Abstract
Sulforaphane, a naturally occurring compound found in cruciferous vegetables, has been shown to be neuroprotective in several neurological disorders. In this study, we sought to investigate the potential protective effects and associated molecular mechanisms of sulforaphane in an in vivo Parkinson’s disease (PD) model, based on rotenone-mediated neurotoxicity. Our results showed that sulforaphane inhibited rotenone-induced locomotor activity deficiency and dopaminergic neuronal loss. Additionally, sulforaphane treatment inhibited the rotenone-induced reactive oxygen species production, malondialdehyde (MDA) accumulation and resulted in an increased level of total glutathione and reduced glutathione (GSH): oxidized glutathione (GSSG) in the brain. Western blot analysis illustrated that sulforaphane increased the expression of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), heme oxygenase-1 (HO-1) and NAD(P)H quinone oxidoreductase (NQO1), the latter two of which are anti-oxidative enzymes. Moreover, sulforaphane treatment significantly attenuated rotenone-inhibited mTOR-mediated p70S6K and 4E-BP1 signalling pathway, as well as neuronal apoptosis. In addition, sulforaphane rescued rotenone-inhibited autophagy, as detected by LC3-II. Collectively, these findings demonstrated that sulforaphane exert neuroprotective effect involving Nrf2-dependent reductions in oxidative stress, mTOR-dependent inhibition of neuronal apoptosis and the restoration of normal autophagy. Sulforaphane appears to be a promising compound with neuroprotective properties that may play an important role in preventing PD.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a chronically progressive disease, that most commonly manifests between the fifth and seventh decade with resting tremor on one or both sides, bradykinesia, rigidity and abnormal postural reflexes1. It affects approximately 6 million people worldwide, with the prevalence expected to double over the next two decades in parallel with an increasingly aged population2. Its pathological hallmarks include loss of dopaminergic neurons in specific brain regions and the presence of proteinaceous cytoplasmic inclusions known as Lewy bodies in the remaining dopaminergic neurons3. Although PD has been studied intensively for almost two centuries, since James Parkinson gave the first description of the disease in 18174, the aetiology and pathogenesis of the disease remain unknown. Currently, there is no cure for PD and additional effective treatments for this devastating disease are urgently needed.
Oxidative stress is widely considered to be central to many forms of PD5. Postmortem studies have revealed evidence for oxidative injury in PD brains, demonstrating lipid peroxidation and oxidative modification of proteins and DNA6. The oxidative stress results from an imbalance of pro-oxidant/antioxidant homeostasis that leads to an abnormal production of reactive oxygen species (ROS). The brain is particularly vulnerable to oxidative stress because of its high oxygen consumption, high content of oxidizable polyunsaturated fatty acids and low antioxidant defence capacities; this is especially true for aging brains7. NF-E2-related factor-2 (Nrf2) is a transcription factor that regulates the expression of antioxidant and phase II detoxifying enzymes. One critical enzyme induced by Nrf2 is NAD(P)H quinone oxidoreductase-1 (NQO1), which detoxifies protein-bound quinone and maintains alpha-tocopherol and coenzyme Q10 in their reduced (antioxidant) states. Another Nrf2-regulated protein is the stress responsive inducible enzyme heme oxygenase 1 (HO-1), which metabolizes prooxidant heme to the antioxidant pigment biliverdin, ferrous iron and carbon monoxide8. The Nrf2 pathway responds to ROS by activating the transcription of phase II detoxification enzymes. Thus, activation of Nrf2 appears to be an attractive approach for treating or mitigating PD9.
Mammalian target of rapamycin (mTOR), a serine/threonine (Ser/Thr) protein kinase, is a central controller of cell proliferation, growth and survival. mTOR regulates phosphorylation of p70 S6 kinase (p70S6K) and eukaryotic initiation factor 4E (eIF4E) binding protein 1 (4E-BP1), the two best-characterized downstream effector molecules of mTOR10. Studies have demonstrated that loss of mTOR activity may be detrimental during PD, as mTOR dysfunction has been shown in animal models of PD to lead to the death/apoptosis of dopaminergic neurons11,12. Depression of mTOR signalling by siRNA interference inhibits phosphorylation of both p70S6K and 4E-BP1, which leads to apoptosis13. However, dopaminergic neuronal death/apoptosis can be blocked with agents that increase mTOR activity14. Recently, our group has demonstrated that PD mimetics including 6-hydroxydopamine (6-OHDA), N-methyl-4-phenylpridine (MPP+) and rotenone suppressed mTOR signalling, accelerated cleavage of caspase-3 and PARP in PC12 cells and primary neurons and subsequently triggered neuronal apoptosis. However, restoration of mTOR, p70S6K, or 4E-BP1 was remarkably potent in protecting against neuronal apoptosis caused by these PD mimetics10,15.
Studies have now confirmed that dysregulation of autophagy results in the accumulation of pathologic proteins and/or damaged organelles, as is commonly observed in PD16. In particular, autophagy is deregulated in PD brains17. Many studies have consistently shown that rapamycin is neuroprotective against PD through autophagy enhancement18,19. Microtubule-associated protein light chain 3 (LC3) is one of the most commonly used biomarkers for autophagy process. LC3-I is cytosolic, whereas LC3-II is membrane bound. During autophagy, LC3-I is lipidated by an ubiquitin-like system to generate LC3-II20. Thus, the amount of LC3-II is correlated with the autophagy level and is a reliable marker for monitoring autophagy-related processes21.
Sulforaphane, a naturally occurring compound found in cruciferous vegetables (i.e. broccoli, cabbage, watercress and Brussels sprouts), has been reported to have potential as a therapeutic agent or preventative in PD. For example, sulforaphane was shown in vitro to be cytoprotective against damage evoked by 6-OHDA, MPP+ and rotenone22,23,24,25,26. Using in vivo neurodegeneration models, Jazwa et al. demonstrated that sulforaphane, in an Nrf2-dependent manner, protected against nigral dopaminergic cell death, astrogliosis and microgliosis in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) subacute mouse model of PD27. Morroni et al. reported that sulforaphane exerted a neuroprotective effect in a 6-OHDA-induced acute mouse model and attributed it to the ability of sulforaphane to enhance glutathione levels and its dependent enzymes, to up-regulate Nrf2 and to modulate the ERK1/2 pathway in the brain28. Surprisingly, no data regarding the effectiveness of sulforaphane in a rotenone-induced chronic PD model have been reported.
