Abstract
ZAG has recently been characterized as a potent metabolic regulator, but the effect of anti-diabetic agents on ZAG in humans remains unknown. Our aim was to study the effects of SGLT2 inhibitor on circulating ZAG and ADI in nT2DM. 162 subjects with nT2DM were treated by a placebo or DAPA. After 3-months of DAPA therapy, HbA1c, FBG, 2h-PBG, FFA, TG, blood pressure, BMI, WHR, body weight, FAT%, FINS, and HOMA-IR in T2DM patients decreased significantly, whereas HDL-C was significantly increased. Importantly, circulating ZAG and ADI levels in these patients were also significantly increased after DAPA therapy. Basal ZAG levels were associated with changes in BMI, FAT%, TC, HbA1c, HDL-C and ADI at post-treatment, whereas basal ADI levels were associated with changes in FAT%, TC, HbA1c, FFA and HDL-c. In vitro, DAPA treatment showed increased ZAG expression and secretion in HepG2 cells. When combined with a PPAR-γinhibitor GW9662, the effect of DAPA on ZAG was abrogated. These findings suggest that circulating ZAG can be regulated by DAPA, and DAPA promotes the expression and secretion of ZAG in the liver via the activation of PPAR-γ. The changes in ZAG induced by DAPA may play a physiologic role in enhancing insulin sensitivity.
Similar content being viewed by others
Introduction
Zinc-alpha-2-glycoprotein (ZAG) is a 41-kDa glycoprotein assigned to the Major Histocompatibility complex (MHC) class I family of proteins1 and is a soluble protein first identified in human blood which represents 0.2% of total serum protein2. Initially ZAG was thought to originate solely from tumors, but later studies showed that it is also produced by white adipose tissue (WAT) and brown adipose tissue (BAT) as well as in liver, heart, and lungs3. The biological functions of ZAG are not completely known, but it has been shown that ZAG is a novel adipokine and that its expression is down-regulated in the adipose tissue of obese subjects3. Furthermore, it has also been reported that ZAG contributes to the control of body weight and induces lipolysis in adipocytes4. ZAG-deficient mice are susceptible to weight gain when fed a high fat diet which is associated with decreased lipolysis and unresponsiveness to β3- adrenoreceptor agonists4. Importantly, Balaz et al. reported that silencing ZAG resulted in reduced adiponectin (ADI), insulin receptor substrate-1(IRS-1) and glucosetransporters-4 (GLUT4) gene expression in primary human adipocytes indicating that ZAG plays an important role in modulating whole-body and adipose tissue insulin sensitivity5. Recently, works from our group have shown that circulating ZAG levels are lower in patients with nT2DM and correlated positively with ADI and inversely with body mass index (BMI), waist-to-hip ratio (WHR), and homeostasis model assessment of insulin resistance (HOMA-IR), further suggesting that ZAG may be an adipokine associated with insulin resistance (IR)6. However, since the biological actions of ZAG have not been fully characterized, it is important to study the effects of diabetic treatment, especially new anti-diabetic agents, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors, on the plasma levels of ZAG in T2DM patients. Therefore, the aim of this study is to evaluate the effects of Dapaglifozin (DAPA), a SGLT2 inhibitor, on ZAG in vivo and vitro.
Results
Plasma ZAG and ADI levels and other parameters in study subjects
The main clinical features, biochemical parameters, and circulating ZAG and ADI levels in the study population are displayed in Table 1. As expected, diabetic subjects had significantly higher BMI, WHR, percentage of body fat (FAT %), systolic blood pressure (SBP), total cholesterol (TC), triglyceride (TG), free fatty acid (FFA), HbA1c, HOMA-IR, fasting blood glucose (FBG), 2-h postprandial blood glucose (2-hPBG), fasting insulin (FINS), 2-h insulin after glucose overload (2-hINS) and HOMA-IR as compared with the controls (P < 0.05 or P < 0.01). However, both plasma ZAG and ADI levels in T2DM patients were significantly lower than in the controls (P < 0.01). These differences remained significant after adjustment for age, BMI, and sex.
 ± s).
