Abstract
The extreme salinity and high internal resistance of saline-alkali soil contaminated by petroleum hydrocarbons were two key limitations for using the bioelectrochemical remediation. In order to solve two problems, we simply rinsed soil, added carbon fiber to polluted soil. The charge output was enhanced by 110% with increase of the maximum current densities from 81 to 304 mA·m−2 while hydrocarbons degradation rate enhanced by 484%, especially the high molecular weight fractions (C28–C36 of n-alkanes and 4–6 rings of PAHs). These effects were possibly due to the selective enrichment of species belonged to δ-Proteobacteria (Proteobacteria), Flavobacteriia (Bacteroidetes) or Clostridia (Firmicutes), the activities of biological electron transfer and enzymes. As we know, oxygenase gene that directly decided the process of degradation, was surveyed for the first time in soil bioelectrochemical remediation system. The results confirmed that the bio-current stimulated the activities of naphthalene dioxygenase and xylene monooxygenase and thus the hydrocarbons degradation and the electricity generation. Given that electricity generation and the remediation performance are governed by multiple factors, understanding of microbial community and enzyme gene is crucial to promote the power yield and the bioelectrochemical remediation applicability.
Similar content being viewed by others
Introduction
Remediation of soils contaminated by petroleum hydrocarbons has been an arduous task, which attracts increasing attention since certain highly toxic and recalcitrant compounds are involved1,2. Physical and chemical remediation inevitably destroyed the original habitat and caused secondary pollution3,4. Though regular microbial technology and phytoremediation exert little effect on the pristine environment5,6, they are seriously restricted by ambient conditions (especially remote/extreme environment). Bioelectrochemical remediation, as an emerging technology, applies inexhaustible electrodes as the terminal electron acceptors to enhance biodegradation with the advantage of energy recovery7,8,9,10,11,12,13. Cost of remediation is greatly decreased as well as the scope is extended10,14,15. The soil microbial fuel cell (MFC) is one of commonly electrochemical biostimulation approaches and hardly exerts adverse influence on original soil structures and properties. Low cost, high efficiency and prevalent application are driving soil MFCs to be adopted widely.
Salt-influenced soils represent around 40% of the world’s lands16. Petroleum hydrocarbons contaminated saline-alkali soil is a common polluted environment because great oilfields always locate in coastal area. Due to the unfavorable high osmotic potential imposed by salts, the saline condition limits the activity of microorganism17,18. Qin et al. found that the degradation rate of petroleum hydrocarbons increased by about 30% when the soil salinity decreased from 2.86% to 0.10%18. Furthermore, high internal resistance is a key limiting factor to performance of MFCs19,20,21,22, particular in soil/sediment due to the low electrical conductivity23,24. It was noted that a 57% of decrement in internal resistance lead to a 100% of increment in current density in soil MFCs25. Additionally, our previous study found that U-type soil MFC with 7.4 Ω of internal resistance exhibited better performance of petroleum hydrocarbons degradation and power generation, compared to that with 42.6 Ω10.
Herein, two treatments including were conducted to enhance bioelectrochemical remediation of petroleum hydrocarbons contaminated saline-alkali soil. The performances of soil MFCs were investigated in terms of power generation, soil characteristics, hydrocarbons degradation, bacterial abundances. More importantly, oxygenase genes that have been widely used for the molecular monitoring of hydrocarbons biodegradation, were firstly quantified in soil MFCs. Simultaneously comprehensive correlations were surveyed to further understand the interaction among these factors in the bioeletrochemical remediation system.
Results
Performance of the soil MFC
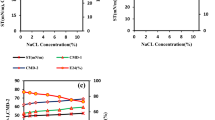
The current densities of all treatments including RS, MC and RM were enhanced compared to CK (Fig. 1a and Figure S1a). The maximum current density of RS (135 ± 2 mA·m−2, averaged over 12 h of peak current) and MC (170 ± 2 mA·m−2, averaged over 12 h of peak current) were 214% and 295% higher than that of CK (43 ± 11 mA·m−2, averaged over 12 h of peak current). The peak of current density of RS was observed after 85 h, which was 16 h earlier than that of MC. Meanwhile, RS had the highest current density during initial 24 hours (Figure S2). For RM, 299 ± 4 mA·m−2 of maximum current density was acquired on day 4 and was 595%, 121% and 76% higher than CK, RS and MC respectively. The accumulated charge output of 260 ± 51 C for CK was gained during 65 days, which was 23%, 64% and 110% lower than these of RS (320 ± 82 C), MC (427 ± 91 C), and RM (546 ± 43 C) respectively (Fig. 1b and Figure S1b).