Rotenone, derived from the roots or leaves of certain plant species, is a pesticide that is widely used to kill insects and nuisance fish in lakes around the world5. Rotenone is extremely lipophilic and thus freely crosses cellular membranes independently of any transporters (unlike MPP+); it easily crosses the blood-brain barrier and enters into the brain rapidly29. Unlike 6-OHDA-based models, generating the chronic oral rotenone model in mice does not require stereotaxic neurosurgical intervention. In fact, accumulated data show rotenone exposure to humans would most commonly involve ingestion1. Interestingly, chronic oral administration of rotenone to rodents in vivo induces many key features of PD, including selectively and progressive nigrostriatal DA neurodegeneration, abnormal behaviours (not seen in the MPTP mouse model) and formation of Lewy bodies (not seen in either the MPTP or 6-OHDA mouse model)30. In this study, by using chronic oral rotenone treatment as an in vivo PD model, we explored the effectiveness of sulforaphane against rotenone-induced motor deficits and dopaminergic neuron loss; we also determined whether the possible underlying mechanisms of its effects were associated with Nrf2-regulated anti-oxidative, mTOR-mediated anti-apoptotic, and/or autophagy systems.
Material and Methods
Chemicals and reagents
Rotenone, sulforaphane, dimethyl sulfoxide (DMSO), 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) and 2′,7′-dichlorofluorescein (DCF) were purchased from Sigma (St. Louis, MO, USA); CMC: (Nacalai Tesque, Kyoto, Japan); Protease and phosphatase inhibitor mini tables (Thermo Scientific, USA); RIPA lysis buffer was purchased from Beyotime, China. The following antibodies were used: phospho-S6K (Thr389), 4E-BP1, Cleaved-caspase-3, LC3A/B (Cell Signalling Technology, Beverly, MA, USA); phospho-mTOR (Ser2448), mTOR (all from sigma); p70S6K; Nrf2, heme oxygenase-1 (HO-1); NAD(P)H dehydrogenase, quinone 1/(NQO1) and monoclonal mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Tyrosine hydroxylase (TH) (Millipore, Billerica, MA). Dulbecco’s Modified Eagle’s Medium (GIBCO, Gaithersburg, MD, USA). Other chemicals were provided by local commercial sources and were of analytical grade quality.
Animals care
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Jiangsu Province Institute of Traditional Chinese Medicine and written up following the ARRIVE guidelines. Experiments were performed in strict accordance with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals. Male C57BL/6 mice (7–8 weeks old, 25–28 g) were purchased from the Institute of Laboratory Animal Science at the Chinese Academy of Medical Sciences (Beijing, China) and housed in a specific pathogen-free facility maintained at 20–22 °C, with cycles of 12 h light-dark and food and water ad libitum. All mice were initially allowed to adapt to the experimental animal laboratory for 1 week.
Cell culture
SH-SY5Y cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum and cultured at 37 °C under humidified 5% CO2 atmosphere.
Experimental design
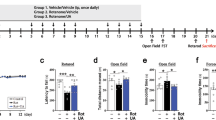
Sulforaphane stock solution was prepared using dimethyl sulfoxide (DMSO) and stored at −20 °C. Immediately prior to experimentation, the stock solution was diluted with sterile saline to a concentration of 50 mg/kg (final DMSO concentration 1%)31,32. The pharmacological effects in vivo were examined using the following groups (n = 10 per group): (a) vehicle control, 0.5% carboxymethyl cellulose sodium salt (CMC) plus saline (containing 1% DMSO) administered once daily; (b) sulforaphane (50 mg/kg dissolved in saline (containing 1% DMSO)) injected intraperitoneally every other day; (c) rotenone (30 mg/kg rotenone suspended in 0.5% CMC) administered orally daily for 60 consecutive days; and (d) rotenone + sulforaphane, rotenone treatment orally alone or followed by sulforaphane injected intraperitoneally every other day. Body weight was measured every five days for the 60-day duration, while the mice were undergoing behaviour tests. Fig. 1b shows the experimental schedules.
Sulforaphane ameliorated rotenone-induced motor function deficits in mice.
(a) Chemical structure of sulforaphane. Animals were submitted to the combined protocol of sulforaphane and rotenone (b). Body weights were measured every five days for the full 60 days (c). Animals underwent behavioural tests on day 60. Spontaneous activity in the cylinder (d), adhesive removal test (e) and challenging beam (f) were measured. Each value is presented as mean ± SEM, n = 3. *P < 0.05, **P < 0.01 vs. control group; #P < 0.05, ##P < 0.01 rotenone group vs. rotenone + sulforaphane treatment group.
Behavioural Tests
The tests below have been validated in PD mouse models33. In each test, mice were tested individually and the equipment cleaned between each animal (except when tests utilised home cages).
Spontaneous activity tests
A mouse was placed in a clear glass cylinder and recorded with a video camera for 3 min in a quiet area, to prevent distractions and accidentally causing freezing behaviour. The videos were used to manually count the number of rears per mouse. A rear is defined as a vertical movement with both forelimbs above shoulder level and making contact with the cylinder wall, followed by removal of both forelimbs from the cylinder wall and contact with the floor34. All mice performed 3 trials.
Adhesive Removal
A mouse was kept in its home cage, but the feeder bin removed to allow the mouse to habituate to the testing environment for 1 hour. Then the mouse was gently restrained and a pair of small forceps used to place one adhesive label onto its snout. The mouse was released and a stopwatch used to record the time until the attempts to contact the label with its forepaws and the time to remove the label (contact and removal time, respectively). The mice were given a maximum of two minutes. All mice performed 3 trials.
Challenging Beam Traversals
This evaluates sensorimotor integration by requiring a mouse to balance steadily on a narrow beam. The test-apparatus consists of four 25 cm beam sections of different widths placed on top of inverted mouse cages. A mouse was trained by being positioned on the widest section of the beam and then trained to step forward on the beam to return to its home cage. The training was performed two consecutive days, until the mouse traversed the full beam on its own without correction. On the test day, a video camera was placed nearby to record the beam travel times. All mice performed 3 trials.
