Abstract
By using density functional theory with generalized gradient approximation, we have carried out detailed investigations of two-dimensional BxNy nanomaterials in the Cairo pentagonal tiling geometry fully composed of pentagons (penta-BxNy). Only penta-BN and BN2 planar structures are dynamically stable without imaginary modes in their phonon spectra. Their stabilities have been further evaluated by formation energy analysis, first-principles molecular dynamics simulation, and mechanical stability analysis. Penta-BN2 is superior to penta-BN in structural stability. Its stability analysis against oxidization and functional group adsorption as well as its synthesizing reaction path analysis show possibilities in fabricating penta-BN2 on experiment. Furthermore, the penta-BN2 could be transferred from metallic to semiconducting by ionizing or covalently binding an electron per dinitrogen. Also, it has been found to have superior mechanical properties, such as the negative Poisson’s ratio and the comparable stiffness as that of hexagonal h-BN sheet. These studies on the stabilities, electronic properties, and mechanical properties suggest penta-BN2 as an attractive material to call for further studies on both theory and experiment.
Similar content being viewed by others
Introduction
Since the discovery of graphene on experiment in 20041, the two-dimensional (2D) nanomaterials have gained explosive researches. Besides the technique of mechanical exfoliation of 2D nanostructures from their parent materials—the corresponding layered bulks, the liquid-phase exfoliation, chemical vapor deposition (CVD), hydrothermal synthesis methods etc. have also been applied in experimental studies. At this point many planar nanostructures beyond graphene have been obtained2,3,4,5. Many exciting unusual properties originated from the quantum confinement have also been confirmed, showing attractive applications and even more revolutionizing many advanced materials.
The structure-property relationship is an important fundamental research issue in the field of 2D materials. The graphene of the atomic monolayer of carbon atoms arranged in a honeycomb lattice gets massless Dirac fermion characters. The Dirac cones located at K and K′ points in Brillouin zone have been protected by both inversion symmetry and time reversal symmetry. Its linear dispersion relationship of π-band at Dirac point makes charge carriers to be continuously tuned between electrons and holes, whose group velocities are comparable with that of light6. However, recent progresses in searching novel 2D carbon allotropes have shown that the hexagonal symmetry is not the necessary conditions for Dirac properties7,8,9,10,11. Topological arrangement of carbon atoms may hybrid px or py orbitals with pz to perturb the isotropic Dirac cones, which may also bring remarkable properties, such as inherent ferromagnetism, high catalytic activity, potential superconductivity, and metal-semiconductor transition12,13,14,15,16. Carbon pentagon, hexagon, and heptagon are commonly used as building units of the 2D carbon allotropes. The graphene consists of carbon hexagons, which in fact often contains carbon pentagons and heptagons as structural defects such as the well-known Stone-Wales defect17. Wang et al. recently studied a novel 2D carbon allotrope with distorted Dirac cone by regularly arranging carbon pentagons, hexagons, and heptagons. Though the carbon pentagons need to be separated from each other by their surrounding hexagons to reduce steric stress according to the isolated pentagon rule (IPR) for fullerenes, considerable effort has been made to stabilize fused-pentagon-based non-IPR fullerenes12,18, which could be rationalized by the “strain-relief” and “local-aromaticity” principles18. Also, some non-IPR fullerenes including the pure pentagon-based C20 cage have been achieved experimentally4,18. Surprisingly, a most recent study performed by Zhang et al. confirmed the pure pentagon-based 2D material penta-graphene resembling the Cairo pentagonal tiling in geometry, which corresponds to the layer structure of T12-carbon bulk19. Inspired by this finding, pentagonal sheet materials of CN220, hydrogenated silicene21, and B2C22 have been recently reported. The pentagonal arrangement of atoms induces high energy density in CN2, superior flexibility and bipolar magnetic properties in hydrogenated silicene, and tunable band gap in B2C. In comparison with graphene, lots of inorganic nanosheets also have hexagonal lattice characters, such as the h-BN, SiC, MoS2 etc., while the non-hexagonal cases are to some sense rare. So, the questions could be raised: could the non-hexagonal lattice be stable for most planar or quasi-planar nanostructures; will the non-hexagonal structures bring new electronic properties in view of the structure-property exploration for advanced nanomaterials? As a contribution to these issues, we have carried out a detailed investigation on the pentagonal penta-BxNy nanosheets. Their thermodynamic and kinetic stabilities have been carefully evaluated. Considering the fabrication conditions, we have also examined their stabilities against oxidization, functional group adsorption, and charge state. The electronic and mechanical properties of the stable penta-BxNy nanomaterials have also been discussed.
