Abstract
We have demonstrated the synthesis of γ-Fe2O3 nano-particles through a facile and novel calcination process in the air. There is no pH regulation, gas atmosphere, additive, centrifugation or other complicated procedures during the preparing process. A detailed formation process of the nano-particles is proposed and DMF as a polar solvent may slower the reaction process of calcination. The structures, morphologies and magnetic properties of γ-Fe2O3 nano-particles were investigated systematically and the pure γ-Fe2O3 nano-particles obtained at 200 °C display uniform morphology good magnetic property. The saturation magnetization of obtained pure γ-Fe2O3 is about 74 emu/g, which is comparable with bulk material (76 emu/g) and larger than other results. In addition, the photocatalytic activity for degradation of methylene blue is also studied, which shows proper photocatalytic activity.
Similar content being viewed by others
Introduction
Magnetic nanomaterials have attracted much interest gradually, since these materials have many potential applications such as information storage, color imaging, magnetic refrigeration, gas sensors, ferrofluids and photocatalysis etc.1,2,3,4,5,6,7 Recently, researches of magnetic nanomaterials are fascinating due to its powerful usefulness for a variety of biomedical8,9 and chemical engineering applications10. Among the magnetic nanomaterials, maghemite (γ-Fe2O3) is considered as one of the most desirable materials for various applications due to its inherent biocompatible nature and stability of oxidation as well as its good magnetic properties1,11,12,13. γ-Fe2O3 also exhibits modest photocatalytic activity and separability7,14. It can be used associated with ZnO or TiO2 to enhance the visible light adsorption and increase the electron/hole separation15,16. High magnetization of γ-Fe2O3 has potential applications for cleaning polluted water with the help of magnetic separation. As a result, magnetic properties, as an important symbolic characteristic of γ-Fe2O3 nano-particles, are noticeable for study.
Therefore, new approaches for the synthesis of γ-Fe2O3 particles as well as the investigation on their properties are of fundamental importance for the development of science and technology. The basic and conventional route of these methods or processes for the synthesis of γ-Fe2O3 nano-particles are controlling the oxidation of Fe3O417,18,19,20 and the total preparation process is shown as follows:

Various methods have been reported for the synthesis of γ-Fe2O3 nano-particles, such as coprecipitation12,21, hydrothermal22, microemulsions4,23, thermal decomposition24,25, aerosol pyrolysis26, sole-gel27, hydrosol chemical reaction20, combustion synthesis7, Massart method2,28, solvothermal method29,30, wet chemical method31,32, sonochemical route33, ultrasonic decomposition34, high-temperature solution reaction1, chemical reaction6 and other chemical process35,36,37,38,39. These synthesis processes or methods are the important routes for the synthesis of γ-Fe2O3 nano-particles and its composite materials. However, suitable pH value, long reaction time and definite additives or surfactants are indispensable for obtaining pureγ-Fe2O3 nano-particles with controllable morphology during those synthesis processes. Furthermore, centrifugation and purification are the vital factor for nano-particles with single products and good dispersity. Those preparation processes were totally complicated and cumbersome. Significantly, the saturation magnetization of γ-Fe2O3 nano-particles of the most methods mentioned above is still dissatisfied.
Herein, we report a unified approach for the synthesis of γ-Fe2O3 nano-particles in the air via a facile and novel calcination process. The method is different from the earlier approaches and there is no pH regulation, gas atmosphere, centrifugation and other supplementary reagents during the preparing process. Various characterizations were measured to perform the obtained pure γ-Fe2O3 nano-particles and γ-Fe2O3 nano-particles show a high saturation magnetization. In addition, the photocatalytic activity of γ-Fe2O3 nano-particles was also studied.
Methods
A unified method was provided using a simple and convenient route to assemble γ-Fe2O3 nano-particles. Ferric nitrate was dissolved in Dimethyl Formamide (DMF), the precursor was 0.6 mol/L and calcined at different temperature (100 °C~400 °C, the interval is 50 °C) for 2 hours in the air. The heating rate was 1 °C/min. The schematic diagram of experiment is shown in Fig. 1.