Current density during initial 22 days (a) and charge output during a period of 65 days (b) of the soil MFCs. The data is the mean value of duplicate soil MFCs (see Figure S1). Nyquist plots of the soil MFCs at open circuit potentials (c). The he resistance analysis of the soil MFCs (d). The charge transfer resistance Rct = Rcathode + Ranode.
RS had the highest internal resistance of 88 ± 5 Ω, which was 238% higher than that of CK (26 ± 4 Ω), while the lowest internal resistance (12 ± 0 Ω) was observed in MC (Fig. 1c,d). There was no significant difference on total internal resistance between CK and RM (26 ± 2 Ω). For the soil samples rinsed salt (RS and RM), the Ohmic resistance (Rs) increased. Particularly, Rs of RS (50 ± 0 Ω) enlarged by more than a factor of 6 compared to CK (7 ± 1 Ω). However, Rs of MC (3 ± 0 Ω) was only 43% of CK. Charge transfer resistances (Rct) exhibited a similar trend to the total internal resistances.
Changes of soil characteristic
In the connected soil MFCs, soil pH decreased compared with original soil (OS, 8.30 ± 0.04) or rinsed salt original soil (RSOS, 9.08 ± 0.02) (Figure S3a). For CK and MC, only a 3–4% drop than OS was observed. While soil pH of RS and RM described a 10–13% lower than RSOS. For the connected MFCs, soil pH exhibited a slight differentiation. The soil electrical conductivities of RS (0.61 ± 0.01 mS∙cm−1) and RM (1.51 ± 0.00 mS∙cm−1) were 110% and 419% higher than that (0.29 ± 0.00 mS∙cm−1) of RSOS (Figure S3b). While CK (0.92 ± 0.00 mS∙cm−1) and MC (1.33 ± 0.00 mS∙cm−1) showed 49% and 27% of decline from OS (1.81 ± 0.04 mS∙cm−1). The soil electrical conductivities of connected soil MFCs showed a 14−49% of decrease from their corresponding controls. Both soil dehydrogenase and polyphenol oxidase activities in connected MFCs were much lower than those of OS or RSOS (Figure S3c,d). In addition, RS, MC or RM depicted lower enzyme activities than CK. Compared to the soil dehydrogenase, the soil polyphenol oxidase showed insignificant differences after bioelectrochemical remediation.
The soluble TC content of RSOS (419 ± 18 μg·g−1) was only 75% of the OS (560 ± 30 μg·g−1), which was partly accounted for the lower TC contents in rinsed salt soil samples (RS and RM) compared to CK and MC (Figure S4). Yet the soluble TN of RSOS (4.4 ± 0.3 μg·g−1) was comparable to the OS (4.8 ± 0.8 μg·g−1) indicating that nonionic nitrogen species were dominated in original soil. For all soil samples, TC and TN in connected MFCs showed 10–25% and 28–72% of reduction from the disconnected MFCs. Total organic carbon (TOC) was the main component of soluble TC in tested soil, and the dominant decrease of TC was inorganic carbon (IC) for CK and MC while TOC for RS and RM. Noticeably, the TN of soil in tested MFCs showed a evident increase (a factor of 16–70) from OS (or RSOS).
Degradation of hydrocarbons
As shown in Fig. 2a, the degradation rates of total petroleum hydrocarbons (TPHs) exhibited 153–484% of increment from CK in the connected MFCs after 65 days. Compared to their controls, the TPHs degradation rates in the connected MFCs were significantly (p < 0.01) higher and enhanced by a factor of 2–6. The highest degradation rate of TPHs was 60 ± 9% in RM, which was more than a factor of 20 as high as that in OSOC (3 ± 1%). The removal rates of TPHs were as follow: RM > MC > RS > CK.
TPH degradation rates (a), concentrations of the n-alkanes (C8–C38) (b) and 16 priority PAHs (c) in soil of MFCs. The abbreviations of PAHs were noted in Figure S5. The different lowercase indicates significant difference at the 0.01 level (2-tailed).
Similar to TPHs, the contents of n-alkanes and PAHs were obviously reduced (Fig. 2b,c) and showed 59–92% and 44–88% of overall degradation rates in the connected MFCs (Figure S6). As expected, the concentration of n-alkanes and PAHs in the connected MFCs were much lower than those in the disconnected MFCs. In addition to the carbon fractions of C16–C19, the high molecular weight fractions (C28–C36) also showed an obvious reduction, particular in RM (Fig. 2b and Figure S6b). Generally the higher degradation rates were observed on low ring of PAHs (1–3 rings, C10–C13) over high ring PAHs (Fig. 2c and Figure S6d). However, even the high molecular weight of PAHs (4–6 rings, C16–C22) were degraded apparently after soil MFC remediation.