Results
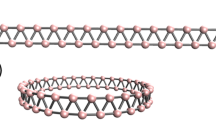
The Cairo pentagonal tiling is the structural geometry fully composed of pentagons, which is schematically shown in Fig. 1 with the repeated unit highlighted by a × b . Arranging B and N atoms at possible positions in the repeated unit, we have carefully optimized the geometrical structures of penta-BxNy nanosheets and calculated their phonon spectra, respectively. In our studies, the number of B atoms ranges from 0 to 6, which simultaneously requires that of N atoms to reversely change from 6 to 0 to meet the requirement of 6 atoms in the primitive unit cell. Only BN (B:N = 3:3) and BN2 (B:N = 2:4) sheets are found to be dynamically stable without imaginary modes, which are presented in Fig. 2. Unlike the Cairo pentagonal tiling, the penta-BN and BN2 sheet structures are found to be slightly buckled to release local steric strain. The penta-BN consists of four atomic layers which are the top layer of dinitrogen, the second layer of boron, the third layer of atomic nitrogen, and the bottom layer of boron again, resulting in four different groups of atoms as marked in Fig. 2a. The atomic arrangement shows CM space symmetry and the thickness between top and bottom layers is measured to be 1.37 Å. As tabulated in Table 1, the charge populations on B1, B2, dinitrogen, and atomic nitrogen species are 2.2, 1.5, 11.9, and 7.0 electrons calculated by Bader analysis23,24, respectively. The lengths are 1.34, 1.60, 1.36, and 1.78 Å for the l1, l2, l3, and l4 bonds, respectively. However, in the penta-BN2 sheet, there are only two composition species. The atomic B atoms form one atomic layer being sandwiched between the top and bottom dinitrogen layers, showing  layer group symmetry. The thickness is 1.26 Å. The boron atom and dinitrogen are calculated to have 1.0 and 12.1 electrons. The l1 and l2 bonds are 1.34 and 1.55 Å in length. For the penta-BN and BN2 nanosheets, the in-plane lattice constants are calculated to be 3.75 and 3.63 Å, respectively.
layer group symmetry. The thickness is 1.26 Å. The boron atom and dinitrogen are calculated to have 1.0 and 12.1 electrons. The l1 and l2 bonds are 1.34 and 1.55 Å in length. For the penta-BN and BN2 nanosheets, the in-plane lattice constants are calculated to be 3.75 and 3.63 Å, respectively.
The structures and the calculated phonon spectra for penta-BN (a) and penta-BN2 (b). The blue and brown balls are for N and B atoms. In (a), B1 and B2 stand for the boron atoms at three- and four-coordinate sites, respectively. N and Ndi are for the atomic nitrogen atom and dinitrogen. The l1, l2, l3, and l4 are used to stand for the non equivalent bonds, respectively. In (b), B1 and Ndi stand for the atomic boron atom and dinitrogen composition species of penta-BN2, respectively. Accordingly, the l1 and l2 account for the non equivalent bonds.
For the kinetically stable penta-BN and BN2 sheets, we have also evaluated the melting temperatures to estimate their heat stabilities by using the first-principles molecular dynamics (MD) simulations. In order to minimize the special constraints due to the periodic conditions, the method of supercell has been adopted in our MD simulations to explore the possibilities in structure reconstruction or melting. The energy barrier protecting the geometrical structure to stay at the local minima on potential surface could be estimated by checking whether the structure reconstruction would happen during the MD simulations. The MD simulations have been performed by heating structures to the temperature of 300 K, with an increase of 50 K for the successive simulations. For each study, the simulation lasts for 6 ps with time step of 1 fs. At the end of each simulation, the final structure has been carefully examined. As shown in Fig. 3, the penta-BN could withstand the temperature as high as 450 K, while the melting of penta-BN2 would not occur below the temperature of 1000 K.