The crystal structure of samples were measured by X-ray diffraction (XRD, PANalytical X’Pert) equipped with Cu-Kα radiation (λ = 1.5406 Å). The morphology of all samples was observed by using field emission scanning electron microscopy (FESEM, Hitachi S-4800) and transmission electron microscopy (TEM, TecnaiTM G2 F30, FEI) equipped with an energy-dispersive spectrometer (EDS). The X-ray photoelectron spectroscopy (XPS, PHI-5702, Physical Electronics) were performed using a monochromatic Al-Kα irradiation and a charge neutralizer. All binding energies were referred to the C1 s peak at 284.6 eV of the surface adventitious carbon. The magnetic properties of the samples were measured by a vibrating sample magnetometer (VSM, Lakeshore 7304). The measurement process of surface areas and photocatalytic activity of the sample were shown in the Supporting Information (SI).
Results and Discussion
On the basis of the below experiments and results, a formation mechanism of the nano-particles in this work is proposed, which is outlined in Fig. 2. It is suggested that the following reactions occur during the calcination process:



As shown in Fig. 2(a), the precursor is composed of DMF and iron nitrate. The solvents begin to volatilize at the beginning of the heating process (Fig. 2b). DMF in precursors plays a role of solvent, which helps the diffusion and contaction of the reactant molecules in the course of volatilization. When calcination temperature (CT) is increased (Fig. 2c), DMF is exhausted gradually and the iron nitrite nonahydrate (Fe(NO3)3·9H2O) loses its water of hydration. The processes of Fig. 2(a–c) reveal that the surface morphology of sample seems not to be changed from their nature. The corresponding SEM picture and XRD pattern at 100 °C show bulk grains, suggesting an amorphous structure. The non-magnetic results (VSM loop) also confirm the amorphous structure. When the specimen is calcined at a moderate temperature (Fig. 2d), Fe(NO3)3 decomposes into γ-Fe2O3 and the nucleation process is observed (See SEM image and XRD spectrum at 150 °C). When CT then increases, a large area of γ-Fe2O3 nucleation is crystallized to the uniform nano-particles, which can be demonstrated by SEM image and XRD spectrum of 200 °C. Thereafter, when CT is about 220~400 °C (Fig. 2e), γ-Fe2O3 nano-particles begin to engulf its surrounded crystals and various sizes of α-Fe2O3 particles are formed. This can be supported by SEM images and XRD spectra of 250~300 °C. As CT is further improved, more γ-Fe2O3 particles are transformed to α-Fe2O3 and the morphologies and structures of samples are mainly composed of α-Fe2O3 particles (see SEM images and XRD spectra of 350~400 °C). Finally, when CT exceeds 500 °C, all γ-Fe2O3 is disappeared and plenty of α-Fe2O3 nano-particles are achieved. The result can be confirmed by the previous phase transformation studies2,38,40.
To sum up, combining all results of γ-Fe2O3 nano-granules, the detailed formation processes are discussed below. DMF is a commendable solvent when compared with water and DMF is associated well with the cation41,42. As a solvent, DMF could disperse the ions and coats each ion during the calcination process. As shown in Figure S1–S2 of SI, α-Fe2O3 particles are produced in the water but it cannot generate in DMF under the same experiment condition (1 °C/min, 200 °C). This indicates DMF may slower the reaction process of calcination, which restrains the transformation process of γ-Fe2O3 to α-Fe2O3. When CT or heating rates is increased, this restraint will be weakened.
Figure 3 shows SEM images of the samples of different CT. It can be seen that the morphologies change obviously with the increased CT. The sample of 100 °C (Fig. 3a) presents a number of disorderly bulk particles and the shape of particles changes to compact and well-regulated nano-particles at 150 °C (Fig. 3b). Especially when CT is 200 °C (Fig. 3c), a large quantity of uniform nano-particles with the size about 60 nm are achieved. However, when CT is further improved (250~400 °C of Fig. 3d–g), the particles display an increased grain growth (Fig. 3d,e). The particle size becomes nonuniform and strong piece of reunited particles are observed with the improvement of CT (Fig. 3f,g). These results are detailedly studied by the following techniques.