Microbial community structures
According to the pyrosequencing of soil samples, the high-quality 16S rRNA gene 10492−20955 reads with an average length of 396 bp were constructed (Table S1). These sequences were assigned into 523−747 OTUs at a 97% similarity. Although new bacterial phylotypes continued to emerge even after 15,000 reads sampling with pyrosequencing, Shannon Index showed a plateau after 10,000 reads (Figure S7). This was also supported by 98–99% of Coverage estimators (Table S1), showing that the sizes of libraries were sufficient to cover the bacterial communities. The connected MFCs described the larger ACE and Chao 1 Index than those of the disconnected MFCs, while an inverse tendency was viewed for Shannon Index. Moreover, the highest ACE and Chao 1 estimators in RS indicated that its community had the greatest richness. The higher Shannon Index was observed in CK than others, showing that its biodiversity was most abundant.
Distinct cluster of bacterial communities were observed between rinsed and not rinsed salt soil samples, as well as between connected and disconnected MFCs from the hierarchical cluster analysis (Fig. 3). This was supported by principal component analysis (PCA) (Figure S8). Principal components 1 and 2 explained 40.32% and 25.62% of the total community variations, respectively. The sum of total observed OTUs in four communities of the connected MFCs was 1340, and 40% (537 OTUs) of the total OTUs were shared by them. OTUs of CK were the most (1073, 80% of total). The rest were as follow: RS (990, 74%) > MC (928, 69%) > RM (851, 64%), directly demonstrated that a selective enrichment was induced by bio-current.
Twenty-two bacterial phyla were viewed from eight libraries (Fig. 4a). The absolute majority of sequences belonged to Proteobacteria that accounted for 34–71% of the total reads in bacterial community. γ-Proteobacteria was the most abundant class (20–85%) within the Proteobacteria, but its ratio reduced by 49–215% in connected MFCs compared to those in disconnected MFCs except RM (Fig. 4b). α-Proteobacteria abundance exhibited a similar trend to γ-Proteobacteria. In addition, the abundance of β-Proteobacteria rose in CK and RS, but declined in MC and RM. In comparison, an increase of composition was observed for δ-Proteobacteria in all connected MFCs compared with the corresponding disconnected MFCs.
Furthermore, the Bacteroidetes, Firmicutes, Acidobacteria and a certain amount of unclassified sequences (at the phylum level) were other major bacterial phyla. Compared to their controls, Bacteroidetes and Firmicutes abundances in connected MFCs increased while Acidobacteria abundance decreased in MC and RM. Within Bacteroidetes, the proportion of Flavobacteriia was the highest (Fig. 4c). Bacteroidia abundance described an increment, but a decrement for Cytophagia. Within Firmicutes, Bacilli and Clostridia were the majority (97.8–99.9%) in all soil MFCs (Fig. 4d). In connected MFCs, the ratio of Bacilli declined, but Clostridia rose.
The distribution of bacterial species at genus level was presented by a heat map (Fig. 3), which accounted for 70–84% of total composition in all libraries (genera that were less than 2% of total composition were ignored, Table S2). Similar to the results from Shannon Index, CK had the higher microbial diversity than any other. There were large number species of Pseudomonas (2.60−34.46%), Salinimicrobium (4.40–15.48%), Bacillus (1.33−11.64%) in all samples. The proportion of Sedimentibacter rose for all soil MFCs, showing a formative anaerobic environment. In CK, RS, MC and RM, the abundances of Azospira, Geobacter and Desulfuromonas significantly increased from their controls, particularly in RM. This increase of composition was also observed for Desulfuromonadales (unclassified), Desulfitobacterium, Alkaliphilus, Castellaniella. Abundance of Clostridium (unclassified) increased from 0.09% in MC to 10.35% in MCOC. In RS, Alcanivoracaceae as well as Weeksella, Zavarzinia and Castellaniella abundances showed some degree of increment, while Bacillus and Pseudomonas exhibited slight decrement compared to RSOC.
Quantification of Oxygenase
Compared to the controls, the number of copies (ng−1 DNA) of total bacteria (16S) showed 20–214% of increase in connected MFCs (Fig. 5), due to the stimulation of bio-current. For the individual soil MFC, there was no apparent difference between investigated naphthalene dioxygenase (nah) and xylene monooxygenase (tol) gene copies (ng−1 DNA), and the total of both enzyme genes accounted for 0.27–0.98% of 16S. In RS and RM, copy numbers (ng−1 DNA) of nah and tol significantly increased from those in corresponding controls (a factor of 5–10 and 3–5 increase for nah and tol respectively). The most copy numbers (ng−1 DNA) of the total of nah and tol were observed in RM, which was a factor of 7 as many as that in CK.