± s).The effects of DAPA on clinical characteristics and ZAG levels in T2DM patients
A total of 180 patients were randomly assigned to receive DAPA or a placebo; 162 completed week 12 and 18 discontinued. The most common reason for discontinuation was withdrawal of consent, adverse event, or three consecutive FBG values > 13 mmol/L. As shown in Table 2, baseline demographics and disease characteristics were similar in the two groups. After three months of treatment with DAPA, HbA1c, FBG, 2hPBG, FFA, TG, blood pressure, BMI, WHR, body weight, FAT%, FINS, and HOMA-IR significantly declined in T2DM patients (P < 0.05 or P < 0.01), whereas high-density lipoprotein cholesterol (HDL-C) significantly increased (P < 0.05). There was no noticeable effect of DAPA on Alanine transaminase (ALT), aspartate transaminase (AST), TG and low-density lipoprotein cholesterol (LDL-C) in this 12-week study (Table 2). More patients achieved >5% body weight reductions with DAPA (n = 11) than with placebo (n = 0). Interestingly, circulating ZAG and ADI levels were significantly increased after DAPA treatment (ZAG: from 35.6 ± 9.1 to 45.0 ± 10.6 mg/L; ADI: 33.3 ± 8.8 to 37.9 ± 8.1 μg/L, both P < 0.01, Fig. 1A,B), whereas circulating ZAG and ADI were unchanged by placebo treatment (ZAG: from 33.4 ± 10.9 to 33.5 ± 10.7 mg/L; ADI: from 32.6 ± 8.3 to 31.8 ± 6.3 μg/L, Fig. 1A,B). In addition, DAPA treatment also led to a significant decrease in circulating tumor necrosis factor-α (TNF-α) levels when compared with pretreatment (9.15 ± 0.33 vs. 13.5 ± 0.55 μg/L, P < 0.01, Fig. 1C), whereas TNF-α levels were unchanged by placebo treatment (11.7 ± 0.32 vs. 12.0 ± 0.63 μg/L, Fig. 1C). Finally, we assessed the association between baseline ZAG and ADI levels and the changes of several parameters related to IR at pre- and post- treatment (∆). Pearson’s correlation analysis showed that baseline ZAG level was associated with ∆BMI (r = 0.2, P < 0.05), ∆FAT% (r = 0.274, P < 0.01), ∆TC (r = 0.228, P < 0.01), ∆HbA1c (r = 0.29, P < 0.05), ∆HDL-C(r = −0.903, P < 0.01) and ∆ADI (r = −0.325, P < 0.01) (Fig. 2A–F), whereas baseline ADI associated with ∆FAT (r = 0.196, P < 0.05), ∆TC (r = 0.442, P < 0.01), ∆HbA1c (r = 0.328, P < 0.01), ∆FFA (r = −0.273, P < 0.01) and ∆HDL-C (r = −0.958, P < 0.01) (Fig. 3A–E). Multiple regression analysis showed that the ∆TC and ∆HDL-c were independent related factors influencing baseline ZAG levels, whereas ∆HDL-C was an independently related factor influencing baseline ADI levels. The multiple regression equations were: YZAG = 9.259 + 4.235 ∆TC-5.480 ∆FFA and YADI = 4.308 + 2.624 ∆HDL-C-4.509 ∆LDL-C.
 ± s).
± s).Effects of DAPA on circulating ZAG, ADI and TNFα levels in T2DM and placebo group before and after treatment.
(A) Circulating ZAG levels. (B) Circulating ADI levels. (C) Circulating TNF-α levels. DAPA, dapaglifozin; ZAG, zinc-α2-glycoprotein; ADI, adiponectin; TNF-α, tumor necrosis factor-α. Values are means ± SD. n = 117 for SGLT2 group; n = 45 for placebo group; **P < 0.01 vs. pre- treatment.
Adverse events were reported at similar frequencies between the two groups. No deaths or drug-related serious adverse events occurred. Hypoglycemic events were reported in 8% of DAPA-treated patients, and in 4% of placebo-treated patients. Infections of the urinary tract were seen in 7% of DAPA-treated patients versus 5% of placebo-treated patients. Genital infections were seen in 3% of DAPA-treated patients versus 0% of placebo-treated patients. The diuretic effect of DAPA was assessed by 24-h urine volume. There were no significant changes in 24-h urine volume and serum creatinine (SCr) at post-treatment. There were also no significant changes in albuminuria after the DAPA treatment (data no shown).
DAPA regulated ZAG and ADI expression and secretion in HepG2 or 3T3-L1cells
In HepG2 cells, DAPA treatment led to the gradual increase in ZAG and ADI mRNA levels, beginning at 0.8 μM and reaching a peak of 4 μM concentration (Fig. 4A,D). Consistent with the results of mRNA, ZAG and ADI protein levels also increased dose-dependently from 0.8 to 4 μM concentration of DAPA in these cells (Fig. 4B,E). In addition, ZAG and ADI secretions in the culture medium were increased dose-dependently by DAPA treatment (from 0.8 to 4 μM) (Fig. 4C,F). Furthermore, DAPA also led to an increase in ADI expression in 3T3-L1cells (Fig. 4G,H). These results suggest that DAPA is an inducer to ZAG and ADI systemic levels.
DAPA regulated of ZAG and ADI expression and secretion in 3T3-L1 adipocytes and HepG2 cells.
HepG2 cells were treated with 0.1% DMSO or DAPA at various concentrations (0.8, 4, 20, 30 μM) for 24 hr. Quantitative RT-PCR and Western blot were performed for the measurement of mRNA and protein. (A,B) ZAG mRNA (A) and protein (B) expression in HepG2 cell lysate. (C) ZAG protein levels in HepG2 culture medium. (D,E) ADI mRNA (D) and protein (E) expression in HepG2 cell lysate. (F) ADI protein levels in HepG2 culture medium. (F,G) ADI mRNA (G) and protein (F) expression in 3T3-L1 adipocytes lysate. DAPA, dapaglifozin; ZAG, zinc-α2-glycoprotein; ADI, adiponectin. The results represent three separate experiments performed in duplicates as means ± SEM. *P < 0.05, **P < 0.01 vs. controls.
