Abstract
The aim of this present study was to investigate if overweight individuals exhibit signs of vascular dysfunction associated with a high risk for cardiovascular disease (CVD). One hundred lean and 100 overweight participants were recruited for the present study. Retinal microvascular function was assessed using the Dynamic Retinal Vessel Analyser (DVA), and systemic macrovascular function by means of flow-mediated dilation (FMD). Investigations also included body composition, carotid intimal-media thickness (c-IMT), ambulatory blood pressure monitoring (BP), fasting plasma glucose, triglycerides (TG), cholesterol levels (HDL-C and LDL-C), and plasma von Willebrand factor (vWF). Overweight individuals presented with higher right and left c-IMT (p = 0.005 and p = 0.002, respectively), average 24-h BP values (all p < 0.001), plasma glucose (p = 0.008), TG (p = 0.003), TG: HDL-C ratio (p = 0.010), and vWF levels (p = 0.004). Moreover, overweight individuals showed lower retinal arterial microvascular dilation (p = 0.039) and baseline-corrected flicker (bFR) responses (p = 0.022), as well as, prolonged dilation reaction time (RT, p = 0.047). These observations emphasise the importance of vascular screening and consideration of preventive interventions to decrease vascular risk in all individuals with adiposity above normal range.
Similar content being viewed by others
Introduction
Excess adipose tissue induces several metabolic changes, including dyslipidaemia, elevated blood pressure (BP), oxidative stress, and increased inflammation, thereby, contributing towards macro- and micro-vascular endothelial dysfunctions, increased arterial stiffness, and atherosclerosis1,2. These changes are traditionally associated with obesity and are not often reported in those with lower levels of adiposity that are classed as overweight. Indeed, it is usually presumed that the cardiovascular risk is not heightened in those with a body mass index (BMI) between 25–29.9 kg/m23. Nevertheless, it is now thought that overweight individuals actually have an approximately 60% higher risk of cardiovascular disease (CVD) mortality when compared to age-matched lean controls4. Indeed, it has been demonstrated that even from early life, an increased BMI is associated, amonfg other changes, with low levels of nitric oxide (NO), the key contributor to normal vascular function5. Overweight children and young adults show evidence of macrovascular endothelial dysfunction and increased arterial stiffness6, which is reversible by increasing NO bioavailability and reduction of abdominal visceral fat7. It seems, therefore, that any excess adiposity would result in a higher chance of future cardiovascular risk.
It is well known that vascular endothelial dysfunction represents an early marker of atherosclerosis and precedes the clinical manifestations of CVD8. In addition, endothelial dysfunction affects the microcirculation much earlier than the macrocirculation in the course of vascular disease development9. This observation is extremely important. Due to the large total surface area of the microvessels, even an early activation of the endothelium at this level could have a large impact on bodily vascular health10. Consequently, detection of functional abnormalities at the microvascular level could serve as a better predictor for future risk of disease than testing macrovasculature.
Dynamic retinal vessel analysis (DVA) represents a non-invasive technique that assesses retinal microvascular motions in response to flickering light and by using this technique a diminished microvascular function has already been demonstrated in obese individuals11. However, no such studies have been carried out in overweight adults that are generally perceived as being at some risk for CVD but not at a level that would demand preventative care. Therefore, the aim of this study was to investigate micro- and macro-vascular function parameters and their relationship with established markers for future cardiovascular risk in otherwise healthy, overweight individuals compared to lean, age- and sex-matched controls.
Methods
Written informed consent was obtained from all participants and ethical approval was granted from the local (Aston University) and NHS ethical committees (COREC West Midlands, UK). This study was designed and conducted in accordance with the tenets of the Declaration of Helsinki.
Study Population
The study population consisted of healthy, normotensive White-European participants aged 30–55 years that were screened and recruited from the Health Clinics at Aston University, Birmingham, UK. Weight classifications were determined according to WHO definitions, whereby normal weight was classed as a BMI of 18.5–24.9 kg/m2 and overweight as a BMI of 25–29.9 kg/m2.