Tissue Preparation
After the behaviour tests, the mouse was euthanized by decapitation. The whole brain, cerebral cortex and striatum were dissected carefully on dry ice as described previously35. The whole brain regions were immediately homogenised for ROS, malondialdehyde (MDA), total glutathione and reduced glutathione (GSH): oxidized glutathione (GSSG) ratio assays. Some of the dissected cerebral cortex and striatum were quickly immersion-perfused with 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) for 2 days and then transferred to 15% sucrose solution in PBS containing 0.1% sodium azide at 4 °C. The other non-perfused brain regions were snap frozen in −80 °C 2-methylbutane and stored in a −80 °C freezer until further processing for protein extraction.
ROS Assay
ROS generation was measured based on the oxidation of 2′7′-dichlorodihydrofluorescein diacetate (DCFH-DA) to 2′7′-dichlorodihydrofluorescein diacetate (DCF)36. Briefly, the brain homogenate was diluted 1:20 (v:v) with ice-cold Locke’s buffer (154 mM NaCcl, 5.6 mM KCl, 3.6 mM NaHCO3, 2.0 mM CaCl2, 10 mM D-glucose and 5 mM HEPES, pH 7.4) to obtain a concentration of 10 mg tissue/ml. The reaction mixture (1 ml) containing Locke’s buffer (pH 7.4), 0.2 ml homogenate and 10 μl of DCFH-DA (5 mM) was incubated for 45 min at room temperature and the fluorescence intensity of DCF was recorded by excitation at 485 nm and emission at 535 nm using a SynergyTM 2 Multi-function Microplate Reader (Bio-Tek Instruments Inc., Winooski, Vermont, USA). Background fluorescence was corrected by the inclusion of parallel blanks. Total protein concentration was determined using the bicinchoninic acid assay kit (Pierce, Rockford, IL, USA). ROS formation was quantified from a DCF-standard curve and data are expressed as pmol DCF formed/min/mg protein.
MDA Assay
This assay assesses lipid peroxidation29. Brain tissue was homogenised with 1:10 (w/v) PBS on ice, then centrifuged at 3,000 rpm for 15 min at 4 °C in an Eppendorf centrifuge and the supernatant was collected. Protein concentration in the supernatant was determined using the bicinchoninic acid assay kit (Pierce, Rockford, IL, USA). The concentration of MDA was measured and expressed as nmol/mg protein using an MDA assay kit (Jiancheng Institute of Biotechnology, Nanjing, China), according to the manufacturer’s protocols.
Total Glutathione Levels and GSH:GSSG Ratio
Total glutathione and GSSG were determined using a GSH and GSSG Assay Kit (Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer’s protocol. Absorbance was measured at 412 nm using a Biotek microplate reader. GSH was calculated as the difference between total glutathione and GSSG and GSH:GSSG ratio was determined. Protein concentration was determined using the bicinchoninic acid assay kit (Pierce, Rockford, IL, USA). Total glutathione content is expressed as nmol/mg protein.
Immunohistochemistry Analysis
The cryoprotected cerebral cortex and striatum were cut into 30 μM slices on a cryostat as previously described27. Sections were rinsed in Tris-buffered saline (TBS). Tissue peroxidase was inactivated by incubating in 3% hydrogen peroxide in TBS for 30 min. After three washes in PBS, sections were incubated for 3 h in blocking solution (3% bovine serum albumin (BSA), 0.3% Triton X-100 in TBS) and then for 24 h at 4 °C with mouse monoclonal antibodies against tyrosine hydroxylase (TH) diluted in PBS containing 1% BSA. Sections were then rinsed in PBS and incubated with the appropriate biotinylated secondary antibody at 1/1000 dilution for 1 h at room temperature. Sections were subsequently developed using the avidin-biotin peroxidase complex system (ABC kit, BOSTER Ltd. Wuhan) followed by counterstaining with haematoxylin. The images of stained sections were taken with a Nikon Eclipse Ti-S microscope equipped with a digital camera. Densitometric quantification of TH-positive dopaminergic neurons in the striatum and fibres in the cerebral cortex was performed using Image-Pro Plus 6.0 software (Media Cybernetics Inc., Newburyport, MA).
Western Blotting Assay
For protein electrophoresis and immunoblotting, the cerebral cortex and striatum were homogenized on ice with lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China). Protease and phosphatase inhibitor min-tablets (Thermo Scientific, USA) were added according to the manufacturer’s instructions. After centrifugation, protein extracts were collected and 50 μg of protein lysates were resolved by SDS-PAGE and electrophoretically transferred onto Immobilon-P membranes (0.22 μM; Millipore). Membranes were blocked with 5% non-fat milk for 2 h, then incubated with primary antibodies overnight at 4 °C. After washing with PBS-T, membranes were incubated with the fluorescent-conjugated secondary antibodies in PBS-T/milk for 2 h at room temperature. Membranes were then rewashed, after which the labelled proteins were detected using an Odyssey infrared fluorescent scanner (LI-COR) with Odyssey ver. 3 software used for analysis.
Quantification and statistics
Results were expressed as mean ± standard error of the mean (mean ± SEM). Student’s t-test for non-paired replicates was used to identify statistically significant differences between treatment means. Group variability and interaction were compared using either one-way or two-way ANOVA followed by Bonferroni’s post-tests to compare replicates means. A level of P < 0.05 was considered to be significant.
Results
Sulforaphane ameliorated rotenone-induced motor function deficits in mice
Sulforaphane did not show any toxicity in our study and has been used previously as a protective reagent at a dose of 50 mg/kg in vivo27,37,38. We used the schematic design depicted in Fig. 1b. Body weight gain was significantly attenuated from day 30 to day 60 in the rotenone-exposed group, compared with the vehicle-treated group. Sulforaphane treatment did not affect body weight gain, but significantly rescued the reduction of weight gain induced by rotenone (Fig. 1c).