Effects of DAPA on gene expression related fat metabolism and lipid accumulations in HepG2 cells
To further investigate whether DAPA regulates fat metabolism, mRNA expression levels of Acetyl-CoA Carboxylase (ACC) and fatty acid synthase (FAS), two lipogenesis-related genes, and hormone sensitive lipase (HSL), a lipolysis-related gene, were examined in vitro. DAPA treatment significantly increased ZAG mRNA expression in palmitic acid (PA)-induced IR HepG2 cells (P < 0.01, Fig. 5A). In PA-treated HepG2 cells, DAPA treatment also significantly attenuated the mRNA expression of FAS (by~ 28%, P < 0.01, Fig. 5B) and ACC (by ~20%, P < 0.01, Fig. 5C). In contrast, DADP treatment increased HSL mRNA expression (by ~20%, Fig. 5D) and phosphorylation (Fig. 5E). Figure 5F showed a representative Oil-Red-O staining exhibiting significant decreased lipid droplet accumulation and Fig. 5G showed a significant decrease in total cell triglyceride content in PA-treated HepG2 cells. These results suggest that DAPA inhibits hepatic lipogenesis and promotes lipolysis.
Effects of DAPA on gene expression related fat metabolism and lipid accumulations in HepG2 cells.
Cells were starved in serum-free medium containing 0.5% bovine serum albumin (BSA) for 12 h, and then treated with 300 μM PA with or without 4 μM DAPA/100 μM metformin, a positive control, for 24 h. Cell lysates were collected for the mRNA, protein or TG content assay. (A) ZAG mRNA expression. (B) FAS mRNA expression. (C) ACC mRNA expression. (D) HSL mRNA expression. (E) HSL phosphorylation. (F) Oil Red O staining of HepG2 cells from different treatments. (G) TG content. 1, control; 2, PA treatment; 3, PA + DAPA treatment; 4, PA + MET treatment. DAPA, dapaglifozin; PA, palmitic acid; ZAG, zinc-α2- glycoprotein; MET, metformin; ACC, Acetyl-CoA Carboxylase; FAS, fatty acid synthase; HSL, hormone sensitive lipase. The results represent three separate experiments performed in duplicates as means ± SEM. *P < 0.05, **P < 0.01.
The effect of DAPA on inflammatory cytokine expression in HepG2 cells and 3T3-L1 adipocytes
To further explore whether SGLT2 inhibitor has anti-inflammatory properties, we examined the effects of DAPA treatment on inflammatory cytokine expression in HepG2 cells and mature 3T3-L1 adipocytes. As shown in Fig. 6A,B, DAPA treatment significantly attenuated the mRNA expression of interleukin-6 (IL-6) (by ~44% in HepG2 cells and by ~63% in 3T3-L1 adipocytes) and C-reactive protein (CRP) (by ~48% in HepG2 cells and by ~39% in 3T3-L1 adipocytes), and TNF-α (by ~35% in HepG2 cells and by ~63% in 3T3-L1 adipocytes), suggesting that DAPA may have an anti-inflammatory role.
The effects of DAPA on inflammatory cytokine and PPAR-γ expression in the vitro.
(A,B) Cells were treated with 0.013% DMSO and 4 μM DAPA for 24 h. mRNA expression was determined by Quantitative RT-PCR. DAPA inhibited IL-6, CRP and TNF-α mRNA expression in HepG2 cells (A) and differentiated 3T3-L1 adipocytes (B). (C) HepG2 cells were treated with 0.013% DMSO and DAPA at various concentrations for 24 h. Cell lysates were collected for the PPARγ mRNA (C) assay. (D–F) HepG2 cells were treated with 4 μM DAPA with or without GW9662 for 24 h. Cell lysates were collected for PPARγ mRNA (D) PPARγ protein (E) and ZAG (F) mRNA assay. IL-6, interleukin-6; CRP, C-reactive protein; TNF-α, tumor necrosis factor-α; DAPA, dapaglifozin; ZAG, zinc-α2-glycoprotein; PPARγ, peroxisome proliferator-activated receptor-γ. The results represent three separate experiments performed in duplicates as means ± SEM. **P < 0.01.
DAPA stimulates ZAG production through activation of PPARγ
To explain why ZAG levels were increased by DAPA treatment, we examined whether the effect of DAPA on ZAG is mediated by peroxisome proliferator-activated receptor-γ (PPARγ) in vitro. As shown in Fig. 6C–E, DAPA treatment led to a significant increase in PPARγmRNA and protein expression in HepG2 cells, suggesting that DAPA induces the activation of PPARγ signaling pathways. Importantly, when the HepG2 cells were treated with DAPA, expression of ZAG was up-regulated (Fig. 6F), accompanied by an increase in PPARγ expression (Fig. 6C), but when combined with the PPARγinhibitor GW9662, the stimulative effect of DAPA on ZAG expression was significantly abrogated, followed by a decrease in PPARγ expression (Fig. 6D,E). However, PPARα expression in HepG2 cells was unchanged by DAPA treatment (Supplemental Figure 1A,B), and SREBP-1c expression was decreased in DAPA treated HepG2 (Supplemental Figure 1C).