Subjects were excluded if they were classified as obese (BMI > 30 kg/m2), as well as, if they had a positive diagnosis of, or were taking medication for, cardio- or cerebro-vascular disease, coronary artery disease, heart failure, arrhythmia, stroke, transient ischaemic attacks, peripheral vascular disease, diabetes, hypertension or severe dyslipidaemia (defined as plasma TG > 6.00 mmol/L or cholesterol levels > 7.00 mmol/L). Smokers (including previous history of smoking) and all subjects taking vasoactive substances including dietary/vitamin/anti-oxidant supplementation, bronchodilators were also excluded from the present study. In addition, a standard 75 g oral glucose tolerance test (OGTT) was performed according to the WHO protocols on all participants a week prior to all other measurements and only normoglycaemic participants were asked to return for subsequent tests.
Furthermore, subjects were excluded if they had a refractive error of more than ±3 Dioptre Spherical (DS – depicting the convergent or divergent refractive power of the eye, i.e. hyperopia or myopia) and more than ±1 Dioptre Cylindrical (DC – depicts the degree of astigmatism, i.e. the curvature of the cornea and or lens of the eye) equivalent to minimise magnification error brought on by high refractive errors, and intraocular pressure (IOP) higher than 24 mmHg, cataract or any other media opacities, as well as, if they had a history of intraocular surgery or any form of retinal or neuro-ophthalmic disease affecting the ocular vascular system.
According to an already established procedure, when examining endothelial function, female participants were asked to fill in a validated menstrual cycle questionnaire and their investigations were carried out during the first week of the menstrual cycle (follicular phase)12.
Investigations
All participants were screened for cardiovascular disease by a physician. Prior to the date of the study, participants were asked to fast and refrain from caffeine, alcohol, chocolate, and carbonated drinks, and to not exercise for 12 hours before the measurements were taken.
General measurements
Anthropometric measures including height and weight were recorded using standard procedures. Body composition was measured using bioelectrical impedance (Biostat 220, Biospace, UK) to determine BMI, percentage body fat (PBF), waist-to-hip ratio (WHR), total fat mass, and fat free mass.
Blood sampling and analyses
A qualified phlebotomist carried out the blood sampling, and all samples were obtained during the morning of the study, between 9:00 and 10:00 AM. Fasting plasma glucose, TG, total and HDL-C were measured using standard routine laboratory techniques using the Reflotron Desktop Analyser (Roche Diagnostics, UK). The TG/HDL cholesterol ratio13 and Total/HDL cholesterol ratio alongside Framingham score as a means of cardiovascular risk were also determined from the above values14.
Laboratory-validated protocols15 for in-house ELISA-testing were adopted to carry out plasma sampling of von-Willebrand Factor (vWF) to investigate possible signs of endothelial damage in overweight individuals.
Micro- and macro-vascular studies
Ambulatory Blood Pressure
Ambulatory BP was measured using a 24-hour computer-operated ambulatory BP and electrocardiography (ECG) monitor (Cardiotens-01, Meditech Ltd., Hungary) for each subject. Measurements were performed in ambulatory conditions and programmed to measure BP oscillometrically every 15 minutes during the subject’s active period and every 30 minutes during the passive period. The 24-hour data was later downloaded and systolic BP (SBP), diastolic BP (DBP) and mean BP [MBP ≈ ( X DBP) + ( X SBP)], were calculated using the Medibase software (Meditech Version 1.42).
Intima-media thickness
Real-time intima-media thickness measurements for both carotid arteries were obtained for all participants through analysis of ultrasound images taken from the right and left common carotid arteries at the neck using a high-resolution B-mode ultrasound system (Acuson Sequoia, 5 MHz linear transducer, Siemens, USA) according to an already published protocol16.
Retinal vascular function
Retinal vessel reactivity was measured with the dynamic retinal vessel analyser (DVA, IMEDOS GmbH, Jena, Germany) using an already established and recommended protocol17. All measurements were performed in one randomly selected eye for each subject between 8:00 and 11:00 AM, and in a quiet, temperature-controlled room (22 °C)17,18. Retinal vessel reactivity, in the form of arterial and venous reaction time (RT) and maximum diameter (MD), were determined using our newly defined method of Sequential and Diameter Response Analysis (SDRA), which is described elsewhere19.