Discussion
The current generation of all soil MFCs peaked at around 4 days and then declined until the end of experiment, which was due to the depletion of easily biodegradable hydrocarbons25. Better performance (power production and hydrocarbons degradation) for RS and MC indicated that either salinity decrease or conductivity increase of soil had positive effect for MFC remediation in saline-alkali soil. After rinsed salt, the soluble salt content of soil decreased from 2.8% to 0.3% (Table S3) so that the suppress effect from salinity on the bacterial populations (especially exoelectrogen and degradation microbe) was relieved18,26. This was supported by higher current density during initial 24 h and shorter time of reaching peak current for RS. Furthermore, the internal resistance of soil blended with carbon fiber significantly declined especially Rct (Fig. 1d), due probably to carbon fiber provided several conductive channels and thus promoted electron transfer and current collection, which enhanced the current density and remediation efficiency23. Simultaneously the best performance was detected when two amendments were combined (Figs 1 and 2). As an evidence, the power generation (current density or charge output) had obviously positive correlations (0.909–0.993**) with the degradation of petroleum hydrocarbons (TPH, Alkanes and PAHs) (Table 1), confirming that bioelectrochemical remediation of soil contaminated by hydrocarbons was a feasible approach with the advantage of energy recovery7,9,12,13. After rinsed salt, the soil osmotic pressure decreased, which was beneficial to the microbial activity. Yet the soil internal resistance significantly increased, which was adverse to the power production of soil MFC. Fortunately, the amendment treatment of blending carbon fiber completely overcame this defect. Consequently, the internal resistance of RM was comparable to that of CK (Fig. 1d). Compared to the Ohmic resistance (Rs), the charge transfer resistance (Rct) had 154% and 323% more significantly negative correlations with the accumulated charge output and TPH degradation, indicating that the activity of biological electron transfer mainly determined the performance of bioelectrochemical remediation system20,27.
The statistical analysis showed that the soil pH showed a lower significant correlation with the TPH degradation or the charge output than the soil electrical conductivity (Table 1), indicating that the soil electrical conductivity is a vital property in soil bioelectrochemical remediation. Additionally, the significant correlations (−0.724*–−0.887**) between enzymatic activity and petroleum hydrocarbons degradation confirmed the biodegradation stimulated by bio-current was responsible for removal of hydrocarbons. Although the soil polyphenol oxidase activity showed a less decline from the original soil (Figure S3d), a more significant correlation was viewed between soil dehydrogenase activity and TPH degradation (−0.764*) or charge output (−0.839). Similar to previous reports7, these result also indicated that dehydrogenase activity may act as an bioindicator during the process of bioelectrochemical remediation.
Among abundances of top ten phyla, only Proteobacteria, Firmicutes and Gemmatimonadetes were positively correlated with the charge output (Table 1), indicating that a selective enrichment (e.g. exoelectrogen) was induced by bio-current7,13 and more importantly some species may grow by electron transfer or grow in association with specific members of the electrically active community28. As an evidence, γ- and δ-Proteobacteria that contained the common exoelectrogens (such as Pseudomonas and Geobacter7,13) showed the positive correlations with the electricity generation. Beside Clostridia and Negativicutes (Within Firmicutes), Bacteroidia abundances (within Bacteroidetes) were also positively correlated with the current density and charge output (0.965* and 0.902) (Table S4). These results suggested the certain distribution of electroactive microbes. Notably, only Proteobacteria, Firmicutes and Bacterioidetes abundances among top ten phyla had positively correlations to hydrocarbons degradation, indicated that the main hydrocarbons degraders belonged to them29,30,31. Significantly positive correlations between petroleum hydrocarbons degradations (TPH, alkane and PAH) and Negativicutes abundances (0.746*–0.803*) was observed (Table S4), confirmed that Negativicutes play a favorable role in hydrocarbon biodegradation32. Furthermore, similar correlations were viewed between petroleum hydrocarbons degradation and δ-Proteobacteria (0.494–0.543), Bacilli (0.404–0.586). Several species of these classes found to serve an effective in the removal of petroleum hydrocarbons31,33. Besides, Planctomycetes (−0.625), Cyanobacteria (−0.626), Bacteria_norank (−0.671), Chloroflexi (−0.671) closely related to n-alkanes degradation. These results suggested certain species (rather than all) of Proteobacteria, Bacteroidetes or Firmicutes played an affirmative role in degradation of hydrocarbons.