Discussion
SGLT2 inhibitors are a new class of anti-diabetes treatment, with a novel and insulin- independent mechanism7. SGLT2 is a sodium-solute cotransport protein located in the kidney proximal tubule that reabsorbs the majority of glomerular-filtered glucose8,9,10,11. Therefore, inhibition of SGLT2 presents two novel mechanisms that reduce hyperglycemia independent of insulin secretion or action12 and which promotes mild osmotic diuresis leading to weight loss13. In the present study, we studied the effects of 3-month DAPA treatment on glucose, blood fat levels, blood pressure, body weight and HOMA-IR in nT2DM patients. We found that HbA1c and body weight significantly decreased by 0.53% and by 2.1 kg, respectively. These results are consistent with previous findings14,15,16. In addition, we also observed significant decreases in blood pressure, TG, FFA, FBG, 2h-PBG and FINS, and a significant increase of HDL-C in these patients post-treatment. Consistent with published data in rats17, DAPA treatment decreased plasma ALT levels by approximately 10% and AST levels by approximately 7%, suggesting a beneficial effect in the liver. We suspect that weight reduction during therapy represents both fluid loss and decreased fat mass. As expected, from the in vitro study, we found that DAPA treatment inhibited hepatic lipogenesis and promoted lipidolysis, and also decreased lipid droplet accumulation in PA-treated HepG2 cells. Similarly, veterinary literature suggests that chronic administration of phlorizin, a nonspecific renal glucose reabsorption inhibitor, in lactating cows induces lipolysis18, and DAPA reduced adiposity in obese rats19. In addition, the weight loss in T2DM patients after the DAPA treatment could have been caused by the increase in ZAG plasma levels which is a well known lipolytic agent and a lipid mobilizing factor3,20. This could also explain, at least in part, the loss of fat mass in these patients. Importantly, along with an improvement in glucose and lipid metabolism, we observed a significant decrease in HOMA-IR after treatment with DAPA, suggesting that SGLT2 inhibitor treatment may improve IR in T2DM patients. This important finding has not been reported by previous studies21.
To further investigate the correlation between SGLT2 inhibitors and IR, we measured the levels of circulating ADI, a known insulin sensitizer and cardio-protective adipokine22,23,24, and TNFα, an inflammatory marker at pre- and post-treatment in our patients with T2DM. Consistent with published data in mice25, we found that circulating ADI levels in DAPA–treated patients were significantly increased compared with those treated with a placebo, whereas circulating TNF-α levels were significantly decreased in DAPA-treated patients. Accordingly, from the in vitro study, we found that treatment of 3T3-L1 adipocytes and HepG2 cells with DAPA increased the expression and secretion of ADI. Additionally, DAPA treatment also downregulated the expression of inflammatory cytokine, including IL-6, CRP and TNF-α. Therefore, it is hypothesized that increasing ADI and decreasing TNFα levels following DAPA treatment may, at least in part, account for the improvement in a chronic low-grade inflammatory state and insulin sensitivity observed in db/db mice25 and our T2DM patients. These results support the notion that SGLT2 inhibitor treatment can improve IR through weight loss or other conceivable mechanisms, such as increasing insulin sensitizer levels and/or decreasing inflammatory cytokine levels.
Although ZAG was initially identified as a protein secreted by the liver, breast, lung and prostate26, a growing body of evidence suggests that ZAG is also an adipokine, like ADI. It is also expressed and secreted by human adipocytes27 and mouse and human adipose tissues3. Recently, ZAG has been reported to be related to ADI, insulin receptor substrate- 1(IRS-1) and glucose transporters-4 (GLUT4) gene expression and plays an important role in modulating insulin sensitivity5,28. In addition, Mracek, et al. found that ZAG mRNA positively correlates with ADI in adipose tissue in humans, and recombinant ZAG stimulates ADI release from human adipocytes29. Therefore, to extend the relationship between the SGLT2 inhibitor and IR, we further examined the effect of 12 wks of DAPA treatment on circulating ZAG levels in T2DM subjects. Before the start of DAPA treatment, the plasma ZAG levels in T2DM subjects were lower than those in healthy subjects. After DAPA treatment, the circulating ZAG increased in diabetic subjects by 26%. The increase in plasma ZAG levels was accompanied by an increase of ADI levels and a decrease in TNF-α levels and HOMA-IR. As with circulating ZAG and ADI levels, the expression and secretion of ZAG and ADI gene in HepG2 or 3T3-L1 cells were significantly increased by DAPA treatment. These data suggest that some aspect of DAPA positively regulates ZAG and ADI expression and their release into the circulation. These results further support that whole-body insulin sensitivity is increased after SGLT2 inhibitor treatment and the increase in ZAG and ADI may contribute to insulin sensitization.
Experimental studies evaluating the effect of ZAG on lipid metabolism have been previously reported. ZAG was shown to act as a lipid-mobilizing factor23. Treatment with ZAG stimulated lipolysis in isolated mouse and human adipocytes, and it selectively reduces body fat in both normal and ob/ob mice30. ZAG-treated animals also showed increased expression of HSL and adipose triglyceride lipase (ATGL) in white adipose tissue31. Recently, our data has shown that circulating ZAG correlates positively with HDL-C and negatively with BMI, WHR, FAT% and TG in T2DM patients6. In our vitro study, we found that DAPA treatment in PA-induced IR HepG2 cells decreased intracellular TG content and lipid droplet, increased HSL phosphorylation and decreased FAS and ACC gene expression. These changes were accompanied by the increase in ZAG expression and secretion. Therefore, the effects of DAPA on lipid metabolism could be due to the increased ZAG expression and secretion.
