Abstract
The influence of spin-orbit coupling (SOC) on the physical properties of the 5d2 system Sr2MgOsO6 is probed via a combination of magnetometry, specific heat measurements, elastic and inelastic neutron scattering and density functional theory calculations. Although a significant degree of frustration is expected, we find that Sr2MgOsO6 orders in a type I antiferromagnetic structure at the remarkably high temperature of 108 K. The measurements presented allow for the first accurate quantification of the size of the magnetic moment in a 5d2 system of 0.60(2) μB –a significantly reduced moment from the expected value for such a system. Furthermore, significant anisotropy is identified via a spin excitation gap and we confirm by first principles calculations that SOC not only provides the magnetocrystalline anisotropy, but also plays a crucial role in determining both the ground state magnetic order and the size of the local moment in this compound. Through comparison to Sr2ScOsO6, it is demonstrated that SOC-induced anisotropy has the ability to relieve frustration in 5d2 systems relative to their 5d3 counterparts, providing an explanation of the high TN found in Sr2MgOsO6.
Similar content being viewed by others
Introduction
There is a great deal of interest in materials with inherently frustrated magnetic exchange interactions and the resulting unusual magnetic ground states1,2. One class of materials currently under investigation in this context is the double perovskites, formula A2BB′O6, where A and B are non-magnetic cations and B′ is a magnetic 4d or 5d transition metal cation3. The resulting network of B′ cations forms a quasi-face-centered cubic (fcc) type lattice where antiferromagnetic interactions are highly frustrated as each B′ cation has twelve nearest neighbor B′ cations3. Particularly complex magnetic states can arise in frustrated systems due to the presence of significant spin-orbit coupling (SOC) that typically accompanies cations of the 4d and 5d transition metals. It’s imperative to understand the role SOC plays in such materials due to its role in lifting the orbital degeneracy and enhancing multipolar interactions, leading to rich phase diagrams4,5,6,7.
In the case of double perovskites containing a single magnetic cation with a d3 electronic configuration, the relatively large S = 3/2 spins are expected to have a quenched orbital contribution resulting in classical behavior6. While experimental results indicate that SOC is allowed in the d3 configuration for 4d or 5d cations, the reduction in the magnetic moment due to SOC is small relative to the effects of covalency, which is large for cations with high oxidation states8,9. Double perovskites with 4d3 or 5d3 ions tend to exhibit frustration indexes (|Θ|/TN) ranging from 4 to 14 and adopt a type I antiferromagnetic structure upon ordering8,10,11,12,13, although incommensurate behavior has also been reported14,15.
In the case of the much smaller S = 1/2 d1 configuration, SOC is expected to play a significant role and antiferromagnetic, ferromagnetic and quadrupolar order have all been predicted as a result5,16. These predictions appear to be in line with measurements of antiferromagnetic order in Ba2LiOsO6 and ferromagnetic order in Ba2NaOsO617,18. However, there are additional examples such as spin glass Sr2MgReO619 and spin singlet Ba2YMoO620,21 which do not easily fit into this framework. Investigation of magnetism due to the d4 configuration has also begun, such as in A2BIrO6 (A = Sr, Ba; B = Sc, In, Y), where questions have arisen concerning the strength of SOC and the magnetism of the resulting ground state22,23,24,25.
In this work, we investigate the influence of SOC on the magnetic state for the intermediate 5d2 S = 1 configuration, by studying the double perovskite Sr2MgOsO626,27,28. Theoretical work6 has predicted a rich phase diagram with seven different phases/regions for the present 5d2 S = 1 scenario, however there has been difficulty in sorting known materials in this context. Ba2YReO6, Sr2YReO6 and Ca2MgOsO6 appear to be spin glasses, which have not been predicted28,29,30, La2LiReO6 and Sr2InReO6 host non-predicted spin singlet states29,30, while μSR experiments indicate that cubic Ba2CaOsO6 orders antiferromagnetically, though with moments too small for detection in the reported neutron scattering experiment31. By combining magnetization, specific heat and neutron scattering measurements with first principles calculations for Sr2MgOsO6, we are able to provide insight into the nature of frustration and influence of SOC in this material.
Sr2MgOsO6 orders at 108 K with a type I antiferromagnetic structure, shown in Fig. 1, a results that was previously predicted in a model considering the influence of SOC6. The Os6+ moments of 0.60(2) μB are significantly reduced from the 2 μB spin only value expected for an S = 1 ion. Density functional theory (DFT) confirms that this substantial reduction in moment occurs through a combination of both covalency and SOC and furthermore predicts that SOC-induced anisotropy is essential in the selection of the magnetic ground state. The presence of this anisotropy is experimentally confirmed by the observation of a spin gap in the magnetic excitation spectrum via inelastic neutron scattering. Sr2MgOsO6 is therefore a rare example of a compound where the Néel order, rather than just the anisotropy, is set by the spin-orbit interaction. Furthermore, we find that SOC-induced anisotropy is responsible for the reduced magnetic frustration in Sr2MgOsO6 relative to d3 double perovskites, therefore explaining the enhanced TN in Sr2MgOsO6.
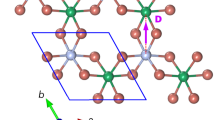
The crystal and magnetic structure of Sr2MgOsO6 with Mg and Os shown as grey and red spheres located within octahedra of the same color with O ions positioned at the corners.
Sr cations are omitted for clarity. The magnetic moment on Os6+ is shown as a black arrow and while shown along the b axis, is known only to be in the a-b plane.