Two hours after administration of the last dose of drugs, mice were observed for sensorimotor function changes. We found no difference in neurobehavioral performance between the sulforaphane and the vehicle group (Fig. 1d–f), whereas, the rotenone group showed a robust decrease in hind limb stepping in the cylinder (Fig. 1d). At the same line, the time taken by the rotenone group was significantly longer in the adhesive removal and challenging beam traversal tests (Fig. 1e,f). However, intraperitoneal administration of sulforaphane at 50 mg/kg every other day before rotenone exposure significantly rescued motor function (Fig. 1d,f). These results suggest that sulforaphane can reverse the loss of body weight gain and PD-related motor deficits caused by rotenone.
Sulforaphane attenuated rotenone-induced dopaminergic neurodegeneration
Since the pathological changes in PD include a loss of dopaminergic neurons (TH-positive neurons) and a decrease in TH expression on the lesioned side, we measured the integrated intensity of labelling in the cerebral cortex and striatum. After 60 days of rotenone exposure, a marked loss of TH-positive neurons and fibres were observed (Fig. 2a). The optical density was decreased in TH-positive neurons by 60% in the stratum and TH-stained fibres, by 53% in the cerebral cortex, respectively (Fig. 2d). Interestingly, co-administration of sulforaphane significantly rescued the rotenone-induced the loss of TH-stained neurons and fibres (Fig. 2a,d). To further confirm the effects of sulforaphane in neuroprotection after rotenone injury, the TH protein levels were analysed by western blotting. Rotenone elicited a 75% and a 55% reduction of TH expression in the extracted cerebral cortex and striatum (Fig. 2b,c). More importantly, sulforaphane rescued TH protein levels, to over 50% of vehicle control group levels, in both lesioned brain regions (Fig. 2e). These results indicate that sulforaphane attenuated rotenone-induced dopaminergic neurodegeneration.
Sulforaphane attenuated rotenone-induced dopaminergic neurodegeneration.
(a) Immunohistochemistry with anti-tyrosine hydroxylase (TH) antibody in the brain. Pictures show 30 μM-thick sections of cerebral cortex and striatum between different groups. (b) Immunoblots of cerebral cortex protein extracts blotted with TH and GAPDH antibodies. (c) Immunoblots of striatal protein extracts blotted with TH and GAPDH antibodies. (d) Densitometric quantification of TH-positive neurons and fibres. (e) Blots for TH were semi-quantified using NIH ImageJ. Relative density expressed as the ratio TH/GAPDH. Each value is presented as mean ± SEM, n = 3. aP < 0.01 vs. control group; bP < 0.05, cP < 0.01 rotenone group vs. rotenone + sulforaphane treatment group.
Sulforaphane rescued rotenone-induced oxidative damage by activating the Nrf2 pathway
ROS generation is a necessary by-product of oxidative metabolism and is tightly regulated in cells by various oxidant and antioxidant enzymes39. MDA is a product of lipid peroxidation and a good indicator of oxidative stress in a cell or tissue29. We firstly investigated the effects of sulforaphane on rotenone-induced ROS production and MDA levels. Results indicated rotenone induced robust ROS generation (Fig. 3a). In addition, exposure of mice to 30 mg/kg rotenone for 60 day significantly increased MDA levels (Fig. 3b). Rotenone also resulted in a consistent, significant decrease in total glutathione content (Fig. 3c) and GSH:GSSG ratio (Fig. 3d). However, co-administration of sulforaphane potently inhibited all of the above pro-oxidative stress effects (Fig. 3a–d). These results imply that sulforaphane may be able to prevent oxidative damage in the brain after rotenone exposure.
Sulforaphane rescued rotenone-induced oxidative damage by activating the Nrf2 pathway.
(a) Reactive oxygen species (ROS) level was measured based on the oxidation of DCFH-DA to DCF using a microplate reader. The malondialdehyde (MDA) levels (b), total glutathione activity (c) and reduced:oxidized glutathione (GSH/GSSG) ratio (d) in the brain. GAPDH was used as loading control for western blots. At least three independent experiments were performed. Representative immunoblots for Nrf2, HO-1, NQO1 and GAPDH in cerebral cortex (e) and striatum (f). Blots for Nrf2, HO-1 and NQO1 were semi-quantified using NIH imageJ. Relative density is expressed as the ratio Nrf2/GAPDH, HO-1/GAPDH, NQO1/GAPDH in cerebral cortex (g) and striatum (h), respectively. Each value is presented as mean ± SEM, n = 3. aP < 0.05, bP < 0.01 vs. control group; cP < 0.05, dP < 0.01 rotenone group vs. rotenone + sulforaphane treatment group.
It is well known that the Nrf2 pathway is able regulate antioxidant enzymes, especially HO-1 and NQO13,8. To explore the molecular mechanisms underlying the protective effects of sulforaphane in the rotenone mouse model of PD, we next analysed Nrf2 pathway in both cerebral cortex and striatum. Western blot analysis demonstrated that exposure to rotenone for 60 days resulted in a strong inhibition of Nrf2 expression, which was profoundly restored by co-administration of sulforaphane (50 mg/kg), in the cerebral cortex and striatum, respectively (Fig. 3e,f). We also analysed the expression of Nrf2-dependent anti-oxidative and detoxifying enzymes after sulforaphane treatment in the rotenone model mice. Compared with the rotenone group, the sulforaphane + rotenone group demonstrated increased protein expression of HO-1, 1.4-fold higher in the cerebral cortex and 1.6-fold higher in the striatum, respectively (Fig. 3e–h). Similarly, sulforaphane partially rescued rotenone-decreased expression of NQO1 level in the cerebral cortex, improving expression by 2.2-fold and striatum, by 7.3-fold (Fig. 3e–h). Collectively, treatment with sulforaphane rescued rotenone induced oxidative damage by activating the Nrf2 pathway.
Sulforaphane inhibited rotenone-induced neuronal apoptosis by restoring the mTOR pathway
Disturbances in the balance of mTOR activity in the brain can impair neuronal functions and have detrimental consequences on neuronal regeneration after damage40. p70S6K and 4E-BP1 are the two best-characterized downstream effector molecules of mTOR10. In the nervous system, inhibition of mTOR pathway, including its downstream effectors p70S6K and 4E-BP1, usually promotes neuronal apoptosis11. Previously, we found that rotenone could negatively regulate mTOR-mediated p70S6K and 4E-BP1/Eif4E pathways, that leads to caspase-3 dependent neuronal apoptosis in vitro15. Here, we tested the hypothesis that sulforaphane prevents rotenone-induced neuronal apoptosis by targeting the mTOR pathway in vivo. Western blot analysis revealed that sulforaphane alone did not obviously alter the expression of mTOR signalling components, but was highly resistant to the inhibition of phosphorylation of mTOR, p70S6K and 4E-BP1 by rotenone, in both the cerebral cortex and striatum (Fig. 4a,b).