XRD data were used to determine the structural parameters of all the samples. Figure 4 shows XRD patterns of all the samples at different CT. It can be observed that when CT is 100 °C, the sample is not crystallized due to the slow evaporation of DMF. Thus, there are no peaks could be seen in XRD pattern. Afterwards, the diffraction spectra of samples reveal the good single γ-Fe2O3 phases (150 °C and 200 °C) with the cubic crystal system (JCPDS#39-1346) and all the diffraction peaks can be well indexed. It can be confirmed that the particles are γ-Fe2O3 rather than α-Fe2O3, due to the different XRD pattern of α-Fe2O3 (JCPDS#80-2377) and γ-Fe2O317,19. The average crystalline size estimated from Scherrer analysis is about 36 nm (150 °C) and 32 nm (200 °C) for γ-Fe2O3. In addition, the peak intensity of γ-Fe2O3 calcined at 200 °C is stronger than that of 150 °C, which indicates that the nano-particles present higher crystallization at 200 °C. However, the samples display α-Fe2O3 phases when CT exceeds to 250 °C. The emergence of additional impurity phase, i.e., α-Fe2O3, is more obvious with the enhanced temperature (300~400 °C). These XRD results indicate that the oxide of iron cannot be formed when CT is 100 °C. When CT is increased to 150~200 °C, γ-Fe2O3 nano-particles can be achieved and α-form is observed as CT exceeds 250 °C. It is well-known that γ-Fe2O3 can be further transformed into α-Fe2O3 at higher temperature33,40 and the phase transformation temperature in our research (250 °C) is similar to the previous literature2,40,43.
In order to distinguish the chemical composition of γ-Fe2O3 as opposed to Fe3O4, XPS measurement of pure γ-Fe2O3 nano-particles is further performed, which is displayed in Fig. 5. The full scanned XPS spectra of γ-Fe2O3 sample of 200 °C in the range of 0–1200 eV were shown in Fig. 5(a). Except for Fe 2p, O 1s and C 1s peaks in the spectra, no redundant peaks appear together in γ-Fe2O3 nano-particles and C element belongs to the carbon contaminants absorbed on the surface of the tested samples. In particular, Fe 2p3/2 spectra (Fig. 5b) exhibit two peaks at 710.6 and 724.1 eV, which are the characteristic peaks of the 3+ ion of γ-Fe2O33,19 and there is no signal or shoulder at smaller binding energies as would be expected for the presence of the Fe2+ ion (~708 eV)44,45. Furthermore, an additional peak at about 718.7 eV is the shakeup satellite peak, which also indicates the absence of the Fe2+ ion3,44. XPS results are consistent with the judgment of XRD spectra.
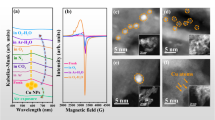
As a representative, the morphology and structure of the pure γ-Fe2O3 nano-particles (200 °C) are further characterized by TEM. As shown in the Fig. 6(a,b), the results indicate large and black areas of near-spherical γ-Fe2O3 nano-particles and the granules present a low dispersity, which may be due to the reuniting of the nano-particles. HRTEM characterizations show the lattice fringes of the obtained ferrites and the interfringe distance shown in Fig. 6(d,e) are 0.252 nm and 0.295 nm, which are correspond well to {311} and {220} planar spaces of γ-Fe2O3 nano-particles, respectively. Both the lattice fringes and SAED (Fig. 6c) clearly presents a group of atomic planes within each particle, revealing the highly crystalline nature of these nano-particles. Meanwhile, Fig. 6(f) gives EDX data of γ-Fe2O3 nano-particles and the appearance of Cu peaks results from copper net used in the experiment. The element ratio of Fe:O is calculated to be 17.9:30.8, which is very close to the stoichiometry of γ-Fe2O3, which further confirms that the composition and structure are coincident with the chemical formulation of γ-Fe2O3. The particle size distributions, obtained from TEM micrographs, are shown in Fig. 6(g) and the histograms show that the samples display uniform particle distributions. The mean particle sizes obtained from the Gaussian fit of the histograms are 27 ± 2 nm, which is comparable with XRD line width results. However, size distributions obtained from SEM are larger than that of TEM, which may be due to the superimposed crystal or the compact arrangement of particles and the shadow or astigmatism of the nano-particles could also cause the measurement error46. Some reports47 also show a relatively large particle size distribution.