The following retinal vessel reactivity and time course parameters (using one-second averaged data for 3 flicker cycles) were calculated (Fig. 1): baseline-diameter fluctuation (BDF), as the difference between maximum and minimum baseline vessel diameter; the maximum diameter (MD), as the maximal vessel dilation in response to flicker light stimulation expressed as a percentage from baseline; the MD reaction time (MDRT), as the time taken (seconds) to reach the maximum vessel diameter during the 20-second flicker exposure; the maximum constriction (MC), as the percentage to baseline minimal vessel diameter within 30 seconds of the recovery period; and the maximum constriction reaction time (MCRT), as the time taken (seconds) to reach maximal vessel constriction. In addition, the dilation amplitude (DA), as the difference between maximal dilation and constriction responses, and the baseline-corrected flicker response (bFR), which accounted for the BDF were also determined19.
The copyright of the original image rests with the Association for Research in Vision and Ophthalmology19.
Brachial artery Flow Mediated Dilation (FMD)
Brachial artery flow-mediated dilation (FMD) was measured using high-resolution ultrasonography, with a 7 mm 8 MHz linear-array (Siemens; Acuson Sequoia, UK). Briefly, vessel diameter was continuously measured, from the anterior to the posterior interface between the media and adventitia, using specialised wall-detection and artificial neural networking software (VIA® Software, UK), and the data was recorded on a personal computer. The procedure was carried out according to a previously validated and universally accepted protocol comprising of a 2-minute baseline recording, followed by 5 minutes of occlusion, and a 2-minute recovery period. Following a subsequent 10-minute re-acclimatisation period, an exogenous NO donor (300 mcg sublingual glyceryl trinitrate – GTN tablet) was administered. The FMD% was determined as absolute change from baseline using real-time raw data, as well as, GTN-induced changes20.
Numerous factors have been known to contribute to the variability of FMD, mainly equipment- and operator-related, as well as, physiological influences. To minimise equipment- and operator-related influences, a standardised protocol was adhered to, and all analyses were performed off-line by a blinded observer. To minimise physiological influences known to exert profound effects on endothelial function, i.e., diurnal variation and dietary substances, we further standardised environmental factors (temperature, noise, and excluded the use of vasoactive substances.
Statistical analysis
All analyses were performed using Statistica® software (StatSoft Inc.; Version 9, USA). Prior to any analysis, all data was tested for normal distribution and thus a suitable test was adopted. Differences in mean values for each of the measured biochemical, demographic, and anthropometric variables were compared by independent samples t-test for continuous variables. A multivariate analysis was performed to test the influence of BMI, BP, and circulating markers on the measured variables. Comparison of retinal vessel reactivity for each of the 3 repeated flicker periods was made by Friedman analysis of variance (ANOVA) following within-group analysis as the data was not normally distributed even following log transformation. Differences in brachial artery and FMD responses (also not normally distributed) were assessed by Kruskal-Wallis testing. Differences between groups in retinal and systemic vascular function were computed by analysis of variance (ANOVA) or covariance (ANCOVA) where appropriate. A p value of <0.05 was considered statistically significant for the retinal and brachial parameters, and a stricter criteria was adopted for within-group and multiple comparisons of the biochemical and demographic variables (p ≤ 0.01 to account for multiple comparisons and thereby minimise bias towards Type II errors).
Results
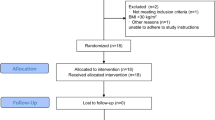
In the present study, an initial 226 individuals were screened for inclusion, following which 26 were excluded as they were diagnosed with impaired glucose tolerance and referred back to appropriate outpatient management. A final 200 subjects were recruited, based on the diagnosis criteria, and allocated to either the normal (n = 100; men = 48, women = 52) or overweight (n = 100; men = 51, women = 49) group. As evident in Table 1, there were no statistically significant differences between the two groups with regards to age and gender distribution of the participants (p > 0.05).