Sulforaphane inhibited rotenone-induced neuronal apoptosis by restoring mTOR pathway activity.
Representative immunoblots for p-mTOR, mTOR, p-p70S6K, p-70S6K, 4E-BP1 and GAPDH in cerebral cortex (a) and striatum (b). Blots for p-mTOR, p-p70S6K and 4E-BP1 were semi-quantified using NIH imageJ. Relative density expressed as the ratios of p-mTOR/GAPDH (c), p-p70S6K/GAPDH (d) and 4E-BP1/GAPDH (e) in cerebral cortex and striatum, respectively. Representative immunoblots for cleaved-caspase-3 in cerebral cortex (f) and striatum (g). Blots for cleaved-caspase-3 were semi-quantified using NIH imageJ. Relative density expressed as the ratio cleaved-caspase-3/GAPDH (h). Each value is presented as mean ± SEM, n = 3. aP < 0.05, bP < 0.01 vs. control group; cP < 0.05, dP < 0.01 rotenone group vs. rotenone + sulforaphane treatment group.
To further assess the role of mTOR in neuronal survival and confirm the apoptosis signalling pathways regulated by sulforaphane, we analysed the level of cleaved-caspase-3. As shown in Fig. 4f,g, sulforaphane alone was not toxic to brain tissues. By contrast, the rotenone group demonstrated increased protein expression of cleaved-caspase-3 relative to the control group. In addition, co-administration of sulforaphane potently blocked rotenone-increased expression of cleaved-caspase-3 (Fig. 4f,g). Collectively, our findings support the notion that sulforaphane inhibited rotenone-induced neuronal apoptosis by restoring mTOR pathway.
Sulforaphane protected against rotenone neurotoxicity via modulating autophagy
Recent reports indicate that the pathology of PD is closely related to the accumulation of aberrant proteins, which in theory could be reduced by autophagy16. Therefore, we evaluated whether sulforaphane protected against rotenone neurotoxicity by modulating autophagy using the LC3-II biomarker (see Introduction). In the rotenone group, LC3-II expression was significantly decreased compared to the vehicle-injected control. Though sulforaphane alone did not increase LC3-II levels, it blocked the significant decrease in LC3-II expression when co-administered with rotenone in vivo. (Fig. 5a,b). We further verified these results in vitro. SH-SY5Y cell line cultures were pre-treated with sulforaphane (10 μM) for 2 h and then exposed to rotenone (0.5 and 1 μM) for 24 h. Consistent with the observation in vivo, sulforaphane alone did not affect LC3-II expression, but significantly rescued rotenone-inhibited LC3-II expression. In addition, sulforaphane inhibited rotenone-induced neuronal apoptosis, as detected by the protein expression of cleaved-caspase-3 in the SH-SY5Y cell line (Fig. S1).
Discussion
PD is a commonly occurring neurodegenerative disorder that produces muscular rigidity, bradykinesia, tremor in resting limbs and loss of postural balance1,39. The basic neuropathology of PD involves the selective degeneration of dopaminergic cells in specific brain regions like the striatum; when degeneration in these neurons reaches a threshold reduction of 80% dopamine, the motor symptoms of PD emerge29. The cerebral cortex, which is abundant with neurons and easily accessible, demonstrates its importance in modelling PD3.
The plant-based pesticide rotenone is a prototypical toxin that can mimic clinical and pathological features of PD in an animal model5. We successfully utilized the rotenone-based model established by Inden et al.30, inducing PD-like symptoms characterised by evident motor deficits and the loss of TH-positive neurons in mice (Figs 1 and 2). Sulforaphane, derived from the corresponding glucoraphanin glucosinolate found in abundance in cruciferous vegetables, has recently been recognised as an effective neuroprotective compound. Tarozzi et al. found that pre-treatment with sulforaphane effectively abolished 6-OHDA and H2O2 induced oxidative stress and cytotoxicity in a dopaminergic-like neuroblastoma SH-SY5Y cell line24. Denzer et al. indicated that sulforaphane was protective effect against rotenone-induced cell damage in PC12 cells26. In addition, sulforaphane crosses the blood brain barrier and was neuroprotective in the MPTP and 6-OHDA mouse model of PD27,28. Consistent with the above results, we found that sulforaphane also effectively protected against rotenone-induced motor dysfunction and TH–positive neurons loss in vivo (Figs 1 and 2).
Patients with PD exhibit prominent evidence of oxidative stress, including decreased levels of reduced glutathione and increased oxidative modification to DNA, lipids and protein5. Recently, we showed that rotenone elicited ROS overproduction in PC12 cell line and primary neurons, which led to neurotoxicity15. In this study, long-term administration of rotenone in C57BL/6 mice triggered marked oxidative stress in the brain. However, pre-treatment with sulforaphane markedly inhibited rotenone-mediated increases in every oxidative marker we tested (Fig. 3a–d). These results are in line with a recent study demonstrating that sulforaphane decreased dopaminergic cell loss, as well as enhanced GSH synthesis and conjugation, in both in vitro and in vivo drosophila models of PD41. In addition, the reduced total glutathione content and lower ratio of GSH:GSSG were analysed and interpreted as evidence of redox imbalance and has been associated with various human disease, including PD42,43. Several important findings suggest Nrf2 activity declines is a significant risk factor for PD27. Similarly, rotenone triggered a robust decrease of Nrf2, HO-1 and NQO1 expression in the cerebral cortex and striatum, which was potently rescued by sulforaphane (Fig. 3e–h). Some studies suggested that sulforaphane treatment alone could induce Nrf2 and its dependent enzymes in vivo44,45. However, in our experiment, even a 2-month repeated injection protocol did not appear to activate the Nrf2 pathway in healthy animals. Interestingly, our results are consistent with other studies showing that repeated sulforaphane treatment could result in a long-term elevation of free radical defence, though the sustained elevation of expression of Nrf2, HO-1 and NQO1 seems unlikely27,46,47. Collectively, our data support the notion that sulforaphane protects against rotenone-induced oxidative stress by activating the Nrf2 pathway.