On the base of good understanding of the microstructure and chemical phase of nano-particles, the room temperature magnetic performance of products is discussed below. As shown in Fig. 7(a), the non-crystalline sample (100 °C) has no magnetism. When CT is 150 °C, i.e. γ-Fe2O3 appears, the nano-particles emerge strong magnetism immediately and the saturation magnetization (Ms) is about 61 emu/g. Particularly, when CT reaches to 200 °C, Ms increases to 74 emu/g, which is comparable with bulk γ-Fe2O3 sample (Ms = 76 emu/g)34,48,49 but larger than other γ-Fe2O3 nanoparticles11,30,33,50. The higher values of Ms are due to the better crystals of the nanoparticles, consistent with both the XRD and HRTEM data. A recent work also shows that the higher crystallinity is benefited to enhanced Ms of the sample51. Typical comparative results of variable quantity of Ms are shown in Fig. 7(d). It can be seen Ms of this work is higher than other nanoparticles, but less when compared with nanoplate and nanocluster. However, when CT exceeds 250 °C, Ms is decreased monotonously. That is because that the presence of non-magnetic α-Fe2O3 leads to the relative reduction of magnetic γ-Fe2O3 and the magnetic moments total quality drop. When CT is further increased to 250~400 °C, the impurity α-Fe2O3 appears more obviously (which can be confirmed by XRD results) and Ms is reduced gradually. Furthermore, low temperature hysteresis loops of γ-Fe2O3 (200 °C) have also been carried out at 80 K, 180 K and 300 K, which are shown in Fig. 7(c). The low temperature is realized by the liquid nitrogen. As expected, Ms and coercivity are also enhanced at low temperature. It is well-known that the coercivity and Ms will increase when the temperature decreases34,50.
(a,b) Room temperature M-H loops for γ-Fe2O3 nano-particles and its corresponding Ms of different CT; (c) Temperature dependence of hysteresis loops measured at 80 K, 180 K and 300 K of the pure γ-Fe2O3 nano-particles of 200 °C. (d) Comparisons of our work and other typical results of the particles size and Ms of γ-Fe2O3 nano-particles. The samples have been marked with circle (nano-particles) and pentagon (others), the values of the size represents the diameter of the samples.
The degradation of methylene blue (MB) was performed as a model reaction to investigate the photocatalytic activity of the sample, which was shown in Figure S3. The results reveal that γ-Fe2O3 has a little adsorption ability of MB with proper photocatalytic activity, which can degrade 16% MB dye in 60 min under UV irradiation. The photocatalytic activity of this work is comparable with the previous report52. As a result, the results provide the fabrication of Fe-based nanocomposites as proper performance photocatalysts and high magnetization of γ-Fe2O3 also has potential in addressing environmental protection issues. The Brunauer-Emmett-Teller (BET) surface areas of γ-Fe2O3 nano-particles were measured to be 24.8 m2/g, which is smaller than the mesoporous nano-particles24,29,30. This suggests that the content of mesopores in the sample is considerably low. BJH average pore diameters calculated from the adsorption branch of the isotherms is 4.7 nm for the γ-Fe2O3 nano-particles and the corresponding total pore volume is 0.04 cm3/g.
Various above investigations have demonstrated that a number of uniform and smooth γ-Fe2O3 nano-particles are obtained using calcination process in the air. Significantly, some typical methods or processes as the comparative results are discussed and the comparative data are presented in Table 1. Except for the iron source of the preparing process, Table 1 detailedly shows the experiment parameters of solvent, additive or surfactant, pH, reaction time, centrifugation and other parts of various methods. These methods or processes are not limited to the literatures we provided. As a result, although the dispersity and size of nano-particles in our research are not good with some of the previous reports, this technique only needs one solvent during the preparing process and other additional processes are omitted. The mehtod realizes a simple, rapid and convenient route for assembling γ-Fe2O3 nano-particles when compared with others.
Conclusions
We reported a unified approach for the synthesis of γ-Fe2O3 nano-particles via a facile and novel calcination process in the air. The process is no pH regulation, gas atmosphere, additive, centrifugation or other procedure during the experiment. The obtained pure γ-Fe2O3 nano-particles at 200 °C display good uniformity and α-Fe2O3 will be observed when CT exceeds 250 °C. As a result, DMF is a commendable solvent when compared with water, which could well disperse the ions and coats each ion during the calcination process. DMF may slower the reaction process of calcination, which restrains the transformation process of γ-Fe2O3 to α-Fe2O3. The saturation magnetization pure γ-Fe2O3 is about 74 emu/g, which is comparable with bulk material. In addition, the photocatalytic activity of the obtained nano-particles for the degradation of methylene blue shows proper photocatalytic properties.
Additional Information
How to cite this article: Cao, D. et al. High saturation magnetization of γ-Fe2O3 nano-particles by a facile one-step synthesis approach. Sci. Rep. 6, 32360; doi: 10.1038/srep32360 (2016).