The anthropometric characteristics of the normal and overweight group are also presented in Table 1. Compared to lean individuals, overweight subjects showed significantly greater right and left c-IMT (p = 0.005 and p = 0.002, respectively), 24-hr BP values (SBP, DBP, MBP, all p < 0.001), fasting glucose (p = 0.008) and TG (p = 0.003) levels, alongside higher TG: HDL-C ratio (p = 0.010). In addition, overweight individuals exhibited higher body composition indices (weight, BMI, WHR, PBF, fat mass, all p < 0.001) and plasma levels of vWF (p = 0.01) than the age-matched controls.
Microvascular function
A multiple regression analysis found that weight and BMI had a positive effect on retinal arterial MD (b = 0.203; p = 0.017, and b = 0.190; p = 0.025, respectively), but not on the other measured microvascular parameters.
The measured retinal parameters presented in Table 2 indicate that, after correcting for all influential variables (BMI, BP), overweight individuals exhibited a reduced arterial MD (p = 0.039) and bFR (p = 0.022), as well as, an increased MDRT (p = 0.047) compared to the lean subjects. There were no differences in retinal venous reaction parameters between the two study groups (all p > 0.05).
Macrovascular function
The brachial artery results presented in Table 3 show that after correcting for influential variables (BMI, BP), the baseline vessel diameter was larger in the overweight individuals when compared to the age- and gender matched lean controls (p = 0.018). All the other measured brachial parameters were, however, comparable between the two groups (p > 0.05).
Discussion
This study demonstrates, for the first time, that otherwise healthy overweight individuals present with signs of microvascular functional impairment, as well as, increased circulatory plasma markers of endothelial dysfunction when compared to lean individuals. Although presenting with a larger baseline diameter, the brachial artery function was not affected in our overweight individuals cohort.
Consistent with previous research21, the present study demonstrates that overweight subjects show elevated BP values, higher TG, cholesterol, and fasting blood glucose. All these factors either separate or in conjunction, are well known contributors to cardiovascular risk and pre-diabetes in both adults and children7,21. In particular, triglyceride-rich lipoprotein remnants may cause endothelial damage22. In addition, the TG: HDL-C ratio was also increased in our overweight subjects and this parameter is a known independent determinant of increased arterial stiffness23. An elevated TG: HDL-C ratio has also been shown to have a strong correlation with fasting plasma insulin concentrations and when used alongside TG levels, represents a good surrogate marker for the presence of insulin resistance (IR)24. It is known that abnormal IR plays an important role in the development of endothelial dysfunction through a reduced production of NO, as well as, by accelerating the onset of atherosclerosis25. Indeed, our overweight cohort demonstrated signs of pre-clinical atherosclerotic changes as measured by c-IMT and a modified baseline diameter of the brachiar artery, as measured by FMD; some of these parameters are well-known predictors for future cardiovascular complications26,27,28. However, we have not observed any correlation between c-IMT and FMD changes and this could further support previous assumptions that in younger patients without overt disease, but with certain risk for cardiovascular pathologies, there is no direct relationship between functional (FMD) and structural(c-ITM) vascular modifications29. Indeed, vascular dysfunction is known to precede the occurrence of atherosclerosis and signals the risk for future vascular disease30. Our overweight group also demonstrated abnormal vWF levels, possibly showing pathophysiological changes of the vascular endothelium. Nevertheless, neither of the measured macrovascular parameters correlated with this circulatory parameter.
An important finding of the present study was the detection of an impaired retinal vessel function in response to flickering stimulus (a reduced bFR and arterial MD and an increased MDRT) in overweight but not in lean individuals. It has been previously reported that a high BMI can be associated with narrower retinal arteriolar calibres31,32. In addition, complex alterations of the retinal microvascular function were already observed in obese individuals of various age groups11,33. Nevertheless, to our knowledge, this is the first report showing functional retinal vascular changes in overweight but not yet obese individuals, therefore, opening a new opportunity for early detection and prevention of cardiovascular complication in this type of population. Although the precise mechanisms behind our results need further elucidation we can, however, formulate some hypotheses. For instance, there is evidence to suggest that the changes observed in our measured MD and MDRT parameters could be the result of either early atherosclerosis, increased arterial stiffness, or reduced NO bioavailability to peripheral tissues34. Indeed, a high BMI has been found to be associated with reduced NO5 and increased levels of vasoconstrictor substances such as endothelin-1 (ET-1) and angiotensin-II (Ang-II)35. Therefore, our observation showing that both weight and BMI had significant influences on retinal arterial MD parameters could possibly be explained by the detrimental effect of BMI on the vascular dilatory capacity of the retinal microvessels through the above mentioned mechanisms. Although neither NO or vasoconstrictor substances were measured in the present study, one could hypothesize that abnormal levels of vasodilatory or vasoconstrictory mediators are, at least in part, responsible for our findings. More research is necessary to confirm our hypothesis.