PPAR-γ ligands have been implicated in the expression of several adipokines, repressing those linked to IR, such as resistin and ADI32,33. Bao, et al. found that treatment with rosiglitazone, a selective PPAR-γ agonist, induced a 3-fold increase in ZAG mRNA level in SGBS cells, paralleling its effect on ADI mRNA, thus indicating that PPAR-γ is involved in the regulation of ZAG synthesis27. To gain new insights into how the molecular mechanisms responsible for the increased expression and secretion of ZAG by DAPA, we used GW9662, an irreversible PPAR-γ antagonist, to investigate the role played by PPAR-γ in the ZAG mRNA changes induced by DAPA. We found that the treatment of HepG2 cells with DAPA increased the mRNA expression of ZAG and PPAR-γ, whereas when combined with GW9662, the positive effect of DAPA on ZAG and PPAR-γ was abrogated. These results indicate that the effect of DAPA on ZAG is dependent on classic PPAR-γ pathway activation. We therefore propose that the DAPA-mediated increase in ZAG could be caused by the activation of PPAR-γ in insulin target tissues, and this may counteract the decreased activation of PPAR-γ that occurs in response to increasing IR in T2DM patients.
It appears to be a conflicting result that DAPA treatment increases PPAR-γ but not FAS or ACC. In fact, we found that DAPA treatment did not lead to an increase in SREBP-1c, a transcription factor located downstream of PPAR-γ. Therefore, DAPA treatment did not increase the mRNA expression of FAS or ACC. Based on, Bing, et al.’s report, we postulate that the increasing in ZAG by DAPA treatment can induce the activation of β3 adrenoreceptors that, in turn, decreases FAS or ACC expression (Fig. 7)34.
In the current study, daily DAPA therapy was well tolerated with no major adverse events. The low incidence of hypoglycemia supports the potential for SGLT2 inhibitors to achieve meaningful glycemic efficacy with relatively low hypoglycemic risk. The number of reported urinary tract infections was similar between SGLT2 inhibitor and placebo group and is consistent with the rates reported in T2DM patients12,14.
Collectively, our results demonstrate that 12 wks of DAPA treatment decreases HbA1c, FBG, 2hPBG, FFA, TG, WHR, body weight, FAT%, and plasma FFA levels, reduces the HOMA-IR and circulating TNFα levels, and increases circulating ADI and ZAG concentration in T2DM patients. The DAPA-mediated increase in circulating ZAG could be caused by the increase in ZAG expression and secretion in insulin target tissues, whereas PPAR-γ may be a key regulator of the effect of DAPD on ZAG. Our current data along with previous evidence indicate that SGLT2 inhibitor therapy in T2DM patients improves blood glucose control with a low risk of hypoglycemia. Importantly, our findings provide novel insights into the relationship between IR and SGLT2 inhibitor in T2DM in the contexts of ZAG and ADI biology.
Research design and methods
Ethics Statement
This study was carried out in accordance with the recommendations of the Declaration of Helsinki and was approved by the Human Research Ethics Committee of Chongqing Medical University. The study was also registered at chictr.org (Registration number: CHICTR- OCC-11001422, Date of Registration: 23-June-2011). An informed consent was obtained from all participants in this study.
Subjects
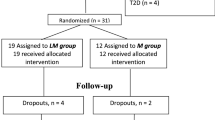
This study was a two-center, prospective, double-blinded, randomized, placebo-controlled study that took place at the Second Affiliated Hospital, Chongqing Medical University Chongqing, and the affiliated Hospital, Zunyi Medical University, Guizhou, China. This trial included 180 patients with T2DM and 100 normal subjects and was conducted from February 2012 to December 2013. The diagnosis of T2DM was based on World Health Organization 1998 diagnostic criteria35. Subjects with T2DM were newly diagnosed and had not been treated with oral hypoglycemic agents or diet control. Inclusion criteria were age 40–75 yr, with BMI of 20–40 kg/m2 and HbA1c levels between 6.5 and 9.0%. Exclusions included patients with type 1 diabetes or ketoacidosis, malignant disease in the previous 10 yr, liver cirrhosis, hypertension, hepatic and renal failure, or other known major diseases. 180 patients with T2DM were randomized (DAPA: placebo, 2:1) by using a computer-generated random- number sequence (simple randomization). Before breakfast, patients were administered with DAPA (AstraZeneca, n = 120) 10 mg once daily, or placebo (n = 60) once daily for 3 months. 162 patients (DAPA: n = 117; placebo: n = 45) completed week 12, and 18 discontinued. Treatment allocation was blinded to patients and study personnel until the database was unlocked for analysis. All patients were in good general health without evidence of cardiac, hepatic, renal, or other chronic diseases as determined by history, examination, and screening blood tests. Participants were requested to adhere to pre-study lifestyle and dietary habits throughout the course of the study. The dietary information was recorded from these subjects during the study. To maintain the compliance of patients, subjects were contacted by phone weekly to collect information on blood glucose and adverse effects. Study drug adherence was assessed at each study visit by pill count and calculated as percentage of pills taken. The adherence rates were 90%. 100 age-matched healthy subjects without clinical evidence of major disease were recruited from an unselected population that underwent routine medical check-ups and were used as controls. These subjects had FBG levels <6.1 mmol/L and a 2-h oral glucose tolerance tests (OGTT) glucose levels <7.8 mmol/L, had no family history of T2DM, and were not using medications. To prevent acute complication, subjects with three consecutive FBG values >13 mmol/L were withdrawn from the study. This study was carried out in accordance with the recommendations of the Declaration of Helsinki and was approved by the Human Research Ethics Committee of Chongqing Medical University. The study was also registered at chictr.org (CHICTR-OCC-11001422). An informed consent was obtained from all participants in this study.