In addition to γ-Proteobacteria and Bacilli, the higher correlations between enzymatic activities and microbial abundances were observed in Deinococcus-Thermus and Cyanobacteria, nevertheless they were not the dominant hydrocarbons degraders or exoelectrogens, suggesting a subtle interaction existed between degraders and concomitant microbes. Furthermore, there were significantly positive correlations between Deinococcus-Thermus and Cyanobacteria, Gemmatimonadetes and Cyanobacteria, pointing out that a complex relationship occurred34. Furthermore, the charge transfer resistance described something with the special microbial community, such as 0.957* of correlation with α-Proteobacteria, 0.997* with Sphingobacteriia. The change of soil characteristics directly induced the evolution of community structure35. For example, increase of soil pH clearly reduced the abundance of Acidobacteria (Fig. 4). Additionally, soil pH had a distinctly positive correlation (0.707) with abundance of γ-Proteobacteria that involved in most reported exoelectrogens7. Moreover, there were significant correlation between soil electrical conductivity and abundance of Bacilli (0.715*), between soil polyphenol oxidase and abundances of Negativicutes (−0.753*), between soil dehydrogenase and Deinococcus-Thermus (0.733*) (Table 1 and Table S4), which need to be further addressed in the succedent plan.
In bioelectrochemical remediation system, copies of naphthalene dioxygenase (nah), xylene monooxygenase (tol) exhibited a high correlation (0.899 and 0.827) with current density of soil MFCs (Table 2). Compared to nah, copies of tol had a more distinct correlation with hydrocarbon degradation (alkane and PAH). By contrast, copies of total bacteria populations (16S) obviously positively related to hydrocarbon degradation (0.896**–0.975**) and electricity generation (0.972**–1.000**), implying that biodegradable hydrocarbons was the strong determinant of total bacterial abundance and power output26. Previous reports showed that amount of nah and tol could use as bioindicator in degradation of petroleum hydrocarbons contaminated soil36. In soil MFCs, it was also demonstrated that the quantitative determination of key enzyme gene was feasible as a monitoring or predicting index during pollution remediation.
Methods
Tested soil and treatment
Petroleum hydrocarbon contaminated soil was collected from the topsoil (<10 cm) near the oil well in Dagang Oilfield of Binhai New Area (Tianjin, China). The soil was air-dried, passed 2 mm sieve and marked as original soil (OS). The soil properties were summarized as Table S3. The OS was rinsed with 1:1.2 (w/v) of distilled water by water leaching method18 and marked as RSOS. The connected MFCs filled with OS and RSOS were marked as CK and RS, while the corresponding disconnected controls as OSOC and RSOC. The connected MFC filled with the mixture of OS and carbon fiber pieces (1 cm of length, 5 μm of diameter, Jilin Carbon Factory; acetone clean overnight; 2% in mass ratio to soil) was marked as MC and the corresponding disconnected control as MCOC. The MFC filled with soil that was rinsed salt and followed by blended of carbon fiber (2%), was marked as RM and its disconnected control as RMOC. The abbreviations used in this study were shown in Table 3. All soil MFCs were filled with 100 g of dry soil without adding buffer solution or any exogenetic inoculation.
Soil MFC configuration
Soil MFCs were constructed as previous describe13 with only one layer of anode as showed in Figure S9. Carbon mesh (Jilin Carbon Factory, Jilin, China) was used as the anode after acetone clean overnight37. Activated carbon air-cathode was prepared according to the modified rolling-press method19,20. All soil MFCs were placed in an incubator (30 °C) and sealed with distilled water during the experiment. Each treatment including their disconnected control had a duplicate.
Electrochemical analysis
Voltage (U) across the external resistor of 100 Ω (R) was recorded by a data acquisition system (PISO-813, ICP DAS Co., Ltd, Shanghai, China). A potentiostat (Autolab PGSTAT 302N, Metrohm, Switzerland) was used to perform electrochemical impedance spectrum (EIS) from 100 kHz to 1 Hz with a 10 mV of amplitude at the open circuit potential (stabilized for 4 h after installed). The carbon mesh anode was used as the working electrode while the air-cathode as the counter and reference electrode. ZsimpWin 3.10 was employed to simulate Nyquist plots according to an equivalent circuit in Figure S10.
Chemical analysis
The soil pH and the electrical conductivity were measured in a mixture with soil to distilled water ratio (w/v) as 1:5. Organic matter, available N, P and K were evaluated according to regular methods38. The soluble salt content was measured as previous description18. The contents of metal elements were attained using ICP-OES (Vista MPX Varian, US)39. The soil dehydrogenase was extracted by 2,3,5-Triphenyl Tetrazolium Chloride reduction and Triphenyl Formazone calibration (485 nm). Soil polyphenol oxidase was analyzed by catalyzing pyrogallol (430 nm) as previous methods40. The soluble total carbon (TC) and total nitrogen (TN) were determined by an Analytik jena multi N/C 3100 (Germany) (Details found in the supporting information). The total petroleum hydrocarbon (TPH) and carbon fractions (n-alkanes and PAHs) were analyzed according to our previous procedures6,10.