Despite recording a modified baseline diameter of the brachial artery, we could not demonstrate any signs of macrovascular dysfunction in our overweight cohort. The literature reports conflicting results on the effect of overweight abd obesity on macrovascular function6,36,37,38. It is possible that the vascular effect of the adipokines secrated by the perivascular adipose tissue (PVAT) at this level varies according to the degree of obesity39. Moreover, in physiological conditions, the PVAT can also have a vasorelaxant effect with a protective role40. This effect could have been responsible for our finding of an increased brachial artery baseline diameter in our overweight individuals it which PVAT was not yet dysfunctional enough to exert detrimental effects on the macrovascular function41. Nevertheless, the macro- and microvascular beds’ functions are governed by different physiological mechanisms. Moreover, it is the microcirculation that may be a better target for the assessment of risk as it is the microvessels that are considered to be the first to be affected in the course of vascular disease9. Therefore, our finding of abnormal vascular function at the retinal microvascular level in overweight individuals without any overt diseases is relevant and points towards the need for a much earlier screening for vascular detrimental effects that any excess adiposity could have.
As discussed above, microvascular dysfunction can be trigerred in areas with PVAT by subclinical inflammation mediated via adipokines/cytokines and infiltrating macropahges42. Nevertheless, these molecules can also trigger remote inflammatory effects withy potential vascular changes as far as the retinal circulation, usually devoided of PVAT, therefore, resulting in abnormal vascular function at this level43. Further research is necessary to elucidate this point.
Dietary and social factors (such as ingestion of high saturated fats or lack of exercise) have not been assessed in the present study and this could therefore be perceived as a limitation. Moreover, larger studies are needed to investigate the effect of other possible confounding factors such as insulin sensitivity and menopause status. Nevertheless, even in the presence of these limitations, the results of our study show that in overweight but not yet obese individuals, signs of microvascular dysfunction exist alongside with abnormal circulatory levels of cardiovascular risk markers. We suggest that the assessment of retinal microcirculation may be a good, easy and non-invasive alternative for assessing vascular dysfunction associated with excess adiposity44. Therefore, the clinical relevance of screening for early CVD risk in overweight individuals using this method is supported by these preliminary, but promising results.
Additional Information
How to cite this article: Patel, S. R. et al. Overweight status is associated with extensive signs of microvascular dysfunction and cardiovascular risk. Sci. Rep. 6, 32282; doi: 10.1038/srep32282 (2016).
References
Klein, S. et al. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation 110, 2952–2967 (2004).
Nanchahal, K., Morris, J. N., Sullivan, L. M. & Wilson, P. W. Coronary heart disease risk in men and the epidemic of overweight and obesity. International journal of obesity 29, 317–323 (2005).
Wannamethee, S. G., Shaper, A. G. & Walker, M. Overweight and obesity and weight change in middle aged men: impact on cardiovascular disease and diabetes. Journal of epidemiology and community health 59, 134–139 (2005).
Katzmarzyk, P. T. et al. Body mass index and risk of cardiovascular disease, cancer and all-cause mortality. Canadian journal of public health = Revue canadienne de sante publique 103, 147–151 (2012).
Stapleton, P. A., James, M. E., Goodwill, A. G. & Frisbee, J. C. Obesity and vascular dysfunction. Pathophysiology: the official journal of the International Society for Pathophysiology/ISP 15, 79–89 (2008).
Pierce, G. L. et al. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension 52, 72–79 (2008).
Kelly, A. S. et al. Inflammation, insulin, and endothelial function in overweight children and adolescents: the role of exercise. The Journal of pediatrics 145, 731–736 (2004).