Anthropometry and blood samples
Anthropometric measurement was performed in the morning, before breakfast, with the subjects wearing light clothing, without shoes. Body weight and height were measured in all subjects using a scale and a wall mounted stadiometer to the nearest 0.5 kg and 0.5 cm respectively. Waist and hip circumferences were measured, and the WHR was calculated. BMI was calculated as weight divided by height squared. The FAT % was measured by bioelectrical impedance (BIA-101; RJL Systems, Shenzhen, China). The HOMA-IR = fasting insulin (FIns, microunits per milliliter) ×FBG (millimoles per liter)/22.536. Plasma glucose and HbAlc were measured immediately by the glucose oxidase method and anion exchange high-performance liquid chromatography, respectively. After 10 h overnight fasting, blood samples were collected, serum and plasma were isolated (EDTA) and stored at −80 °C for the measurements of ZAG, insulin, ADI, FFA, and blood fat levels.
Measurements of biochemical parameters and adipokines
Circulating ZAG levels were determined with an ELISA obtained from RayBiotech, Inc. (Wuhan, China) following the manufacturer’s protocol. The limit of detection was 21pg/mL, and intra- assay and inter-assay variations were <10% and <15, respectively. Circulating ADI level was also measured by ELISA as we previously described37. The limit of detection was 1.102 ng/mL, and intra-assay and inter-assay variations were <5% and 10%, respectively. Insulin was measured by RIA using human insulin as standard (Institute of Atomic Energy, China) according to previous report37. FFAs were measured with a commercial kit (Randox Laboratories Ltd., Antrim, UK). Circulating TNFα levels were measured by ELISA (4A Biotech Co. Ltd, Beijing, China). ALT and AST levels in serum were measured by an auto-analyzer (Hitachi High-Technologies Corp., Tokyo, Japan). TC, HDL-C, LDL-C, and TG were determined enzymatically using an autoanalyzer (Hitachi 747; Hitachi, Tokyo, Japan).
Cell culture, treatment and oil red O staining
Mice 3T3-L1 preadipocytes and human hepatoma cells (HepG2) were purchased from American Type Culture Collection (ATCC; Manassas, VA). 3T3-L1 preadipocytes were cultured and induced to be differentiated to mature adipocytes as previously described38. HepG2 cells were cultured in DMEM medium supplemented with 10% fetal serum and 1% antibiotics. The glucose concentration of the DMEM medium was 25 mmol/L. For study on the regulation of ZAG ADI expression and secretion, HepG2 or 3T3-L1 cells were treated with 0.1% DMSO or DAPA at various concentrations (0.8, 4, 20, 30 μM) for 24 hr. The supernatants and cell lysates were collected and stored at −80 °C until analysis. For studying the effects of DAPA on lipid metabolism in vitro, HepG2 cells were starved in serum-free medium containing 0.5% bovine serum albumin (BSA) for 12 h, and then treated with 300 μM palmitic acid (PA) with or without 4 μM DAPA/100 μM metformin, a positive control, for 24 h. Cell lysates were collected for ZAG and gene related fat metabolism mRNA assay. For study on the regulation of inflammatory cytokine induced by DAPA in vitro, HepG2 cells and differentiated 3T3-L1 adipocytes were treated with 0.013% DMSO and 4 μM DAPA for 24 h. For study of PPARγ signaling in vitro, HepG2 cells were treated with 2 μM DAPA or 0.013% DMSO for 2 h, then incubated with or without GW9662 (Sigma), a PPARγ inhibitor, for 24 h. Cell lysates were collected for expression assay. For intracellular TG content measurement, HepG2 cells were homogenized and supernatants were collected. TG contents were by a spectrophotometric procedure and HepG2 cells were stained with Oil-Red-O as previously described39.
Quantitative real-time PCR
Total RNA was extracted with Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Purified RNA was used as the template for first-strand cDNA synthesis with the PrimerScript TM RT reagent Kit (Takara Bio, Otsu, Japan). Quantitative realtime PCR was performed with a SYBR Premix Ex Taq II kit (Takara Bio), and a Corbett Rotor Gene 6000 real-time PCR system (Corbett Research, Sydney, Australia), according to the manufacturer’s instructions. Gene expression levels were analyzed with the comparative Ct method, and normalized with β-actin. Forward and reverse primer pairs are as listed in Supplemental Table 1.