Biological analysis
Genomic DNA was extracted using a Power Soil DNA Isolation Kit (Mobio, USA) according to the manufacturer’s instruction. 1% agarose gel electrophoresis was employed to confirm the purity of genomic DNA.
Bacterial 16S rRNA gene fragments were PCR-amplified using primers of 515F (5′-GTGCCAGCMGCCGCGG-3′) and 907R (5′-CCGTCAATTCMTTTRAGTTT-3′) covering V4 and V5 regions. PCR was carried out in triplicate 20 μL reactions containing 4 μL of 5× FastPfu Buffer, 2 μL of 2.5 mM dNTPs, 0.8 μL of each Primer (5 μM), 0.4 μL of FastPfu Polymerase, 10 ng of Template DNA, and added ddH2O to bring up the final volume to 20 μL. The PCR temperature procedures were 95 °C for 3 min; 25 cycles consisted of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 45 s; 72 °C for 10 min and 4 °C until halted. After confirmed by 2% agarose gel electrophoresis, PCR products were pooled and purified using AxyPrepDNA (Axygen, USA).
Pyrosequencing was performed on a Genome Sequencer instrument (MiSeq) as the standard procedure of Majorbio Bio-Pharm Biotechnology Co., Ltd. (Shanghai, China). Quality filters were used to remove low quality sequences which were shorter than 50 bp, involved in any ambiguous bases, showed a homopolymer of longer than 10 bp, or were chimera. Finally, the valid sequences were trimmed to an average length of 396 bp. Then these sequences were clustered into Operational Taxonomic Units (OTUs) based on a 97% similarity threshold. Ribosomal Database Project classifier was used to align for their taxonomy at different levels (http://www.arb-silva.de).
The quantitative determination of naphthalene dioxygenase (nah), xylene monooxygenase (tol) and total bacteria populations (16S) were determined by ABI7500 FAST system with the primers41 listed in Table S5. A 25 μL real-time PCR mixture contained 12.5 μL of 2× SybrGreen qPCR Master Mix, 0.5 μL of each primer (10 μM), 1 μL of Template DNA and 10.5 μL of ddH2O. PCR conditions were as follow: initial denaturation at 95 °C for 2 min; 40 cycles consisted of 95 °C for 10 s and 60 °C for 40 s.
Calculation
The current density (mA·m−2) of MFC was obtained as I´ = U/(R·A), where A is the area of air-cathode (0.0036 m2). The charge output was evaluated as Q = ∫0T(U/R)dt. The degradation rate of hydrocarbons was gained as η = (COS – C)/COS, where COS is the hydrocarbons concentration in original soil (OS) or rinsed salt original soil (RSOS) as shown in Figure S10 and C is the hydrocarbons concentration in tested soil. The overall degradation rate of PAHs (or n-alkanes) was calculated by adding the contents of 16 PAHs (or 31 kinds of n-alkanes). The community richness and diversity estimates (Rarefaction, Chao 1, ACE and Shannon Index) were calculated according to the assigned methods (http://www.mothur.org/wiki/). Relative abundance was defined as that the number of sequences affiliated with that phylum, genus, or class divided by the total sequence number of per sample or dominant phyla (Proteobacteria, Bacteroidetes or Firmicutes).
Additional Information
How to cite this article: Li, X. et al. Salinity and Conductivity Amendment of Soil Enhanced the Bioelectrochemical Degradation of Petroleum Hydrocarbons. Sci. Rep. 6, 32861; doi: 10.1038/srep32861 (2016).
References
Ayotamuno, M., Kogbara, R., Ogaji, S. & Probert, S. Bioremediation of a crude-oil polluted agricultural-soil at Port Harcourt, Nigeria. Applied Energy 83, 1249–1257 (2006).
Chen, M. et al. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: applications, microbes and future research needs. Biotechnology Advances 33, 745–755 (2015).
Riser-Roberts, E. Remediation of petroleum contaminated soils: biological, physical, and chemical processes. (CRC Press, 1998).
Zhou, Q., Sun, F. & Liu, R. Joint chemical flushing of soils contaminated with petroleum hydrocarbons. Environment International 31, 835–839 (2005).
Tang, J., Wang, R., Niu, X. & Zhou, Q. Enhancement of soil petroleum remediation by using a combination of ryegrass (Lolium perenne) and different microorganisms. Soil and Tillage Research 110, 87–93 (2010).