Experimental
Powder samples of Sr2MgOsO6 were synthesized by grinding stoichiometric amounts of SrO2, MgO, Os and OsO2 together using a mortar and pestle according to the following chemical equation:

Ground mixtures of up to 3 g were contained in high-density alumina tubes and sealed in evacuated silica ampoules (approximate volume 40 mL with 3 mm thick walls) for heatings of 48 hours at 1000 °C in a box furnace located within a fumehood. This was followed by regrinding and identical reheating for an additional two cycles. Larger sample sizes were produced by synthesizing multiple aliquots which would be ground together in the intermittent grindings and redistributed for the subsequent heatings. Powdered Sr2MgWO6 samples were synthesized in air following the procedure outlined in the literature32.
The temperature dependence of the magnetization of Sr2MgOsO6 powders was measured using a Quantum Design MPMS SQUID magnetometer. Data were collected over the temperature range 2.5 to 400 K under zero-field-cooled (ZFC) and field-cooled conditions (FC) in an applied field of 10 kOe. Powders were contained in gel capsules and mounted in straws for insertion into the device for measurement. An analogous data set was collected using an empty sample mount and subtracted from the temperature dependent magnetization data of Sr2MgOsO6 in order to remove the background response.
Powders of Sr2MgOsO6 and Sr2MgWO6 were cold pressed and sintered at their synthesis temperatures overnight (in an evacuated ampoule for Sr2MgOsO6) to prepare polycrystalline pellets. The specific heat measurements were conducted on the pellets mounted with Apiezon grease using a Quantum Design PPMS instrument using a relaxation technique.
Laboratory x-ray powder diffraction measurements were conducted at room temperature on a Bruker D8 Advance equipped with a Ge (111) monochromator and a Cu radiation source. Time of flight neutron powder diffraction (NPD) measurements were conducted on Sr2MgOsO6 at Oak Ridge National Laboratory’s (ORNL) Spallation Neutron Source (SNS) on the POWGEN beamline33 using a sample size of 1.359 g. Data were collected at 10, 50 and 300 K using the POWGEN Automatic Changer (PAC) environment. Separate data sets with the bank 2 and bank 7 chopper settings corresponding to respective d-spacing ranges of 0.2760–3.0906 Å and 2.2076–10.3019 Å were collected at each temperature. Data were analyzed using the Rietveld method as implemented in the GSAS EXPGUI software package34,35. Additional NPD data were collected at High Flux Isotope Reactor (HFIR) facility at ORNL on the triple-axis spectrometer HB-1A using a sample size of 11 g. The sample was sealed under a He atmosphere into a cylindrical can made of aluminum with an inner diameter 0.6 cm. Data were collected at a constant wavelength of λ = 2.37Å using collimation of 40′-40′-40′-80′. The data were analyzed using the Rietveld refinement suite FULLPROF36 and the magnetic form factor for Os6+ from ref. 37 was assumed.
Inelastic neutron scattering experiments were performed on an 11 g sample of Sr2MgOsO6 and on a 16.5 g sample of Sr2ScOsO6 that was previously examined in ref. 38. Measurements were performed on the SEQUOIA chopper spectrometer at the Spallation Neutron Source (SNS) at Oak Ridge National Laboratory (ORNL). The samples were sealed in aluminum cans and an identical empty Al can was measured as a background. A closed-cycle refrigerator was used to reach temperatures between 6 K and 125 K and measurements were performed using an incident neutron energy 20 meV. Empty-can measurements were subtracted from the data sets, which were then normalized by a factor mf.u./ms, where mf.u. is the formula unit mass and ms is the sample mass for each of Sr2MgOsO6 and Sr2ScOsO6. The presented magnetic scattering intensity is therefore per Os ion.
First principles calculations were performed using the generalized gradient approximation (GGA) of Perdew, Burke and Ernzerhof (PBE)39 with the general potential linearized augmented planewave (LAPW) method40 as implemented in the WIEN2k code41. We used the experimental 10 K crystal structure and highly converged basis sets, including local orbitals with LAPW sphere radii of 2.0 bohr for the metal atoms and 1.55 bohr for O. We did calculations both with the PBE-GGA itself and with an additional Coulomb repulsion parameter U = 3 eV in the PBE + U approach with the fully localized limit double counting. The key difference between these treatments is that metallic behavior is predicted with PBE while an insulating gap is obtained with U = 3 eV, consistent with experimental reports for the resistivity of polycrystalline samples28. We focus on results that do not depend on U.
Results
Sr2MgOsO6 crystallizes in the tetragonal I4/m space group as previously reported27,28 and shown in Fig. 1, which is common to a number of other Sr2BOsO6 compositions42,43,44,45,46. The I4/m space group is associated with the a0a0c− Glazer tilt system, where out of phase tilting occurs about the c-axis47. Rietveld refinement of the x-ray powder diffraction data did not indicate any disorder between Mg and Os cations, a typical result considering the charge difference of 4+ between the anticipated oxidation states of Mg2+ and Os6+ 3. Refinements also did not indicate any loss of Os during synthesis within experimental error.