Yoshinaga, K., Manabe, O. & Tamaki, N. Assessment of coronary endothelial function using PET. Journal of nuclear cardiology: official publication of the American Society of Nuclear Cardiology 18, 486–500 (2011).
Gates, P. E., Strain, W. D. & Shore, A. C. Human endothelial function and microvascular ageing. Experimental physiology 94, 311–316 (2009).
Krentz, A. J., Clough, G. & Byrne, C. D. Vascular disease in the metabolic syndrome: do we need to target the microcirculation to treat large vessel disease? Journal of vascular research 46, 515–526 (2009).
Kotliar, K. E. et al. Dynamic retinal vessel response to flicker in obesity: A methodological approach. Microvascular research 81, 123–128 (2011).
Moens, A. L., Goovaerts, I., Claeys, M. J. & Vrints, C. J. Flow-mediated vasodilation: a diagnostic instrument, or an experimental tool? Chest 127, 2254–2263 (2005).
Bertoluci, M. C., Quadros, A. S., Sarmento-Leite, R. & Schaan, B. D. Insulin resistance and triglyceride/HDLc index are associated with coronary artery disease. Diabetology & metabolic syndrome 2, 11 (2010).
Tewari, S. et al. Premature coronary artery disease in North India: an angiography study of 1971 patients. Indian heart journal 57, 311–318 (2005).
Blann, A. D. von Willebrand factor antigen: a radial immunodiffusion method evaluated and compared with an ELISA method. Medical laboratory sciences 49, 141–143 (1992).
Salonen, R., Haapanen, A. & Salonen, J. T. Measurement of intima-media thickness of common carotid arteries with high-resolution B-mode ultrasonography: inter- and intra-observer variability. Ultrasound in medicine & biology 17, 225–230 (1991).
Garhofer, G. et al. Use of the retinal vessel analyzer in ocular blood flow research. Acta ophthalmologica 88, 717–722 (2010).
Nagel, E., Vilser, W. & Lanzl, I. Age, blood pressure, and vessel diameter as factors influencing the arterial retinal flicker response. Investigative ophthalmology & visual science 45, 1486–1492 (2004).
Heitmar, R., Blann, A. D., Cubbidge, R. P., Lip, G. Y. & Gherghel, D. Continuous retinal vessel diameter measurements: the future in retinal vessel assessment? Investigative ophthalmology & visual science 51, 5833–5839 (2010).
Corretti, M. C. et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. Journal of the American College of Cardiology 39, 257–265 (2002).
Iannuzzi, A. et al. Increased carotid intima-media thickness and stiffness in obese children. Diabetes care 27, 2506–2508 (2004).
Hennig, B., Chung, B. H., Watkins, B. A. & Alvarado, A. Disruption of endothelial barrier function by lipolytic remnants of triglyceride-rich lipoproteins. Atherosclerosis 95, 235–247 (1992).
Urbina, E. M. et al. Triglyceride to HDL-C ratio and increased arterial stiffness in children, adolescents, and young adults. Pediatrics 131, e1082–1090 (2013).
Li, C., Ford, E. S., Meng, Y. X., Mokdad, A. H. & Reaven, G. M. Does the association of the triglyceride to high-density lipoprotein cholesterol ratio with fasting serum insulin differ by race/ethnicity? Cardiovascular diabetology 7, 4 (2008).
Rask-Madsen, C. et al. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell metabolism 11, 379–389 (2010).
Simon, A., Gariepy, J., Chironi, G., Megnien, J. L. & Levenson, J. Intima-media thickness: a new tool for diagnosis and treatment of cardiovascular risk. Journal of hypertension 20, 159–169 (2002).
Elias-Smale, S. E. et al. Common carotid intima-media thickness in cardiovascular risk stratification of older people: the Rotterdam Study. European journal of preventive cardiology 19, 698–705 (2012).
Benjamin, E. J. et al. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation 109, 613–619 (2004).
Palmieri, V., Russo, C., Pezzullo, S., Di Minno, M. N. & Celentano, A. Relation of flow-mediated dilation to global arterial load: impact of hypertension and additional cardiovascular risk factors. International journal of cardiology 152, 225–230 (2011).
