles Can Kill. Science 289, 22–23 (2000).
Lelieveld, J., Evans, J. S., Fnais, M., Giannadaki, D. & Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 525, 367–371 (2015).
Cho, A. K. et al. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environ. Res. 99, 40–47 (2005).
Verma, V. et al. Organic Aerosols Associated with the Generation of Reactive Oxygen Species (ROS) by Water-Soluble PM2.5. Environ. Sci. Technol. 49, 4646–4656 (2015).
Kumagai, Y., Shinkai, Y., Miura, T. & Cho, A. K. The chemical biology of naphthoquinones and its environmental implications. Annu. Rev. Pharmacol. Toxicol. 52, 221–247 (2012).
Gurgueira, S. A., Lawrence, J., Coull, B., Murthy, G. G. & Gonzalez-Flecha, B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ Health Perspect 110, 749–755 (2002).
van der Vliet, A. et al. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am. J. Physiol.-Lung C 276, L289–L296 (1999).
Mudway, I. S. & Kelly, F. J. Ozone and the lung: A sensitive issue. Mol. Aspects Med. 21, 1–48 (2000).
Charrier, J. G., McFall, A. S., Richards-Henderson, N. K. & Anastasio, C. Hydrogen Peroxide Formation in a Surrogate Lung Fluid by Transition Metals and Quinones Present in Particulate Matter. Environ. Sci. Tech. 48, 7010–7017 (2014).
Winterbourn, C. C. Reconciling the chemistry and biology of reactive oxygen species. Nature Chem. Biol. 4, 278–286 (2008).
Charrier, J. G. & Anastasio, C. Impacts of antioxidants on hydroxyl radical production from individual and mixed transition metals in a surrogate lung fluid. Atmos. Environ. 45, 7555–7562 (2011).
Wang, Y., Kim, H. & Paulson, S. E. Hydrogen peroxide generation from alpha- and beta-pinene and toluene secondary organic aerosols. Atmos. Environ. 45, 3149–3156 (2011).
Tong, H. et al. Hydroxyl radicals from secondary organic aerosol decomposition in water. Atmos. Chem. Phys. 16, 1761–1771 (2016).
Shiraiwa, M., Pfrang, C. & Pöschl, U. Kinetic multi-layer model of aerosol surface and bulk chemistry (KM-SUB): the influence of interfacial transport and bulk diffusion on the oxidation of oleic acid by ozone. Atmos. Chem. Phys. 10, 3673–3691 (2010).
Corradi, M. et al. Comparison between exhaled and bronchoalveolar lavage levels of hydrogen peroxide in patients with diffuse interstitial lung diseases. Acta Biomed. 79, 73–78 (2008).
Kietzmann, D., Kahl, R., Müller, M., Burchardi, H. & Kettler, D. Hydrogen peroxide in expired breath condensate of patients with acute respiratory failure and with ARDS. Intens. Care Med. 19, 78–81 (1993).
Janssen, N. A. H. et al. Oxidative potential of particulate matter collected at sites with different source characteristics. Sci. Total Environ. 472, 572–581 (2014).
Strak, M. M. et al. Respiratory health effects of airborne particulate matter: the role of particle size, composition, and oxidative potential-the RAPTES project. Environ. Health Perspect. 120, 1183–1189 (2012).
Fang, T., Guo, H., Verma, V., Peltier, R. E. & Weber, R. J. PM2.5 water-soluble elements in the southeastern United States: automated analytical method development, spatiotemporal distributions, source apportionment, and implications for heath studies. Atmos. Chem. Phys. 15, 11667–11682 (2015).
Shuster-Meiseles, T. et al. ROS-generating/ARE-activating capacity of metals in roadway particulate matter deposited in urban environment. Environ. Res. 146, 252–262 (2016).
Pardo, M., Shafer, M. M., Rudich, A., Schauer, J. J. & Rudich, Y. Single exposure to near roadway particulate matter leads to confined inflammatory and defense responses: Possible role of metals. Environ. Sci. Tech. 49, 8777–8785 (2015).
Weschler, C. Chemistry in indoor environments: 20 years of research. Indoor Air 21, 205–218 (2011).
Morawska, L. et al. Indoor aerosols: from personal exposure to risk assessment. Indoor Air 23, 462–487 (2013).
Isaxon, C. et al. Contribution of indoor-generated particles to residential exposure. Atmos. Environ. 106, 458–466 (2015).