The results of the refinement of neutron powder diffraction data on Sr2MgOsO6 from POWGEN are given in Table 1 and the refined pattern at 10 K is given in Fig. 2. The average Os−O bond length is typical of recent results for octahedrally coordinated Os6+ in the double perovskite structure42,46,48. Both the MgO6 and OsO6 octahedra are slightly elongated, with two slightly longer and four slightly shorter M−O bonds. For the d2 cation Os6+, this is the expected Jahn-Teller distortion. The unit cell is tetragonally distorted as compared to the cubic cell, with a c-axis which is greater than √2 of the a-axis. The tetragonal distortion is enhanced at lower temperatures, with a c/√2a ratio of 1.0068 at 300 K and 1.0206 at 10 K. The distortion manifests as a combination of enhanced tetragonal elongation of the octahedra and octahedral tilting as evidenced by a reduced Mg−O−Os bond angle. No structural phase transition or change in symmetry occurs within the temperature range studied. The short Os−O bond lengths compared to the d3 osmates reflect the contraction and increased covalency that can be anticipated as the oxidation state is increased.
Refined neutron powder diffraction pattern of Sr2MgOsO6 at 10 K.
Black symbols, red curves and blue curves correspond to the observed data, the calculated patterns and the difference curve, respectively. The black hashes correspond to the nuclear peak positions of the compound. The inset shows low Q neutron powder diffraction data on Sr2MgOsO6 from POWGEN at 300, 50 and 10 K. Arrows indicate the position of the potential magnetic reflections associated with Type I AFM order.
The temperature dependence of the magnetization of Sr2MgOsO6 is given in Fig. 3a. A clear cusp corresponding to an antiferromagnetic transition occurs with a maximum at 108 K in both the FC and ZFC data sets, in approximate agreement with previous reports27,28, while the Fisher heat capacity, d(χT)/dT, shown in Fig. 3b, indicates a maximum value at 102 K– a lower TN from this approach is typical of double perovskites31. A divergence of the FC and ZFC data at 15 K is noted in Fig. 3a which is absent in previous reports27,28 despite being present in numerous independent samples and measurements by the present authors. A Curie-Weiss fit was conducted in the temperature range 250 to 400 K, which resulted in an effective moment of 1.88 μB, in close agreement with reported values and a Weiss constant of Θ = −269 K, in approximate agreement with reported values27,28. The effective paramagnetic moment is substantially reduced from the theoretical spin-only result of 2.83 μB for S = 1, indicating that the influence of spin-orbit coupling is significant for Os6+. The ratio between the Weiss constant and ordering temperature TN yields a relatively low frustration index, (|Θ|/TN), of 2.5.
(a) The temperature dependence the field-cooled (red filled circles) and zero-field-cooled (blue open circles) magnetization under an applied field of Sr2MgOsO6 plotted against the left axis. The inverse data (black circles) is plotted against the right axis with a red line indicating the higher temperature Curie-Weiss fitting. (b) The Fisher heat capacity of Sr2MgOsO6 derived from the data in panel (a).
The specific heat of Sr2MgOsO6 is shown in Fig. 4, with a clear second-order type anomaly positioned at 108 K indicating that the transition is likely due to long-range antiferromagnetic order. Much like the cusp in magnetic susceptibility, this anomaly is significantly broadened, a possible indication of magnetic frustration in the system. An isostructural nonmagnetic material with similar mass, Sr2MgWO6, was measured to approximate the nonmagnetic lattice contributions to the specific heat. The solid line, also shown in Fig. 4(a), represents the specific heat data of Sr2MgWO6 scaled by mass. The difference of these two data sets, plotted as Cmag/T and shown in Fig. 4b, corresponds to the magnetic component of the specific heat in Sr2MgOsO6. Clearly, there is a large peak at the antiferromagnetic transition, but there is also a significant tail up to temperatures much higher than TN, indicating persistent magnetic fluctuations. The magnetic specific heat Cmag/T is integrated to obtain the magnetic entropy, plotted against the right axis in Fig. 4b. Analysis of the magnetic entropy over the entire temperature range results in Smag ~ 11.2 J/mol K, which is in between the theoretical values for a simple L-S scheme for a d2 cation with an expected total spin J = 2 (Smag = 13.38 J/mol K) and a spin-only scenario with S = 1 (Smag = 9.134 J/mol K). These results clearly contrast from a similar analysis recently conducted on 3d8 S = 1 Sr2NiWO649, where a spin-only analysis in a nominally orbitally quenched material resulted in good agreement with the experimentally determined magnetic entropy. Therefore, we conclude that the magnetic entropy of Sr2MgOsO6 is significantly impacted by the orbital contribution to the magnetic moment in this compound.
(a) The temperature dependence of the specific heat of Sr2MgOsO6 (blue) and diamagnetic Sr2MgWO6 (red) which is used to approximate the lattice specific heat of S2MgOsO6. (b) The magnetic specific heat (black) of Sr2MgOsO6 taken by subtracting the scaled specific heat of Sr2MgWO6 and magnetic entropy (red) obtained by integration.
In order to obtain a microscopic insight into the ordered magnetic structure, we examined the low Q region of the neutron powder diffraction data collected on the POWGEN instrument, shown as the inset of Fig. 2. However, no apparent magnetic reflections were observed arising below the ordering temperature. The specific location of the anticipated peaks associated with the common type I antiferromagnetic order are highlighted in the inset. This is similar to the case of 5d2 Ba2CaOsO6, where NPD data from the C2 diffractometer at the Canadian Neutron Beam Centre at Chalk River National Laboratories did not yield any observable magnetic reflections despite evidence of long range magnetic order from muon spin relaxation experiments31. In that study, it was determined that the ordered moments must be less than an estimated detection limit of 0.7 μB per Os6+.