Neuronal apoptosis is reported to play a predominant role in the pathological progress of neuron loss in PD48. There are two major pathways through which apoptosis are induced, but both pathways converge on caspase-3 activation, resulting in nuclear degradation and cellular morphological change49. mTOR signalling is involved in regulating differentiation and survival in neurons, as well as synaptic plasticity, learning and memory and food uptake in adult brain15. In the nervous system, inhibition of mTOR-mediated p70S6K and 4E-BP1 signalling usually promotes neuronal apoptosis11. Rotenone is assumed to exert its cytotoxicity in part through the induction of apoptosis. Previously, we have found that by inhibiting mTOR signalling, rotenone increased cleaved-caspase-3 expression and led to neuronal apoptosis in vitro10,15. We also demonstrated that ectopic expression of wild-type mTOR, constitutively activate S6K1, or downregulated 4E-BP1 partially prevented rotenone-induced neuronal apoptosis, suggesting that rotenone-induced neuronal loss in PD may be prevented by activating mTOR signalling15. Consistent with these data, we found that rotenone inhibited mTOR-mediated p70S6K and 4E-BP1 activity and triggered neuronal apoptosis, as detected by increased expression of cleaved-caspase-3 in vivo (Fig. 4). Studies have shown that sulforaphane is able to regulate cell cycle arrest and apoptosis50. For example, sulforaphane attenuated cell necrosis and apoptosis in renal injury rats51 and type 1 diabetes mice52 models. Interestingly, it is reported that sulforaphane activated the PI3K/Akt pathway to confer protection against 6-OHDA induction of apoptosis in PC12 cells23. Here, as expected, co-treatment with sulforaphane strongly reversed rotenone-inhibited phosphorylation of mTOR, p70S6K and 4EBP1 (Fig. 4a–e), as well as neuronal apoptosis detected by cleaved-caspase-3 (Fig. 4f–h). Our findings strongly suggest that sulforaphane attenuated rotenone-induced inhibition of mTOR signalling pathway as well as neuronal apoptosis.
Autophagy has been reported to be involved in rotenone-mediated neurotoxicity, based on LC3-II decreases53. Our results herein showed that rotenone treatment triggered a robust decrease of LC3-II, probably through inhibition of autophagy, which aggravated deposition of aberrant proteins and subsequent cell death53. Sulforaphane alone cannot directly stimulate autophagy at the 50 mg/kg dose associated with neuroprotection, but it could rescue rotenone-inhibited autophagy (Fig. 5a,b). It seems this study is in contrast to recent reports that indicate that administration of sulforaphane alone enhances autophagic activities54,55. To address this discrepancy, we further investigated the effects of sulforaphane and rotenone treatments in the SH-SY5Y cell line. Our results were in line with the in vivo data, that sulforaphane itself did not affect LC3-II expression, but significantly rescued the rotenone-inhibited expression of the protein. Furthermore, sulforaphane significantly attenuated rotenone-induced neuronal apoptosis in the SH-SY5Y cell line (Fig. S1). Our observations imply that sulforaphane may not directly affect autophagy at the concentration and over the time course associated with neuroprotection that we used, but it can still rescue rotenone-inhibited autophagy. This is consistent with previous investigations showing that sulforaphane induction of autophagy is cell-type, concentration and time dependent54,56.
The kinase mTOR is a major negative regulator of autophagy57. However, the interplay between mTOR and autophagy is complex in PD. For example, some studies found that rapamycin, an inhibitor of mTOR, protected against neuronal injury through induction of autophagy in vivo16,58,59. A similar scenario was also observed, in which rapamycin-activated autophagy inhibited rotenone-induced neuronal apoptosis19. However, one report showed that the autophagy process is altered independently of mTOR pathway disruption and that the two inhibited could contribute to neuronal damage in PD57. Therefore, though alterations in mTOR signalling are linked to autophagy, the relationship between sulforaphane neuroprotection, mTOR signalling and autophagy processes in PD does not seem mutually dependent. It has been shown that Nrf2 is related to mTOR and our data supported the notion that sulforaphane exerts neuronal protective effects via activating Nrf2 and mTOR. This is consistent with a recent report that salvianolic acid A results in a dramatic activation of the Akt/mTOR signalling, followed by activation of the Nrf2/HO-1 axis in retinal pigment epithelia cells60. Understanding the interactions among these systems may be helpful in revealing the full mechanism of how sulforaphane suppresses rotenone-induced neurotoxicity. A summary of our findings is shown in Fig. 6.
Additional Information
How to cite this article: Zhou, Q. et al. Sulforaphane protects against rotenone-induced neurotoxicity in vivo: Involvement of the mTOR, Nrf2 and autophagy pathways. Sci. Rep. 6, 32206; doi: 10.1038/srep32206 (2016).
References
Uversky, V. N. Neurotoxicant-induced animal models of Parkinson’s disease: understanding the role of rotenone, maneb and paraquat in neurodegeneration. Cell Tissue Res 318, 225–241 (2004).
Zhou, J. et al. Salvianolic acid B attenuates toxin-induced neuronal damage via Nrf2-dependent glial cells-mediated protective activity in Parkinson’s disease models. PLoS One 9, e101668 (2014).
Xiong, N. et al. Mitochondrial complex I inhibitor rotenone-induced toxicity and its potential mechanisms in Parkinson’s disease models. Crit Rev Toxicol 42, 613–632 (2012).
Xiong, N. et al. Stereotaxical infusion of rotenone: a reliable rodent model for Parkinson’s disease. PLoS One 4, e7878 (2009).
Sanders, L. H. & Greenamyre, J. T. Oxidative damage to macromolecules in human Parkinson disease and the rotenone model. Free Radic Biol Med 62, 111–120 (2013).
Greenamyre, J. T., Betarbet, R. & Sherer, T. B. The rotenone model of Parkinson’s disease: genes, environment and mitochondria. Parkinsonism Relat Disord 9 Suppl 2, S59–S64 (2003).
Tarozzi, A. et al. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid Med Cell Longev 2013, 415078 (2013).