References
Capone, S. et al. Fe3O4/γ-Fe2O3 Nanoparticle Multilayers Deposited by the Langmuir–Blodgett Technique for Gas Sensors Application. Langmuir 30, 1190–1197 (2014).
Vichery, C., Maurin, I., Bonville, P., Boilot, J.-P. & Gacoin, T. Influence of Protected Annealing on the Magnetic Properties of γ-Fe2O3 Nanoparticles. J. Phys. Chem. C 116, 16311–16318 (2012).
Rao, P. M. & Zheng, X. Unique Magnetic Properties of Single Crystal γ-Fe2O3 Nanowires Synthesized by Flame Vapor Deposition. Nano Lett. 11, 2390–2395 (2011).
Zhu, H.-Y., Jiang, R., Xiao, L. & Li, W. A novel magnetically separable gamma-Fe2O3/crosslinked chitosan adsorbent: Preparation, characterization and adsorption application for removal of hazardous azo dye. J. Hazard. Mater. 179, 251–257 (2010).
Kostiainen, M. A. et al. Hierarchical Self-Assembly and Optical Disassembly for Controlled Switching of Magnetoferritin Nanoparticle Magnetism. ACS Nano 5, 6394–6402 (2011).
Park, J. et al. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 3, 891–895 (2004).
Apte, S., Naik, S., Sonawane, R., Kale, B. & Baeg, J. Synthesis of Nanosize‐Necked Structure α‐and γ‐Fe2O3 and its Photocatalytic Activity. J. Am. Ceram. Soc. 90, 412–414 (2007).
Nemati, Z., Alonso, J., Khurshid, H., Phan, M. H. & Srikanth, H. Core/shell iron/iron oxide nanoparticles: are they promising for magnetic hyperthermia? RSC Adv. 6, 38697–38702 (2016).
Tseng, P., Judy, J. W. & Di Carlo, D. Magnetic nanoparticle-mediated massively parallel mechanical modulation of single-cell behavior. Nat. Meth. 9, 1113–1119 (2012).
Zywietz, U., Evlyukhin, A. B., Reinhardt, C. & Chichkov, B. N. Laser printing of silicon nanoparticles with resonant optical electric and magnetic responses. Nat. Commun. 5, 1–7 (2014).
Lu, J., Jiao, X., Chen, D. & Li, W. Solvothermal Synthesis and Characterization of Fe3O4 and γ-Fe2O3 Nanoplates. J. Phys. Chem. C 113, 4012–4017 (2009).
Singh, K., Ohlan, A., Kotnala, R. K., Bakhshi, A. K. & Dhawan, S. K. Dielectric and magnetic properties of conducting ferromagnetic composite of polyaniline with gamma-Fe2O3 nanoparticles. Mater. Chem. Phys. 112, 651–658 (2008).
Hu, J. S., Zhong, L. S., Song, W. G. & Wan, L. J. Synthesis of hierarchically structured metal oxides and their application in heavy metal ion removal. Adv. Mater. 20, 2977–2982 (2008).
Wang, C. et al. Magnetic (γ-Fe2O3@SiO2)n@TiO2 Functional Hybrid Nanoparticles with Actived Photocatalytic Ability. J. Phys. Chem. C 113, 4008–4011 (2009).
Zhou, W. et al. Mesoporous TiO2/α-Fe2O3: Bifunctional Composites for Effective Elimination of Arsenite Contamination through Simultaneous Photocatalytic Oxidation and Adsorption. J. Phys. Chem. C 112, 19584–19589 (2008).
Achouri, F. et al. Aqueous synthesis and enhanced photocatalytic activity of ZnO/Fe2O3 heterostructures. J. Phys. Chem. Solids 75, 1081–1087 (2014).
Zhong, L. S. et al. Self‐Assembled 3D flowerlike iron oxide nanostructures and their application in water treatment. Adv. Mater. 18, 2426–2431 (2006).
Ni, Y., Ge, X., Zhang, Z. & Ye, Q. Fabrication and Characterization of the Plate-Shaped γ-Fe2O3 Nanocrystals. Chem. Mater. 14, 1048–1052 (2002).
Zhang, Y. et al. Two-in-One Strategy for Effective Enrichment of Phosphopeptides Using Magnetic Mesoporous γ-Fe2O3 Nanocrystal Clusters. ACS Appl. Mater. Interfaces 5, 614–621 (2013).