Ghio, A. J., Turi, J. L., Yang, F., Garrick, L. M. & Garrick, M. D. Iron homeostasis in the lung. Biol. Res. 39, 67–77 (2006).
Pryor, W. A., Squadrito, G. L. & Friedman, M. The Cascade Mechanism to Explain Ozone Toxicity - The Role of Lipid Ozonation Products. Free Radical Bio. Med. 19, 935–941 (1995).
Shiraiwa, M. et al. The role of long-lived reactive oxygen intermediates in the reaction of ozone with aerosol particles. Nature Chem. 3, 291–295 (2011).
Enami, S., Hoffmann, M. R. & Colussi, A. J. Acidity enhances the formation of a persistent ozonide at aqueous ascorbate/ozone gas interfaces. P. Natl. Acad. Sci. USA 105, 7365–7369 (2008).
Enami, S., Hoffmann, M. R. & Colussi, A. J. How Phenol and α-Tocopherol React with Ambient Ozone at Gas/Liquid Interfaces. J. Phys. Chem. A 113, 7002–7010 (2009).
Enami, S., Hoffmann, M. R. & Colussi, A. J. OH-Radical Specific Addition to Glutathione S-Atom at the Air–Water Interface: Relevance to the Redox Balance of the Lung Epithelial Lining Fluid. J. Phys. Chem. Lett. 3935–3943 (2015).
Crutzen, P. J. & Stoermer, E. F. The Anthropocene. IGBP Global Change Newsl. 41, 17–18 (2000).
Cooper, O. R. et al. Global distribution and trends of tropospheric ozone: An observation-based review. Elem. Sci. Anth. 2, 000029 (2014).
Becker, S., Soukup, J. M., Gilmour, M. I. & Devlin, R. B. Stimulation of human and rat alveolar macrophages by urban air particulates: Effects on oxidant radical generation and cytokine production. Toxicol. Appl. Pharmacol. 141, 637–648 (1996).
Zorov, D. B., Juhaszova, M. & Sollott, S. J. Mitochondrial ROS-induced ROS release: An update and review. B. B. A. - Bioenergetics 1757, 509–517 (2006).
Borcherding, J. et al. Iron oxide nanoparticles induce Pseudomonas aeruginosa growth, induce biofilm formation, and inhibit antimicrobial peptide function. Environ. Sci. Nano 1, 123–132 (2014).
Lucas, K. & Maes, M. Role of the Toll Like Receptor (TLR) Radical Cycle in Chronic Inflammation: Possible Treatments Targeting the TLR4 Pathway. Mol. Neurobiol. 48, 190–204 (2013).
Acknowledgements
We gratefully acknowledge stimulating discussions and exchange with many members of the scientific communities of atmospheric chemistry and aerosols, air quality and climate, toxicology and public health.
Author information
Authors and Affiliations
Contributions
P.S.J.L., U.P. and M.S. designed the research. P.S.J.L., T.B. and M.S. conducted kinetic modeling. P.S.J.L., T.B., H.T., A.M.A., K.L., U.P. and M.S. analyzed data and discussed the results. P.S.J.L., U.P. and M.S. wrote the paper. P.S.J.L., T.B. and M.S. wrote the supplement.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lakey, P., Berkemeier, T., Tong, H. et al. Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract. Sci Rep 6, 32916 (2016). https://doi.org/10.1038/srep32916
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep32916
This article is cited by
-
Toxicological Effects of Fine Particulate Matter (PM2.5): Health Risks and Associated Systemic Injuries—Systematic Review
Water, Air, & Soil Pollution (2023)
-
Characteristics of PM2.5 and Its Reactive Oxygen Species in Heating Energy Transition and Estimation of Its Impact on the Environment and Health in China—A Case Study in the Fenwei Plain
Advances in Atmospheric Sciences (2023)
-
Investigating the nonlinear and non-stationary relationship between PM2.5 and air pollutants by wavelet signal analysis in central Taiwan
Environmental Geochemistry and Health (2023)
-
The role of N6-methyladenosine methylation in PAHs-induced cancers
Environmental Science and Pollution Research (2023)
-
Interaction between ozone and paternal smoking on fetal congenital heart defects among pregnant women at high risk: a multicenter maternal–fetal medicine study
World Journal of Pediatrics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.