Yang, L. et al. Neuroprotective effects of the triterpenoid, CDDO methyl amide, a potent inducer of Nrf2-mediated transcription. PLoS One 4, e5757 (2009).
Tufekci, K. U., Civi Bayin, E., Genc, S. & Genc, K. The Nrf2/ARE Pathway: A Promising Target to Counteract Mitochondrial Dysfunction in Parkinson’s Disease. Parkinsons Dis 2011, 314082 (2011).
Xu, Y. et al. Activation of AMPK and inactivation of Akt result in suppression of mTOR-mediated S6K1 and 4E-BP1 pathways leading to neuronal cell death in in vitro models of Parkinson’s disease. Cell Signal 26, 1680–1689 (2014).
Chong, Z. Z., Shang, Y. C., Wang, S. & Maiese, K. Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Progress in Neurobiology 99, 128–148 (2012).
Malagelada, C., Ryu, E. J., Biswas, S. C., Jackson-Lewis, V. & Greene, L. A. RTP801 is elevated in Parkinson brain substantia nigral neurons and mediates death in cellular models of Parkinson’s disease by a mechanism involving mammalian target of rapamycin inactivation. The Journal of neuroscience 26, 9996–10005 (2006).
Holmes, G. L. & Stafstrom, C. E. Tuberous sclerosis complex and epilepsy: recent developments and future challenges. Epilepsia 48, 617–630 (2007).
Choi, K.-C., Kim, S.-H., Ha, J.-Y., Kim, S.-T. & Son, J. H. A novel mTOR activating protein protects dopamine neurons against oxidative stress by repressing autophagy related cell death. Journal of Neurochemistry 112, 366–376 (2010).
Zhou, Q. et al. Rotenone induction of hydrogen peroxide inhibits mTOR-mediated S6K1 and 4E-BP1/eIF4E pathways, leading to neuronal apoptosis. Toxicol Sci 143, 81–96 (2015).
Liu, K., Shi, N., Sun, Y., Zhang, T. & Sun, X. Therapeutic effects of rapamycin on MPTP-induced Parkinsonism in mice. Neurochem Res 38, 201–207 (2013).
Chu, Y., Dodiya, H., Aebischer, P., Olanow, C. W. & Kordower, J. H. Alterations in lysosomal and proteasomal markers in Parkinson’s disease: Relationship to alpha-synuclein inclusions. Neurobiology of Disease 35, 385–398 (2009).
Xiong, N. et al. Potential autophagy enhancers attenuate rotenone-induced toxicity in SH-SY5Y. Neuroscience 199, 292–302 (2011).
Pan, T. et al. Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience 164, 541–551 (2009).
Dadakhujaev, S. et al. Autophagy protects the rotenone-induced cell death in α-synuclein overexpressing SH-SY5Y cells. Neuroscience Letters 472, 47–52 (2010).
Wang, N., Zhao, J., Liu, Q., Diao, X. & Kong, B. Sulforaphane protects human umbilical vein cells against lipotoxicity by stimulating autophagy via an AMPK-mediated pathway. Journal of Functional Foods 15, 23–34 (2015).
Han, J. M. et al. Protective Effect of Sulforaphane against Dopaminergic Cell Death. Journal of Pharmacology and Experimental Therapeutics 321, 249–256 (2007).
Deng, C., Tao, R., Yu, S. Z. & Jin, H. Sulforaphane protects against 6-hydroxydopamine-induced cytotoxicity by increasing expression of heme oxygenase-1 in a PI3K/Akt-dependent manner. Mol Med Rep 5, 847–851 (2012).
Tarozzi, A. et al. Sulforaphane as an inducer of glutathione prevents oxidative stress-induced cell death in a dopaminergic-like neuroblastoma cell line. Journal of Neurochemistry 111, 1161–1171 (2009).
Deng, C., Tao, R., Yu, S.-Z. & Jin, H. Inhibition of 6-hydroxydopamine-induced endoplasmic reticulum stress by sulforaphane through the activation of Nrf2 nuclear translocation. Molecular medicine reports 6, 215–219 (2012).
Denzer, I., Munch, G., Pischetsrieder, M. & Friedland, K. S-allyl-L-cysteine and isoliquiritigenin improve mitochondrial function in cellular models of oxidative and nitrosative stress. Food Chem 194, 843–848 (2016).
Jazwa, A. et al. Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxidants & redox signaling 14, 2347–2360 (2011).
Morroni, F. et al. Neuroprotective effect of sulforaphane in 6-hydroxydopamine-lesioned mouse model of Parkinson’s disease. Neurotoxicology 36, 63–71 (2013).
Tieu, K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb Perspect Med 1, a009316 (2011).
Inden, M. et al. Parkinsonian rotenone mouse model: reevaluation of long-term administration of rotenone in C57BL/6 mice. Biol Pharm Bull 34, 92–96 (2011).
Ambrecht, L. A. et al. Protection of retinal function by sulforaphane following retinal ischemic injury. Exp Eye Res 138, 66–69 (2015).
Kanematsu, S. et al. Sulforaphane inhibits the growth of KPL-1 human breast cancer cells in vitro and suppresses the growth and metastasis of orthotopically transplanted KPL-1 cells in female athymic mice. Oncol Rep 26, 603–608 (2011).
Fleming, S. M., Ekhator, O. R. & Ghisays, V. Assessment of Sensorimotor Function in Mouse Models of Parkinson’s Disease. Journal of Visualized Experiments: JoVE 50303 (2013).
Cannon, J. R. et al. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol Dis 34, 279–290 (2009).
Jackson-Lewis, V. & Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc 2, 141–151 (2007).
Chen, S. et al. N-acetyl-L-cysteine protects against cadmium-induced neuronal apoptosis by inhibiting ROS-dependent activation of Akt/mTOR pathway in mouse brain. Neuropathol Appl Neurobiol 40, 759–777 (2014).
Lee, S. et al. Sulforaphane alleviates scopolamine-induced memory impairment in mice. Pharmacol Res 85, 23–32 (2014).
Li, B. et al. Sulforaphane ameliorates the development of experimental autoimmune encephalomyelitis by antagonizing oxidative stress and Th17-related inflammation in mice. Exp Neurol 250, 239–249 (2013).