In order to search for the presence of weak magnetic reflections, additional neutron powder diffraction data was collected on the HB-1A beamline using an 11 g sample, nearly 10 times the mass measured on POWGEN and with the sample contained in an aluminum can to minimize incoherent scattering. HB-1A was utilized for this particular investigation because of its excellent signal-to-noise ratio, arising from the combined use of a double-bounce monochromator and an analyzer. Below TN, two magnetic reflections previously anticipated due to type I antiferromagnetic order were observed, see Fig. 5. The diffraction pattern was analyzed using constant structural values as determined from POWGEN, but varying the background and instrument-dependent parameters, resulting in a good fit to the data, Fig. 5a. Extra peaks are visible which are due to the aluminum sample can scattering and a small, unidentified impurity phase is visible in this sample, as indicated in Fig. 5, which is present at all temperatures. The magnetic structure refinement yielded Os6+ moments of 0.60(2) μB, which are aligned within the a-b plane. The resulting value is just below the proposed detection limit from the case of Ba2CaOsO631. The temperature dependence of the Q(001) = 0.78 Å−1 peak is shown in Fig. 5c. A power-law curve was fit to the data to extract TN and confirms the TN = 108(2) K transition temperature associated with this magnetic peak. This is a remarkably high Néel temperature for a double perovskite with B = Mg, since the Os6+ ions are on a quasi-fcc lattice and are separated by more than 5.5 Å.
(a) Neutron powder diffraction data collected on Sr2MgOsO6 using the HB-1A beamline at 125 K. (b) A close view of the low Q data at 125 K (red open circles) and 6 K (blue filled circles) with a Rietveld fitting to the 6 K data (green line) as described in the text. (c) The temperature dependence of the Q(001) = 0.78 Å−1 magnetic reflection with a power-law fitting to the data as described in the text.
For our DFT calculations, we considered different magnetic orders including ferromagnetic, the observed type I order and checkerboard antiferromagnetic order in the basal a-b plane of the tetragonal cell. We find that PBE-GGA calculations without SOC predict an incorrect magnetic order, specifically a ferromagnetic ground state. Only when spin-orbit coupling is included do we obtain the correct type I order as the lowest energy state. This conclusion is robust, as when spin orbit coupling is included we obtain type I order independent of the moment direction and for both PBE and PBE + U calculations. It follows that Sr2MgOsO6 is a rare example of a material where the Néel order itself, rather than just the anisotropy is set by the spin-orbit interaction–a reflection of the strong SOC.
Also for both PBE and PBE + U calculations with SOC we find the lowest energy spin direction to be along the tetragonal <100> direction in the tetragonal I4/m cell. The anisotropy is sizable and increases with U. For the PBE calculations we find that the <110> direction is disfavored by 1 meV/Os, while the <001> direction is disfavored by 5 meV/Os. The easy axis and plane do not depend on U. This anisotropy and the symmetry breaking due to the tetragonal lattice no doubt partly explain the relatively high ordering temperature on a dilute fcc-like lattice. The other needed ingredient in obtaining the ordering is the intersite exchange interaction. The value of this coupling depends on the choice of U and as such we cannot directly predict the precise magnitude of this coupling. However, as seen from the energy differences, regardless of the choice of U the correct ground state is predicted, i.e. there is sufficient intersite exchange coupling. We find that the ferromagnetic ordered state is 31 meV above the ground state in the PBE + U calculation and 168 meV above the ground state without U.
Turning to the moment size, based on integration within the LAPW spheres (2.0 bohr for Os) we obtain moments that are strongly reduced from the nominal values due both to covalency and SOC. The total Os moment in the PBE calculation is 0.48 μB consisting of a spin moment of 0.77 μB and an orbital moment of −0.29 μB. In the probably more realistic PBE + U calculation we obtain 0.57 μB, from a spin moment of 1.07 μB and an orbital moment of −0.50 μB, in close agreement with the experimental result of 0.60(2) μB from NPD. We also find sizable moments on the O ions, which is a result of strong covalency. This can be seen in the density of states, shown for the ground state with PBE + U in Fig. 6. The O 2p bands extend from −7.4 eV to −1.1 eV relative to the valence band maximum. The region from the bottom to −5.1 eV comprise O 2p–Os eg σ bonding states and one can see very strong Os character in this region reflecting the covalency. The Os t2g states, which are the active orbitals here, extend from −0.5 eV to + 1.8 eV and are split by U to give a gap of 0.22 eV. The t2g band width is similar in the PBE calculation. The Os eg states extend from 4.3 eV to 6.3 eV. The large crystal field splitting is another reflection of very strong covalency.
A consequence of this covalency is the presence of sizable moments on the O sites. In the double perovskite structure each O has only one Os nearest neighbor. The O moments are parallel to those of the neighboring Os regardless of the treatment (PBE or PBE + U), the magnetic order or the inclusion of SOC. While Os6+ d orbitals can be regarded as largely inside a 2 bohr LAPW sphere, this is not the case for O2− p orbitals with a 1.55 bohr sphere. In order to estimate the O contribution, we turn to the ferromagnetic case. In the PBE + U calculation the total spin moment in the unit cell is 1.93 μB per formula unit, while the Os spin moment is only 1.14 μB. Thus ~40% of the spin moment is distributed over the six neighboring O ions. This moment will be active in specific heat and susceptibility data, but not in refinements of neutron diffraction data as the moment is spread across neighboring oxide ions and bonds. The O moments provide an explanation for the sizable intersite exchange. Although the double perovskite lattice can be regarded from a Zintl perspective as touching (OsO6)6− anions held together by interstitial Mg2+ and Sr2+ cations, the fact that the O atoms on the exterior of these contacting polyanions carry sizable moments provides a mechanism for the intersite exchange.