Peng, S., Zhou, Q., Cai, Z. & Zhang, Z. Phytoremediation of petroleum contaminated soils by Mirabilis Jalapa L. in a greenhouse plot experiment. Journal of hazardous materials 168, 1490–1496, doi: 10.1016/j.jhazmat.2009.03.036 (2009).
Lu, L., Huggins, T., Jin, S., Zuo, Y. & Ren, Z. J. Microbial metabolism and community structure in response to bioelectrochemically enhanced remediation of petroleum hydrocarbon-contaminated Soil. Environmental Science & Technology 48, 4021–4029 (2014).
Mohan, S. V. & Chandrasekhar, K. Self-induced bio-potential and graphite electron accepting conditions enhances petroleum sludge degradation in bio-electrochemical system with simultaneous power generation. Bioresource Technology 102, 9532–9541 (2011).
Morris, J. M. & Jin, S. Enhanced biodegradation of hydrocarbon-contaminated sediments using microbial fuel cells. Journal of hazardous materials 213, 474–477 (2012).
Wang, X., Cai, Z., Zhou, Q., Zhang, Z. & Chen, C. Bioelectrochemical stimulation of petroleum hydrocarbon degradation in saline soil using U‐tube microbial fuel cells. Biotechnology and Bioengineering 109, 426–433 (2012).
Zhang, T., Gannon, S. M., Nevin, K. P., Franks, A. E. & Lovley, D. R. Stimulating the anaerobic degradation of aromatic hydrocarbons in contaminated sediments by providing an electrode as the electron acceptor. Environmental Microbiology 12, 1011–1020 (2010).
Zhang, Y. et al. Horizontal arrangement of anodes of microbial fuel cells enhances remediation of petroleum hydrocarbon-contaminated soil. Environmental Science and Pollution Research, 1–7 (2014).
Li, X. et al. Extended petroleum hydrocarbon bioremediation in saline soil using Pt-free multianodes microbial fuel cells. RSC Advances 4, 59803–59808 (2014).
Yuan, Y., Chen, Q., Zhou, S., Zhuang, L. & Hu, P. Improved electricity production from sewage sludge under alkaline conditions in an insert‐type air‐cathode microbial fuel cell. Journal of Chemical Technology and Biotechnology 87, 80–86 (2012).
Yuan, Y., Zhou, S. & Zhuang, L. A new approach to in situ sediment remediation based on air-cathode microbial fuel cells. Journal of Soils and Sediments 10, 1427–1433 (2010).
Serrano, R. & Gaxiola, R. Microbial models and salt stress tolerance in plants. Critical Reviews in Plant Sciences 13, 121–138 (1994).
Dendooven, L. et al. Dynamics of carbon and nitrogen in an extreme alkaline saline soil: a review. Soil Biology and Biochemistry 42, 865–877 (2010).
Qin, X., Li, D., Tang, J., Zhang, Q. & Gao, J. Effect of the salt contentin soil on bioremediation of soil by contaminated petroleum. Letter in Applied Microbiology 55, 210–217 (2012).
Li, X., Wang, X., Zhang, Y., Ding, N. & Zhou, Q. Opening size optimization of metal matrix in rolling-pressed activated carbon air–cathode for microbial fuel cells. Applied Energy 123, 13–18 (2014).
Li, N., Liu, Y., An, J., Feng, C. & Wang, X. Bifunctional quaternary ammonium compounds to inhibit biofilm growth and enhance performance for activated carbon air-cathode in microbial fuel cells. Journal of Power Sources 272, 895–899, doi: 10.1016/j.jpowsour.2014.09.008 (2014).
Cheng, S., Liu, H. & Logan, B. E. Increased power generation in a continuous flow MFC with advective flow through the porous anode and reduced electrode spacing. Environmental Science & Technology 40, 2426–2432 (2006).
Liu, H., Cheng, S. & Logan, B. E. Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environmental Science & Technology 39, 5488–5493 (2005).
Domínguez-Garay, A., Berná, A., Ortiz-Bernad, I. & Esteve-Núñez, A. Silica colloid formation enhances performance of sediment microbial fuel cells in a low conductivity soil. Environmental Science & Technology 47, 2117–2122 (2013).
Rezaei, F., Richard, T. L., Brennan, R. A. & Logan, B. E. Substrate-enhanced microbial fuel cells for improved remote power generation from sediment-based systems. Environmental Science & Technology 41, 4053–4058 (2007).
Lu, L. et al. Enhanced bioremediation of hydrocarbon-contaminated soil using pilot-scale bioelectrochemical systems. Journal of hazardous materials 274, 8–15 (2014).
Nie, M. et al. Rhizosphere effects on soil bacterial abundance and diversity in the Yellow River Deltaic ecosystem as influenced by petroleum contamination and soil salinization. Soil Biology and Biochemistry 41, 2535–2542 (2009).