Western Blot Analysis
The cell lysates and medium were collected 24 h after treatment. Protein concentration was measured with a BCA protein quantification kit (Pierce Biotechnology, Rockford, IL, USA). Proteins (50 μg per lane) were separated by SDS/PAGE, and then transferred to polyvinylidene fluoride (PVDF) membranes. Immunoblots were blocked in NaCl/Tris/Tween-20 and 5% skimmed milk for 2 h, at room temperature and incubated with primary antibodies, including: anti-PPAR-γ and anti-phospho-HSL (1: 1000 dilution; Cell Signaling Technology), anti-ADI (1: 1000 dilution; Abcam), anti-ZAG, anti-HSL and anti-β-actin (1: 500 dilution; Santa Cruz Biotechnology), overnight at 4 °C. Following three consecutive 5-min washes in NaCl/Tris/Tween-20, blots were incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. After two washes in NaCl/Tris/Tween-20 and a final wash in NaCl/Tris, the blots were scanned with the Odyssey Infrared Imaging System (LICOR Biosciences, Lincoln, NE, USA), and quantification of antigen–antibody complexes was performed with QUANTITY ONE (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Statistical analysis was carried out using SPSS 8.0 software (SPSS Inc., Chicago, IL). Data are expressed as mean ± SD unless stated otherwise. Variables with a non-normal distribution were transformed logarithmically before analysis. Comparisons between groups were performed by unpaired t test. Differences between values before and after treatment (i.e. within the placebo and within the DAPA groups) were analyzed using the paired Student’s t test at time 0 and 12 wk. All t-tests were two tailed and P < 0.05 was considered statistically significant. All statistical analyses were performed by a single operator who was blinded to treatment group. Simple and multiple regression analyses were used to examine the association between baseline ZAG and/or DIA levels and the changes of other biomarkers at pre- and post- treatment. The chosen covariates for adjustment included age, BMI and sex. In the vitro study, the data were analyzed by 1-way ANOVA and a post-hoc Tukey’s analysis or by a t-test as appropriate.
Additional Information
How to cite this article: Liao, X. et al. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitor Increases Circulating Zinc-A2-Glycoprotein Levels in Patients with Type 2 Diabetes. Sci. Rep. 6, 32887; doi: 10.1038/srep32887 (2016).
References
Tada, T. et al. Immunohistochemical localization of Zn-alpha 2-glycoprotein in normal human tissues. J Histochem Cytochem 39, 1221–1226 (1991).
Burgi, W. & Schmid, K. Preparation and properties of Zn-alpha 2-glycoprotein of normal human plasma. J Biol Chem 236, 1066–1074 (1961).
Bing, C. et al. Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up-regulated in mice with cancer cachexia. Proc Natl Acad Sci USA 101, 2500–2505 (2004).
Rolli, V. et al. Lipolysis is altered in MHC class I zinc-alpha(2)-glycoprotein deficient mice. FEBS Lett 581, 394–400 (2007).
Balaz, M. et al. Subcutaneous adipose tissue zinc-alpha2-glycoprotein is associated with adipose tissue and whole-body insulin sensitivity. Obesity (Silver Spring) 22, 1821–1829 (2014).
Yang, M. et al. Zinc-alpha2-glycoprotein is associated with insulin resistance in humans and is regulated by hyperglycemia, hyperinsulinemia, or liraglutide administration: cross-sectional and interventional studies in normal subjects, insulin-resistant subjects, and subjects with newly diagnosed diabetes. Diabetes Care 36, 1074–1082 (2013).
List, J. F. & Whaley, J. M. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int Suppl, S20–S27 (2011).
Santer, R. et al. Molecular analysis of the SGLT2 gene in patients with renal glucosuria. J Am Soc Nephrol 14, 2873–2882 (2003).
Wright, E. M., Hirayama, B. A. & Loo, D. F. Active sugar transport in health and disease. J Intern Med 261, 32–43 (2007).
Kanai, Y., Lee, W. S., You, G., Brown, D. & Hediger, M. A. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest 93, 397–404 (1994).
Hediger, M. A. & Rhoads, D. B. Molecular physiology of sodium-glucose cotransporters. Physiol Rev 74, 993–1026 (1994).
Idris I Fau-Donnelly, R. & Donnelly, R. Sodium-glucose co-transporter-2 inhibitors: an emerging new class of oral antidiabetic drug. Diabetes Obes Metab 11, 79–88 (2009).
Komoroski, B. et al. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther 85, 513–519 (2009).
List, J. F., Woo, V., Morales, E., Tang, W. & Fiedorek, F. T. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 32, 650–657 (2009).
Henry, R. R. et al. Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract 66, 446–456 (2012).
Fonseca, V. A. et al. Active- and placebo-controlled dose-finding study to assess the efficacy, safety, and tolerability of multiple doses of ipragliflozin in patients with type 2 diabetes mellitus. J Diabetes Complications 27, 268–273 (2013).
Hayashizaki-Someya, Y. et al. Ipragliflozin, an SGLT2 inhibitor, exhibits a prophylactic effect on hepatic steatosis and fibrosis induced by choline-deficient l-amino acid-defined diet in rats. Eur J Pharmacol 754, 19–24 (2015).
Bradford, B. J. & Allen, M. S. Phlorizin induces lipolysis and alters meal patterns in both early- and late-lactation dairy cows. J Dairy Sci 90, 1810–1815 (2007).
Devenney, J. et al. The effect of dapagliflozin, a highly selective SGLT2 inhibitor, on body weight in diet-induced obese rats. Presented at The Obesity Society Annual Scientific Meeting 2007; Abstract 0384, New Orleans, LA (2007).