Having shown that SOC has a significant influence on the moment size observed by neutron scattering, we anticipate that SOC may have a major effect on the magnetic dynamics in Sr2MgOsO6. Confirmation of this can be found by examining the inelastic neutron scattering spectra of both Sr2MgOsO6 and Sr2ScOsO6 shown in Fig. 7. We compare Sr2MgOsO6 to Sr2ScOsO6 because Sr2ScOsO6 also shows high-TN type I AFM order with TN = 92 K, but has Os5+ 5d3 ions which are expected to show significantly reduced SOC due to a S = 3/2 state. In both materials we observe scattering emanating from the type I antiferromagnetic wavevector at Q ≈ 0.8 Å−1, Fig. 7. We identify the development of a spin gap in both materials at low temperatures—compare Fig. 7(a–c) for Sr2MgOsO6 and Fig. 7(b–d) for Sr2ScOsO6. The Sr2MgOsO6 spectrum at 6 K is remarkably similar to that of Sr2ScOsO6, in which the gap has been extensively characterized38, see Fig. 7(a,b), respectively. This suggests that the physical mechanisms controlling each system are more similar than previously predicted6, with SOC having influence in both materials.
The similar size of the gaps in Sr2MgOsO6 and Sr2ScOsO6 does, however, support a picture of SOC having stronger influence in Sr2MgOsO6. The microscopic mechanism by which SOC typically produces the spin gap is associated with either exchange anisotropy or single-ion anisotropy (or a combination)10,15,38,50. For either mechanism, for a fixed strength of SOC the magnitude of the gap observed by neutron scattering scales with the magnetic moment size. Therefore, as Sr2MgOsO6 has a smaller magnetic moment, the similarity of observed gap to that in Sr2ScOsO6 demonstrates that SOC is stronger in Sr2MgOsO6 resulting in a comparable gap.
Despite the similarity in the spectra at 6 K, above TN the intensity of the observed scattering is very different between the two compounds, see Fig. 7(c,d). To examine the temperature dependence further, we present in Fig. 8(a) the integrated intensity of the scattering for the range 0.7 < Q < 1 Å−1 and 3 < E < 12 meV. For both materials the integrated intensity in this region increases with temperature, because of both the modification of the scattering due to the closing of the gap and the Bose thermal population factor. The integrated intensity per Os ion for each of the samples is similar at low temperatures, with a slightly higher value for Sr2ScOsO6, as expected for the larger spin system. However, as the magnetic transition temperatures are approached the integrated intensities diverge dramatically. This difference in fluctuation intensity above TN is indicative of the level of frustration in each system - a strong signal implies strong correlations despite the absence of long range magnetic order.
(a) Comparison of the evolution of normalized per Os integrated neutron scattering intensity of Sr2MgOsO6 and Sr2ScOsO6 and (b) the temperature dependence of the scattering within the gap of Sr2MgOsO6, converted to χ″ as in ref. 10.
In Fig. 8(b) we present the temperature dependence of the scattering at very low energies, i.e. within the gap at low temperatures. The data is converted to χ″(T) for the fixed range 0.7 < Q < 1 Å−1 and 3 < E < 5 meV following the method described in ref. 10, in which the lowest temperature data set has been subtracted as a background and a Bose factor correction has been applied. This confirms that the reduction in the scattering at low energies is far beyond what would be expected due to thermal population, thereby confirming the opening of a gap at low temperature.
Discussion
A phase diagram depicting seven potential magnetic ground states, including three potential antiferromagnetic configurations, has been proposed for double perovskites with a single magnetic 5d2 cation6. Through a combination of reduced paramagnetic effective moment, an enhanced magnetic entropy from a spin-only scenario, a substantially reduced moment refined from neutron diffraction and a significant spin-excitation gap observed in neutron spectroscopy, we have unequivocally shown that SOC plays a major role in the magnetic behavior of the Os6+ 5d2 cation in Sr2MgOsO6. Despite this, we have shown that the ground state remains in the “AFM100” region of the phase diagram predicted in ref. 6, similar to many 4d3 and 5d3 double perovskites8,10,11,12,13. The predicted influence of SOC on the d2 state would inherently infer anisotropic interactions on the Os6+ ions, with the strong Os−O hybridization in Sr2MgOsO6 ensuring that the anisotropy has significant influence on the collective properties. We have confirmed anisotropy is present in Sr2MgOsO6 via observation of the spin gap in the magnetic excitation spectrum, Fig. 7a.
The moment observed by neutron diffraction of 0.60(2) μB is considerably reduced (70%) from the expected high-field spin-only value of 2 μB. Covalency has been shown to play a significant role in the reduction of the moment in the case of 4d3 and 5d3 transition metal oxides, resulting in 37–47% reductions of the 5d3 Os5+ 3 μB moment to 1.6 to 1.9 μB8,9,10,11,12,13 and we expect a similar effect in the Os6+ 5d2 case. Here, however, the magnetization and specific heat analyses strongly suggests that an orbital contribution is also important, consistent with the recent x-ray magnetic circular dichroism (XMCD) study of Os6+ in the related material Ca2CoOsO648. Our DFT results confirm that both SOC and covalency together cause the major reduction in the observed spin-moment, predicting a 47% reduction from covalency and a further 25% reduction from SOC, consistent with the total 70% reduction we observed experimentally. The result is a moment which is challenging to observe with standard neutron diffraction instrumentation, but via a high-flux, low-background experiment we were able to determine both the ground state and the moment size - it would be interesting to revisit Ba2CaOsO6 on a similar instrument in order to conclusively place the magnetic ground state among those known and predicted.