Kang, Y. S., Risbud, S., Rabolt, J. F. & Stroeve, P. Synthesis and characterization of nanometer-size Fe3O4 and gamma-Fe2O3 particles. Chem. Mater. 8, 2209–2211 (1996).
Lin, S., Lu, D. & Liu, Z. Removal of arsenic contaminants with magnetic γ-Fe2O3 nanoparticles. Chem. Eng. J 211–212, 46–52 (2012).
Chen, D. & Xu, R. Hydrothermal Synthesis and Characterization of Nanocrystalline γ-Fe2O3 Particles. J. Solid State Chem. 137, 185–190 (1998).
Hyeon, T., Lee, S. S., Park, J., Chung, Y. & Na, H. B. Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection process. J. Am. Chem. Soc. 123, 12798–12801 (2001).
Asuha, S., Zhao, Y. M., Zhao, S. & Deligeer, W. Synthesis of mesoporous maghemite with high surface area and its adsorptive properties. Solid State Sci. 14, 833–839 (2012).
Asuha, S., Zhao, S., Wu, H. Y., Song, L. & Tegus, O. One step synthesis of maghemite nanoparticles by direct thermal decomposition of Fe-urea complex and their properties. J. Alloys Compd. 472, L23–L25 (2009).
Basak, S., Rane, K. S. & Biswas, P. Hydrazine-Assisted, Low-Temperature Aerosol Pyrolysis Method to Synthesize γ-Fe2O3 . Chem. Mater. 20, 4906–4914 (2008).
Silva, M. F. et al. Optimization of maghemite-loaded PLGA nanospheres for biomedical applications. Eur. J. Pharm. Sci. 49, 343–351 (2013).
El Mendili, Y., Bardeau, J.-F., Randrianantoandro, N., Grasset, F. & Greneche, J.-M. Insights into the Mechanism Related to the Phase Transition from gamma-Fe2O3 to alpha-Fe2O3 Nanoparticles Induced by Thermal Treatment and Laser Irradiation. J. Phys. Chem. C 116, 23785–23792 (2012).
Xu, J.-S. & Zhu, Y.-J. Monodisperse Fe3O4 and γ-Fe2O3 magnetic mesoporous microspheres as anode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 4, 4752–4757 (2012).
Ianos, R., Taculescu, E.-A., Pacurariu, C. & Niznansky, D. gamma-Fe2O3 nanoparticles prepared by combustion synthesis, followed by chemical oxidation of residual carbon with H2O2 . Mater. Chem. Phys. 148, 705–711 (2014).
Pour, Z. S. & Ghaemy, M. Fabrication and characterization of superparamagnetic nanocomposites based on epoxy resin and surface-modified gamma-Fe2O3 by epoxide functionalization. J. Mater. Sci. 49, 4191–4201 (2014).
Darezereshki, E., Ranjbar, M. & Bakhtiari, F. One-step synthesis of maghemite (γ-Fe2O3) nano-particles by wet chemical method. J. Alloys Compd. 502, 257–260 (2010).
Lai, J. et al. Doping γ-Fe2O3 Nanoparticles with Mn(III) Suppresses the Transition to the α-Fe2O3 Structure. J. Am. Chem. Soc. 125, 11470–11471 (2003).
Shafi, K. V. P. M. et al. Magnetic Enhancement of γ-Fe2O3 Nanoparticles by Sonochemical Coating. Chem. Mater. 14, 1778–1787 (2002).
Boal, A. K., Das, K., Gray, M. & Rotello, V. M. Monolayer Exchange Chemistry of γ-Fe2O3 Nanoparticles. Chem. Mater. 14, 2628–2636 (2002).
Andersen, H. L., Jensen, K. M., Tyrsted, C., Bøjesen, E. D. & Christensen, M. Size and Size Distribution Control of γ-Fe2O3 Nanocrystallites: An in situ Study. Cryst. Growth Des. 14, 1307–1313 (2014).
Sakurai, S., Namai, A., Hashimoto, K. & Ohkoshi, S.-i. First Observation of Phase Transformation of All Four Fe2O3 Phases (gamma ->epsilon ->beta ->alpha-Phase). J. Am. Chem. Soc. 131, 18299–18303 (2009).
Laurent, S. et al. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations and biological applications. Chem. Rev. 108, 2064–2110 (2008).