Abdulwahid Arif, I. & Ahmad Khan, H. Environmental toxins and Parkinson’s disease: putative roles of impaired electron transport chain and oxidative stress. Toxicol Ind Health 26, 121–128 (2010).
Greenamyre, J. T., Sherer, T. B., Betarbet, R. & Panov, A. V. Complex I and Parkinson’s disease. IUBMB Life 52, 135–141 (2001).
Trinh, K. et al. Induction of the phase II detoxification pathway suppresses neuron loss in Drosophila models of Parkinson’s disease. J Neurosci 28, 465–472 (2008).
Jia, P. et al. Ferric ion could facilitate osteoclast differentiation and bone resorption through the production of reactive oxygen species. Journal of Orthopaedic Research 30, 1843–1852 (2012).
Lee, K. W. et al. Neuroprotective and anti-inflammatory properties of a coffee component in the MPTP model of Parkinson’s disease. Neurotherapeutics 10, 143–153 (2013).
Zhou, R., Lin, J. & Wu, D. Sulforaphane induces Nrf2 and protects against CYP2E1-dependent binge alcohol-induced liver steatosis. Biochim Biophys Acta 1840, 209–218 (2014).
Wruck, C. J. et al. Nrf2 Induces Interleukin-6 (IL-6) Expression via an Antioxidant Response Element within the IL-6 Promoter. Journal of Biological Chemistry 286, 4493–4499 (2011).
Innamorato, N. G. et al. The Transcription Factor Nrf2 Is a Therapeutic Target against Brain Inflammation. The Journal of Immunology 181, 680–689 (2008).
Bergstrom, P. et al. Repeated transient sulforaphane stimulation in astrocytes leads to prolonged Nrf2-mediated gene expression and protection from superoxide-induced damage. Neuropharmacology 60, 343–353 (2011).
Niso-Santano, M. et al. Activation of apoptosis signal-regulating kinase 1 is a key factor in paraquat-induced cell death: Modulation by the Nrf2/Trx axis. Free Radical Biology and Medicine 48, 1370–1381 (2010).
Ueda, S. et al. Redox control of cell death. Antioxid Redox Signal 4, 405–414 (2002).
Fukaya, Y. & Yamaguchi, M. Overexpression of regucalcin suppresses apoptotic cell death in the cloned rat hepatoma H4-II-E cells induced by a naturally occurring isothiocyanate sulforaphane. Int J Mol Med 15, 853–857 (2005).
Negrette-Guzman, M. et al. Sulforaphane attenuates gentamicin-induced nephrotoxicity: role of mitochondrial protection. Evid Based Complement Alternat Med 2013, 135314 (2013).
Jiang, X., Bai, Y., Zhang, Z., Xin, Y. & Cai, L. Protection by sulforaphane from type 1 diabetes-induced testicular apoptosis is associated with the up-regulation of Nrf2 expression and function. Toxicology and Applied Pharmacology 279, 198–210 (2014).
Wu, F. et al. Rotenone impairs autophagic flux and lysosomal functions in Parkinson’s disease. Neuroscience 284, 900–911 (2015).
Liu, Y. et al. Sulforaphane enhances proteasomal and autophagic activities in mice and is a potential therapeutic reagent for Huntington’s disease. Journal of Neurochemistry 129, 539–547 (2014).
Jo, C. et al. Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat Commun 5, 3496 (2014).
Watson, G. W. et al. Analysis of autophagic flux in response to sulforaphane in metastatic prostate cancer cells. Mol Nutr Food Res 59, 1954–1961 (2015).
Heras-Sandoval, D., Pérez-Rojas, J. M., Hernández-Damián, J. & Pedraza-Chaverri, J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cellular Signalling 26, 2694–2701 (2014).
Decressac, M. & Bjorklund, A. mTOR inhibition alleviates L-DOPA-induced dyskinesia in parkinsonian rats. J Parkinsons Dis 3, 13–17 (2013).
Jiang, J., Zuo, Y. & Gu, Z. Rapamycin protects the mitochondria against oxidative stress and apoptosis in a rat model of Parkinson’s disease. Int J Mol Med 31, 825–832 (2013).
Zhang, H. et al. Salvianolic acid A protects RPE cells against oxidative stress through activation of Nrf2/HO-1 signaling. Free Radical Biology and Medicine 69, 219–228 (2014).
Acknowledgements
This work was supported by grant BK20140049 from the Jiangsu Province Funds for Distinguished Young Scientists, BK20161082 from the Natural Science Foundation of Jiangsu Province and from the National Natural Science Foundation of China(NOs 81274150, 81403092, 81573964).
Author information
Authors and Affiliations
Contributions
Q.Z. Conception and design, acquisition of data, analysis and interpretation of data, writing of the manuscript. B.C. Acquisition of data, analysis and interpretation of data. Y.Y. Development of methodology, revision of the manuscript. X.W., L.W., X.C., Z.H., X.C., J.Y., X.S., W.L. and H.Y., supported several experiments. J.C. and J.Y. Statistical analysis and revision of the manuscript. J.S. Technical support, proof-reading of the manuscript. P.C. and Q.Z. Obtained funding, conception and design, revision of the manuscript. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhou, Q., Chen, B., Wang, X. et al. Sulforaphane protects against rotenone-induced neurotoxicity in vivo: Involvement of the mTOR, Nrf2 and autophagy pathways. Sci Rep 6, 32206 (2016). https://doi.org/10.1038/srep32206
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep32206
This article is cited by
-
A Complex Interplay of DJ-1, LRRK2, and Nrf2 in the Regulation of Mitochondrial Function in Cypermethrin-Induced Parkinsonism
Molecular Neurobiology (2024)
-
Neuroprotective actions of a fatty acid nitroalkene in Parkinson’s disease
npj Parkinson's Disease (2023)
-
SARS-CoV-2 infection and dysregulation of nuclear factor erythroid-2-related factor 2 (Nrf2) pathway
Cell Stress and Chaperones (2023)
-
Inhibition of carnitine palmitoyl-transferase 1 is a potential target in a mouse model of Parkinson’s disease
npj Parkinson's Disease (2023)
-
Long-term sulforaphane-treatment restores redox homeostasis and prevents cognitive decline in middleaged female and male rats, but cannot revert previous damage in old animals
Biogerontology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.