For 5d2 double perovskites the type I AFM structure was anticipated via theory including strong SOC in ref. 6. The predicted influence of SOC on the d2 state would inherently infer anisotropy, with the strong Os−O hybridization in Sr2MgOsO6 ensuring that the anisotropy has significant influence on the collective properties. Via inelastic neutron scattering we have indeed observed anisotropy in Sr2MgOsO6 via the spin gap.
It is interesting to investigate the comparison between 5d2 and 5d3 systems. Sr2MgOsO6 orders at a higher temperature than other double perovskites with a single magnetic ion28. While 4d3 and 5d3 double perovskites like Sr2ScOsO6 have larger magnetic moments, which should yield stronger interactions and higher ordering temperatures, they also have significantly larger frustration indices ranging from 4 to 148,10,11,12,13 in comparison to 2.5 in Sr2MgOsO6. The similarity of the QE-space dependence and the intensity of the excitation spectra of Sr2MgOsO6 and Sr2ScOsO6 at 6 K, shown in Fig. 7, indicates that similar interaction mechanisms are responsible for the collective properties in each. However, Fig. 8a shows that the intensity of fluctuations in Sr2ScOsO6 above TN is far greater than the intensity in Sr2MgOsO6 above TN. This implies strong correlations persist in Sr2ScOsO6 in the absence of long range magnetic order–a hallmark of frustration. While the tetragonal symmetry of Sr2MgOsO6 may play some role in reducing the geometric frustration, it is less distorted than monoclinic Sr2ScOsO6, which has angles that deviate more substantially from 180° 8.
The question, therefore, is why is the frustration relieved in Sr2MgOsO6 compared to Sr2ScOsO6? The strength of hybridization plays a major role in determining the strength of the interactions, but does not directly explain relief of frustration. Similarly, the unit cell volume and B/B′ site disorder affect interaction strengths and all these mechanisms likely contribute to differences in TNs amongst the AFM d2 DPs Sr2MgOsO6 (|Θ|/TN = 2.5) [this work], Ba2CaOsO6 (|Θ|/TN = 3.1)51 and Ca2CaOsO6 (|Θ|/TN = 3.0)52, but in all of these materials the frustration index is low compared to d3 DP Sr2ScOsO6. Our DFT results reveal that the SOC induced anisotropy leads to selection of the magnetic ground state, as also found experimentally for Sr2ScOsO638, therefore stronger anisotropy promotes a more robust ground state. Given the observation of the large spin gap in Sr2MgOsO6 and the multiple sources of evidence we have presented for stronger SOC in this d2 material, we conclude that SOC induced anisotropy is the dominant factor in the relieved frustration in Sr2MgOsO6.
This conclusion is supported by an earlier theoretical prediction that TN should vary with the strength of anisotropy in such systems, due to reduced competition between possible ground states53. The anisotropy term is proportional to the gap size (probed by INS) divided by the moment size (probed by neutron diffraction). Given that the gap is of the same magnitude in Sr2MgOsO6 and Sr2ScOsO6, Fig. 7, but the moment is less than half the size in Sr2MgOsO6, we conclude that the anisotropy is indeed much larger in Sr2MgOsO6. Therefore, the results presented demonstrate the relief of frustration via SOC induced anisotropy and represent the experimental demonstration of the evolution of TN with SOC.
Conclusion
The double perovskite osmate Sr2MgOsO6 has been synthesized and characterized by magnetometry, specific heat measurements and elastic and inelastic neutron scattering. The combined results demonstrate that spin-orbit coupling is essential to describe the magnetic properties of the system. The Os6+ moments order antiferromagnetically at 108 K in a type I configuration on the Os fcc sublattice as theoretically predicted6. For the first time in such a 5d2 material, refinements of neutron powder diffraction data yields a moment of 0.60(2) μB per Os cation which is significantly reduced due to the combined effects of SOC and covalency. Through comparison of inelastic neutron spectra, it is shown that SOC induced anisotropy has the ability to relieve frustration in d2 systems, relative to analogous d3 materials which have systematically higher frustration indices, promoting high magnetic transition temperatures.
Additional Information
How to cite this article: Morrow, R. et al. Spin-orbit coupling control of anisotropy, ground state and frustration in 5d2 Sr2MgOsO6. Sci. Rep. 6, 32462; doi: 10.1038/srep32462 (2016).
References
Ramirez, A. P. Strongly geometrically frustrated magnets. Annu. Rev. Mater. Sci. 24(1), 453–480 (1994).
Balents, L. Spin liquids in frustrated magnets. Nature 464(7286), 199–208 (2010).
Vasala, S. & Karppinen, M. A2B′B″O6 perovskites: a review. Prog. Solid State Chem. 43, 1–36 (2015).
Witczak-Krempa, W., Chen, G., Kim, Y. B. & Balents, L. Correlated quantum phenomena in the strong spin-orbit regime. Annu. Rev. Condens. Matter Phys. 5, 57–82 (2014).
Chen, G., Pereira, R. & Balents, L. Exotic phases induced by strong spin-orbit coupling in ordered double perovskites. Phys. Rev. B 82, 174440 (2010).
Chen, G. & Balents, L. Spin-orbit coupling in d2 ordered double perovskites. Phys. Rev. B 84, 094420 (2011).