Synek, P. et al. Plasmachemical synthesis of maghemite nanoparticles in atmospheric pressure microwave torch. Mater. Lett. 65, 982–984 (2011).
Yen, F. S., Chen, W. C., Yang, J. M. & Hong, C. T. Crystallite Size Variations of Nanosized Fe2O3 Powders during γ- to α-Phase Transformation. Nano Lett. 2, 245–252 (2002).
Pastoriza-Santos, I. & Liz-Marzán, L. M. N,N-Dimethylformamide as a Reaction Medium for Metal Nanoparticle Synthesis. Adv. Funct. Mater. 19, 679–688 (2009).
Muzart, J. N. N-Dimethylformamide: much more than a solvent. Tetrahedron 65, 8313–8323 (2009).
Gallagher, K. Mechanism of oxidation of magnetite to gamma-Fe2O3 . Nature 217, 1118–1121 (1968).
Fujii, T. et al. In situ XPS analysis of various iron oxide films grown by NO2-assisted molecular-beam epitaxy. Phys. Rev. B 59, 3195–3202 (2001).
Gao, Y. & Chambers, S. A. Heteroepitaxial growth of alpha-Fe2O3, gamma-Fe2O3 and Fe3O4 thin films by oxygen-plasma-assisted molecular beam epitaxy. J. Cryst. Growth 174, 446–454 (1997).
Sadhu, A. & Bhattacharyya, S. Enhanced Low-Field Magnetoresistance in La0.71Sr0.29MnO3 Nanoparticles Synthesized by the Nonaqueous Sol–Gel Route. Chem. Mater. 26, 1702–1710 (2014).
Guo, P. et al. Facile Synthesis of ZnFe2O4 Nanoparticles with Tunable Magnetic and Sensing Properties. Langmuir 29, 8997–9003 (2013).
Wang, J., Ma, Y. & Watanabe, K. Magnetic-Field-Induced Synthesis of Magnetic γ-Fe2O3 Nanotubes. Chem. Mater. 20, 20–22 (2008).
Berkowitz, A. E., Schuele, W. J. & Flanders, P. J. Influence of Crystallite Size on the Magnetic Properties of Acicular γ-Fe2O3 Particles. J. Appl. Phys. 39, 1261–1263 (1968).
Park, K. et al. Structural and Magnetic Properties of Gold and Silica Doubly Coated γ-Fe2O3 Nanoparticles. J. Phys. Chem. C 111, 18512–18519 (2007).
Das, R. et al. Tunable High Aspect Ratio Iron Oxide Nanorods for Enhanced Hyperthermia. J. Phys. Chem. C 120, 10086–10093 (2016).
Chen, F. & Zhao, J. Preparation and photocatalytic properties of a novel kind of loaded photocatalyst of TiO2/SiO2/γ‐Fe2O3 . Catal. Lett. 58, 246–247 (1999).
Acknowledgements
This work is supported by National Basic Research Program of China (Grant No. 2012CB933101), National Science Fund of China (11574121, 51371092), PCSIRT (Grant No. IRT1251).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. D.C. prepared the samples and wrote the main manuscript text; D.C., H.L., L.P., P.J., J.L., X.W. and X.C. processed data; D.C., H.L., J.W. and Q.L. discussed the synthesis mechanism and magnetic properties of samples. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Cao, D., Li, H., Pan, L. et al. High saturation magnetization of γ-Fe2O3 nano-particles by a facile one-step synthesis approach. Sci Rep 6, 32360 (2016). https://doi.org/10.1038/srep32360
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep32360
This article is cited by
-
High-throughput electrochemical strategy for synthesis of iron-based nanostructures for electrocatalytic water splitting
Journal of Materials Science (2024)
-
Microwave assisted green synthesis of γ-Fe2O3 nanoparticles and their application for photodegradation of ternary dye mixture
Journal of Materials Science: Materials in Electronics (2023)
-
Nano-sized hematite-assembled carbon spheres for effectively adsorbing paracetamol in water: Important role of iron
Korean Journal of Chemical Engineering (2023)
-
Adsorptive removal of anionic azo dye by Al3+-modified magnetic biochar obtained from low pyrolysis temperatures of chitosan
Environmental Science and Pollution Research (2023)
-
Ultralight, hierarchical metal–organic framework derivative/graphene hybrid aerogel for electromagnetic wave absorption
Advanced Composites and Hybrid Materials (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.