Zhu, H. J. et al. Inhibition of preadipocyte differentiation and adipogenesis by zinc- alpha2-glycoprotein treatment in 3T3-L1 cells. J Diabetes Investig 4, 252–260 (2013).
Goring, S. et al. Dapagliflozin compared with other oral anti-diabetes treatments when added to metformin monotherapy: a systematic review and network meta-analysis. Diabetes Obes Metab 16, 433–442 (2014).
Weyer, C. et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86, 1930–1935 (2001).
Tschritter, O. et al. Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes 52, 239–243 (2003).
Matsubara, M., Maruoka, S. & Katayose, S. Decreased plasma adiponectin concentrations in women with dyslipidemia. J Clin Endocrinol Metab 87, 2764–2769 (2002).
Chen, L., Klein, T. & Leung, P. S. Effects of combining linagliptin treatment with BI- 38335, a novel SGLT2 inhibitor, on pancreatic islet function and inflammation in db/db mice. Curr Mol Med 12, 995–1004 (2012).
Garrido-Sanchez, L. et al. Zinc-alpha 2-glycoprotein gene expression in adipose tissue is related with insulin resistance and lipolytic genes in morbidly obese patients. PLoS One 7, e33264 (2012).
Bao, Y. et al. Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed and secreted by human (SGBS) adipocytes. FEBS Lett 579, 41–47 (2005).
Russell, S. T. & Tisdale, M. J. Antidiabetic properties of zinc-alpha2-glycoprotein in ob/ob mice. Endocrinology 151, 948–957 (2010).
Mracek, T. et al. The adipokine zinc-alpha2-glycoprotein (ZAG) is downregulated with fat mass expansion in obesity. Clin Endocrinol (Oxf) 72, 334–341 (2010).
Wargent, E. T. et al. Contrasts between the effects of zinc-alpha2-glycoprotein, a putative beta 3/2-adrenoceptor agonist and the beta3/2-adrenoceptor agonist BRL35135 in C57Bl/6 (ob/ob) mice. J Endocrinol 216, 157–168 (2013).
Russell, S. T. & Tisdale, M. J. Studies on the antiobesity effect of zinc-alpha2- glycoprotein in the ob/ob mouse. Int J Obes (Lond) 35, 345–354 (2011).
Shojima, N. et al. Humoral regulation of resistin expression in 3T3-L1 and mouse adipose cells. Diabetes 51, 1737–1744 (2002).
Maeda, N. et al. PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 50, 2094–2099 (2001).
Bing, C. et al. Zinc-α2-glycoprotein: an adipokine modulator of body fat mass? Int J Obes (Lond) 34, 1559–1565 (2010).
Alberti, K. G. & Zimmet, P. Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15, 539–553 (1998).
Albareda, M., Rodriguez-Espinosa, J., Murugo, M., de Leiva, A. & Corcoy, R. Assessment of insulin sensitivity and beta-cell function from measurements in the fasting state and during an oral glucose tolerance test. Diabetologia 43, 1507–1511 (2000).
Hu, W. et al. Circulating Sfrp5 is a signature of obesity-related metabolic disorders and is regulated by glucose and liraglutide in humans. J Clin Endocrinol Metab 98, 290–298 (2013).
Li, K. et al. The effects of fibroblast growth factor-21 knockdown and over- expression on its signaling pathway and glucose-lipid metabolism in vitro. Mol Cell Endocrinol 348, 21–26 (2012).
Li, K. et al. Effect of short hairpin RNA-mediated adiponectin/Acrp30 down- regulation on insulin signaling and glucose uptake in the 3T3-L1 adipocytes. J Endocrinol Invest 33, 96–102 (2010).
Acknowledgements
This work was supported by research grants from the Natural Science Foundation Key Project of CQ cstc (cstc2012 jjB10022) to G. Y. and L. G.
Author information
Authors and Affiliations
Contributions
X.L., H.L., X.W., G.Z., M.Y., L.Y. and L.L. performed the experiments and/or collected researched data. H.L. reviewed and edited the manuscript. G.Y. and L.G. contributed to the study conception, design, data analysis and discussion, and reviewed and edited the manuscript. L.G. and G.Y. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Liao, X., Wang, X., Li, H. et al. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitor Increases Circulating Zinc-Α2-Glycoprotein Levels in Patients with Type 2 Diabetes. Sci Rep 6, 32887 (2016). https://doi.org/10.1038/srep32887
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep32887
This article is cited by
-
Effect of sodium–glucose cotransporter 2 inhibitors on insulin resistance; a systematic review and meta-analysis
Acta Diabetologica (2022)
-
Development of a prediction model for mortality and cardiovascular outcomes in older adults taking into account AZGP1
Scientific Reports (2021)
-
A randomized controlled trial of two diets enriched with protein or fat in patients with type 2 diabetes treated with dapagliflozin
Scientific Reports (2021)
-
Efficacy and Safety of Luseogliflozin in Patients with Type 2 Diabetes Complicated by Hepatic Dysfunction: A Single-Site, Single-Arm, Open-Label, Exploratory Trial
Diabetes Therapy (2021)
-
Zinc-alpha2-glycoprotein, dysglycaemia and insulin resistance: a systematic review and meta-analysis
Reviews in Endocrine and Metabolic Disorders (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.