Cook, A. M., Matern, S., Hickey, C., Aczel, A. A. & Paramekanti, A. Spin-orbit coupled jeff = 1/2 iridium moments on the geometrically frustrated fcc lattice. Phys. Rev. B 92, 020417(R) (2015).
Taylor, A. E. et al. Magnetic order and electronic structure of the 5d3 double perovskite Sr2ScOsO6 . Phys. Rev. B 91, 100406(R) (2015).
Tian, W. et al. High antiferromagnetic transition temperature of the honeycomb compound SrRu2O6 . Phys. Rev. B 92, 100404(R) (2015).
Kermarrec, E. et al. Frustrated fcc antiferromagnet Ba2YOsO6: structural characterization, magnetic properties and neutron scattering studies. Phys. Rev. B 91, 075133 (2015).
Paul, A. K. et al. Magnetically frustrated double perovskites: synthesis, structural properties and magnetic order of Sr2BOsO6 (B = Y, In, Sc). Z. Anorg. Allg. Chem. 641, 197 (2015).
Aczel, A. A. et al. Coupled Nd and B′ spin ordering in the double perovskites Nd2NaB′O6 (B′ = Ru, Os). Phys. Rev. B 88, 014413 (2013).
Aharen, T. et al. Magnetic properties of the S = 3/2 geometrically frustrated double perovskites La2LiRuO6 and Ba2YRuO6 . Phys. Rev. B 80, 134423 (2009).
Aczel, A. A. et al. Frustration by competing interactions in the highly distorted double perovskites La2NaB′O6 (B′ = Ru, Os). Phys. Rev. B 87, 014435 (2013).
Aczel, A. A. et al. Exotic magnetism on the quasi-fcc Lattices of the d3 double perovskites La2NaB′O6 (B′ = Ru, Os). Phys. Rev. Lett. 112, 117603 (2014).
Ishizuka, H. & Balents, L. Magnetism in S = 1/2 double perovskites with strong spin-orbit interactions. Phys. Rev. B 90, 184422 (2014).
Stitzer, K. E., Smith, M. D. & zur Loye, H. C. Crystal growth of Ba2MOsO6 (M = Li, Na) from reactive hydroxide fluxes. Solid State Sci. 4(3), 311–316 (2002).
Erickson, A. S. et al. Ferromagnetism in the mott insulator Ba2NaOsO6 . Phys. Rev. Lett. 99, 016404 (2007).
Wiebe, C. R. et al. Frustration-driven spin freezing in the S = 1/2 fcc perovskite Sr2MgReO6 . Phys. Rev. B 68, 134410 (2003).
Aharen, T. et al. Magnetic properties of the geometrically frustrated S = 1/2 antiferromagnets, La2LiMoO6 and Ba2YMoO6, with the B-site ordered double perovskite structure: Evidence for a collective spin-singlet ground state. Phys. Rev. B 81, 224409 (2010).
Carlo, J. P. et al. Triplet and in-gap magnetic states in the ground state of the quantum frustrated fcc antiferromagnet Ba2YMoO6 . Phys. Rev. B 84, 100404(R) (2011).
Cao, G. et al. Novel Magnetism of Ir5+ (5d4) Ions in the Double Perovskite Sr2YIrO6 . Phys. Rev. Lett. 112, 056402 (2014).
Ranjbar, B. et al. Structural and Magnetic Properties of the Iridium Double Perovskites Ba2–xSrxYIrO6 . Inorg. Chem. 54, 10468–10476 (2015).
Laguna-Marco, M. A. et al. Electronic structure, local magnetism and spin-orbit effects of Ir(IV)-, Ir(V)- and Ir(VI)-based compounds. Phys. Rev. B 91, 214433 (2015).
Dey, T. et al. Ba2YIrO6: a cubic double perovskite material with Ir5+ ions. Phys. Rev. B 93, 014434 (2016).
Sleight, A. W., Longo, J. & Ward, R. Compounds of osmium and rhenium with the ordered perovskite structure. Inorg. Chem. 1, 245 (1962).
Angelina, S. et al. Sr2MgOsO6: a frustrated Os6+ (5d2) double perovskite with strong antiferromagnetic interactions. Z. Anorg. Allg. Chem. 641(5), 769–771 (2015).
Yuan, Y. et al. High-Pressure Synthesis, Crystal Structures and Magnetic Properties of 5d Double-Perovskite Oxides Ca2MgOsO6 and Sr2MgOsO6 . Inorg. Chem. 54(7), 3422–3431 (2015).
Aczel, A. A. et al. Structural and magnetic properties of the 5d2 double perovksites Sr2BReO6 (B = Y, In). Phys. Rev. B 93, 214407 (2016).
Aharen, T. et al. Structure and magnetic properties of the S = 1 geometrically frustrated double perovskites La2LiReO6 and Ba2YReO6 . Phys. Rev. B 81, 064436 (2010).
Thompson, C. M. et al. Long-range magnetic order in the 5d2 double perovskite Ba2CaOsO6: comparison with spin-disordered Ba2YReO6 . J. Phys.: Condens. Matter 26, 306003 (2014).
Achary, S. N. et al. Anisotropic thermal expansion behavior in tetragonal Sr2MgWO6 . Mater. Res. Bulletin 41(3), 674 (2006).
Huq, A., Hodges, J. P., Gourdon, O. & Heroux, L. Powgen: A third-generation high-resolution high-throughput powder diffraction instrument at the Spallation Neutron Source. Z. Kristallogr. Proc. 1, 127–135 (2011).