Gupta, A. K. et al. Endothelial Dysfunction: An Early Cardiovascular Risk Marker in Asymptomatic Obese Individuals with Prediabetes. British journal of medicine and medical research 2, 413–423 (2012).
De Boer, M. P. et al. Microvascular dysfunction: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Microcirculation (New York, N.Y.: 1994) 19, 5–18 (2012).
Boillot, A. et al. Obesity and the microvasculature: a systematic review and meta-analysis. PloS one 8, e52708 (2013).
Schiel, R. et al. Retinal vessel response to flicker light in children and adolescents with type 1 diabetes mellitus and overweight or obesity. Diabetes Res Clin Pract 83, 358–364 (2009).
Patel, S. R. et al. Abnormal retinal vascular function and lipid levels in a sample of healthy UK South Asians. The British journal of ophthalmology 95, 1573–1576 (2011).
Stepp, D. W. et al. Obesity augments vasoconstrictor reactivity to angiotensin II in the renal circulation of the Zucker rat. American journal of physiology. Heart and circulatory physiology 293, H2537–2542 (2007).
Arkin, J. M. et al. Relation of cumulative weight burden to vascular endothelial dysfunction in obesity. Am J Cardiol 101, 98–101 (2008).
Faintuch, J., Marques, P. C., Bortolotto, L. A., Faintuch, J. J. & Cecconello, I. Systemic inflammation and cardiovascular risk factors: are morbidly obese subjects different? Obes Surg 18, 854–862 (2008).
Biasucci, L. M. et al. Paradoxical preservation of vascular function in severe obesity. Am J Med 123, 727–734 (2010).
Temiz, A. et al. Epicardial adipose tissue thickness, flow-mediated dilatation of the brachial artery, and carotid intima-media thickness: Associations in rheumatoid arthritis patients. Herz 40 Suppl 3, 217–224 (2015).
Fernandez-Alfonso, M. S. et al. Mechanisms of perivascular adipose tissue dysfunction in obesity. International journal of endocrinology 2013, 402053 (2013).
Ozen, G., Daci, A., Norel, X. & Topal, G. Human perivascular adipose tissue dysfunction as a cause of vascular disease: Focus on vascular tone and wall remodeling. Eur J Pharmacol 766, 16–24 (2015).
Koller, A., Balasko, M. & Bagi, Z. Endothelial regulation of coronary microcirculation in health and cardiometabolic diseases. Internal and emergency medicine 8 Suppl 1, S51–54 (2013).
Bagi, Z., Broskova, Z. & Feher, A. Obesity and coronary microvascular disease - implications for adipose tissue-mediated remote inflammatory response. Current vascular pharmacology 12, 453–461 (2014).
Czernichow, S. et al. Microvascular dysfunction in healthy insulin-sensitive overweight individuals. Journal of hypertension 28, 325–332 (2010).
Acknowledgements
The authors would like to thank Mrs Tahsin Shaikh, Mrs Bhopinder Sagoo and Miss Robeela Begum for their support and work in recruiting participants for this study.
Author information
Authors and Affiliations
Contributions
S.R.P. study design, data collection and analysis and manuscript writing-up. S.B. study design and specialist input. S.K. data analysis and manuscript writing up. D.G. project initiation, principal investigator, study design, overall project supervision, data analysis, manuscript writing-up and corrections. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Patel, S., Bellary, S., Karimzad, S. et al. Overweight status is associated with extensive signs of microvascular dysfunction and cardiovascular risk. Sci Rep 6, 32282 (2016). https://doi.org/10.1038/srep32282
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep32282
This article is cited by
-
Pilot study of optical coherence tomography angiography-derived microvascular metrics in hands and feet of healthy and diabetic people
Scientific Reports (2023)
-
Normative data and standard operating procedures for static and dynamic retinal vessel analysis as biomarker for cardiovascular risk
Scientific Reports (2021)
-
The role of the adipocytokines vaspin and visfatin in vascular endothelial function and insulin resistance in obese children
BMC Endocrine Disorders (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.