Wetser, K., Sudirjo, E., Buisman, C. J. & Strik, D. P. Electricity generation by a plant microbial fuel cell with an integrated oxygen reducing biocathode. Applied energy 137, 151–157 (2015).
Wrighton, K. C. et al. A novel ecological role of the Firmicutes identified in thermophilic microbial fuel cells. The ISME journal 2, 1146–1156 (2008).
Kostka, J. E. et al. Hydrocarbon-degrading bacteria and the bacterial community response in Gulf of Mexico beach sands impacted by the Deepwater Horizon oil spill. Applied and environmental microbiology 77, 7962–7974 (2011).
Röling, W. F. et al. Robust hydrocarbon degradation and dynamics of bacterial communities during nutrient-enhanced oil spill bioremediation. Applied and environmental microbiology 68, 5537–5548 (2002).
Al-Mailem, D., Kansour, M. & Radwan, S. Bacterial communities associated with biofouling materials used in bench-scale hydrocarbon bioremediation. Environmental Science and Pollution Research 22, 3570–3585 (2015).
de Menezes, A., Clipson, N. & Doyle, E. Comparative metatranscriptomics reveals widespread community responses during phenanthrene degradation in soil. Environmental microbiology 14, 2577–2588 (2012).
Trejo-Hernandez, M., Ortiz, A., Okoh, A., Morales, D. & Quintero, R. Biodegradation of heavy crude oil Maya using spent compost and sugar cane bagasse wastes. Chemosphere 68, 848–855 (2007).
Logan, B. E. Exoelectrogenic bacteria that power microbial fuel cells. Nature Reviews Microbiology 7, 375–381 (2009).
Lauber, C. L., Strickland, M. S., Bradford, M. A. & Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biology and Biochemistry 40, 2407–2415 (2008).
DeBruyn, J. M., Chewning, C. S. & Sayler, G. S. Comparative quantitative prevalence of Mycobacteria and functionally abundant nidA, nahAc, and nagAc dioxygenase genes in coal tar contaminated sediments. Environmental Science & Technology 41, 5426–5432 (2007).
Wang, X. et al. Use of carbon mesh anodes and the effect of different pretreatment methods on power production in microbial fuel cells. Environmental Science & Technology 43, 6870–6874 (2009).
Liu, G. Soil physical and chemical analysis and description of soil profiles. (China Standard Press, 1996).
Sun, Y., Zhou, Q., Xie, X. & Liu, R. Spatial, sources and risk assessment of heavy metal contamination of urban soils in typical regions of Shenyang, China. Journal of hazardous materials 174, 455–462 (2010).
Zhou, L. Soil enzymology. (Science and Technology Press, 1987).
Baldwin, B. R., Nakatsu, C. H. & Nies, L. Detection and enumeration of aromatic oxygenase genes by multiplex and real-time PCR. Applied and Environmental Microbiology 69, 3350–3358 (2003).
Acknowledgements
This research work was financially supported by the Natural Science Foundation of Tianjin (No. 16JCQNJC08800), the Ministry of Science and Technology as an 863 major project (No. 2013AA06A205), the National Natural Science Foundation of China (No. 41601536, 21577068 and 51409052), and the Special Fund for Agro-Scientific Research in the Public Interest of China (No. 201503107-7).
Author information
Authors and Affiliations
Contributions
X.L. and X.W. designed the research; X.L., Y.Z., Q.Z., B.Y. and Y.L. performed the research; X.L. and Q.Z. analyzed the data and wrote the paper; and X.W. and Q.Z. revised the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, X., Wang, X., Zhang, Y. et al. Salinity and Conductivity Amendment of Soil Enhanced the Bioelectrochemical Degradation of Petroleum Hydrocarbons. Sci Rep 6, 32861 (2016). https://doi.org/10.1038/srep32861
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep32861
This article is cited by
-
Long-term egret (Egretta garzetta) habitation alters topsoil and subsoil phosphorus fractions and bacterial communities in coastal wetlands
Biology and Fertility of Soils (2023)
-
Hydrocarbons in the water and bottom sediments of Sivash Bay (the Azov Sea) during its salinization
Environmental Science and Pollution Research (2022)
-
Shifting interactions among bacteria, fungi and archaea enhance removal of antibiotics and antibiotic resistance genes in the soil bioelectrochemical remediation
Biotechnology for Biofuels (2019)
-
Environmental applications of microbial extremophiles in the degradation of petroleum hydrocarbons in extreme environments
Environmental Sustainability (2019)
-
Cathodic microbial community adaptation to the removal of chlorinated herbicide in soil microbial fuel cells
Environmental Science and Pollution Research (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.