Larson, A. C. & Von Dreele, R. B. GSAS Los Alamos National Laboratory Report LAUR 86–748 (1994).
Toby, B. H. EXPGUI, a graphical user interface for GSAS. J. Appl. Cryst. 34, 210–213 (2001).
Rodriguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 192, 55 (1993).
Kobayashi, K., Nagao, T. & Ito, M. Radial integrals for the magnetic form factor of 5d transition elements. Acta Cryst. A 67, 473 (2011).
Taylor, A. E. et al. Exchange interactions and the origin of the spin gap in Sr2ScOsO6 . Phys. Rev. B 93, 220408(R) (2016).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Singh, D. J. & Nordstrom, L. Planewaves, Pseudopotentials and the LAPW Method, 2nd ed., Springer, Berlin (2006).
Blaha, P., Schwarz, K., Madsen, G., Kvasnica, D. & Luitz, J. WIEN2k (Tech. Univ. Wien, Austria, 2001).
Morrow, R. et al. Independent ordering of two interpenetrating magnetic sublattices in the double perovskite Sr2CoOsO6 . J. Am. Chem. Soc. 135, 18824–18830 (2013).
Yan, B. et al. Lattice-Site-Specific Spin Dynamics in Double Perovskite Sr2CoOsO6 . Phys. Rev. Lett. 112, 147202 (2014).
Morrow, R., Freeland, J. W. & Woodward, P. M. Probing the Links between Structure and Magnetism in Sr2–xCaxFeOsO6 Double Perovskites. Inorg. Chem. 53, 7983–7992 (2014).
Paul, A. K. et al. Lattice Instability and Competing Spin Structures in the Double Perovskite Insulator Sr2FeOsO6 . Phys. Rev. Lett. 111, 167205 (2013).
Macquart, R. et al. Synthesis, structure and magnetic properties of Sr2NiOsO6 and Ca2NiOsO6: Two new osmium-containing double perovskites. Inorg. Chem. 44, 9676–9683 (2005).
Glazer, A. M. The classification of tilted octahedra in perovskites. Acta Cryst. B 28, 3384–3392 (1972).
Morrow, R. et al. Effects of chemical pressure on the magnetic ground states of the osmate double perovskites SrCaCoOsO6 and Ca2CoOsO6 . Phys. Rev. B 92, 094435 (2015).
Blum, C. G. F. et al. Flux growth and characterization of Sr2NiWO6 single crystals. J. Cryst. Growth 421, 39–44 (2015).
Carlo, J. P. et al. Spin gap and the nature of the 4d3 magnetic ground state in the frustrated fcc antiferromagnet Ba2YRuO6 . Phys. Rev. B 88, 024418 (2013).
Yamamura, K., Wakeshima, M. & Hinatsu, Y. Structural phase transition and magnetic properties of double perovskites Ba2CaMO6 (M = W, Re, Os). J. Solid State Chem. 179, 605–612 (2006).
Feng, H. L. et al. High-pressure crystal growth and electromagnetic properties of 5d double-perovskite Ca3OsO6 . J. Solid State Chem. 201, 186–190 (2013).
Kuz’min, E. V., Ovchinnikov, S. G. & Singh, D. J. Effect of frustrations on magnetism in the Ru double perovskite Sr2YRuO6 . Phys. Rev. B 68, 024409 (2003).
Acknowledgements
Support for this research was provided by the Center for Emergent Materials an NSF Materials Research Science and Engineering Center (DMR-1420451) and in the framework of the materials world network (Deutsche Forschungsgemeinschaft DFG project no. WU595/5-1 and National Science Foundation (DMR-1107637)). S. Wurmehl gratefully acknowledges funding by DFG in project WU 595/3-3 (Emmy Noether program) and by DFG in SFB 1143. Research using Oak Ridge National Laboratory’s Spallation Neutron Source and High Flux Isotope Reactor facilities was sponsored by the US Department of Energy, Office of Science, Basic Energy Sciences (BES), Scientific User Facilities Division. Work at the University of Missouri (DJS) was funded through the Department of Energy S3TEC Energy Frontier Research Center, award DE-SC0001299/DE-FG02-09ER46577. The authors would like to acknowledge S. Calder and M. D. Lumsden for helpful discussions and the authors also thankfully acknowledge Ashfia Huq for experimental assistance with POWGEN data collection. This manuscript has been authored by UT-Battelle, LLC under Contract No. DE-AC05-00OR22725 with the U.S. Department of Energy. The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. The Department of Energy will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
Author information
Authors and Affiliations
Contributions
R.M. synthesized all samples as well as conducted and analyzed XRD and POWGEN NPD data. R.M. and J.X. collected and analyzed magnetometry data. S.R., A.U.B.W., S.W. and B.B. were responsible for the specific heat data collection and analysis. A.E.T. and A.A.A. conducted the HB-1A NPD experiment. A.E.T., M.B.S. and A.I.K. measured the SEQUOIA INS data. A.E.T. analyzed the results from the HB-1A and SEQUOIA experiments. D.J.S. performed and analyzed DFT calculations. A.D.C. supervised neutron scattering activities and P.M.W. supervised synthesis and property characterization activities. R.M. and A.E.T. led the manuscript preparation and all coauthors contributed.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Morrow, R., Taylor, A., Singh, D. et al. Spin-orbit coupling control of anisotropy, ground state and frustration in 5d2 Sr2MgOsO6. Sci Rep 6, 32462 (2016). https://doi.org/10.1038/srep32462
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep32462
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.