In Fig. 4, the mechanical effects on the stabilities of penta-BN and BN2 nanostructures have been estimated. Beside the primitive unit cell, the 4 × 4 supercell has been adopted to release the special constraints due to the periodic conditions. Our calculations clearly show that both of them could withstand biaxial strain up to 12% before the structures start to collapse, suggesting nice static stabilities. However, before mechanical failure, phonon instability known as Kohn anomaly might occur25. After applying tensile strain, we have also calculated phonon spectra correspondingly. As shown in Fig. 5, one of the acoustical phonon branches start to have imaginary modes for penta-BN under 6.2% strain and penta-BN2 under 7.8% strain, whose deformation energies are calculated to be 0.58 eV and 1.06 eV, respectively. These suggest penta-BN2 to be stiffer than penta-BN, agreeing with the conclusions obtained in our MD simulations. The melting temperature for penta-BN is quite lower as compared with that of penta-BN2. Also, by applying the finite distortion method19, we have calculated the linear elastic constants. Following the standard Voigt notation26, the elastic strain energy per unit area can be written as

where  and
and  are the uniaxial strains respectively applied along x and y directions, and
are the uniaxial strains respectively applied along x and y directions, and  is the equi-biaxial strain. C11, C22, C12, and C66 are the components of elastic modulus tensor, which could be obtained by calculating the second partial derivative of strain energy with respect to strain. According to Born-Huang criteria27, the mechanically stable 2D nanostructure requires the elastic constants to satisfy
is the equi-biaxial strain. C11, C22, C12, and C66 are the components of elastic modulus tensor, which could be obtained by calculating the second partial derivative of strain energy with respect to strain. According to Born-Huang criteria27, the mechanically stable 2D nanostructure requires the elastic constants to satisfy  and
and  . Our calculated elastic constants are presented in Table 2. One can see that both penta-BN and BN2 are mechanically stable. By calculating the
. Our calculated elastic constants are presented in Table 2. One can see that both penta-BN and BN2 are mechanically stable. By calculating the  , we have also estimated the in-plane Young’s moduli in Table 2. The penta-BN2 has a value of 224 N/m being close to the 271 N/m of h-BN monolayer, which is stiffer than the penta-BN, being in the line of the above studies28. Interesting, the Poisson’s ratio C12/C11 = −0.03 of penta-BN2 is negative to render it attractive in view of both scientific and technological investigations29.
, we have also estimated the in-plane Young’s moduli in Table 2. The penta-BN2 has a value of 224 N/m being close to the 271 N/m of h-BN monolayer, which is stiffer than the penta-BN, being in the line of the above studies28. Interesting, the Poisson’s ratio C12/C11 = −0.03 of penta-BN2 is negative to render it attractive in view of both scientific and technological investigations29.
Discussion
The penta-BN consists of the fragments of 3-coordinate B atom, 4-coordinate B atom, dinitrogen Ndi, and atomic N, which have 6.85, 4.60, −0.70, and 7.86 eV formation energies as calculated by below formula.

where Ep-BxNy, Efrag, and E□ are the total energies calculated for the penta-BxNy nanostructure, the composition fragment, and the vacancy defected penta-BxNy structure optimized after removing the fragment. In the calculation, a supercell of 5 × 5 has been adopted to minimize the structural deformation effects from the neighboring images of vacancy defect. Though the reactions of the atomic B and N atoms are exothermic, the reaction of dinitrogen is endothermic as referred to N2 molecule. As to its electronic properties, our bandstructure calculations show penta-BN to be indirect band gap semiconductor (see Fig. 6a). Its conduction band minimum and valence band maximum are located at Γ and M points, respectively. Here, we must point out that the composition fragments include both dinitrogen and atomic nitrogen, which in combination with the above studied stabilities may challenge its fabrication on experiment.
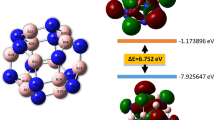
In comparison, the penta-BN2 nanostructure is composed of only atomic boron and dinitrogen fragments, which is in form similar to the experimentally fabricated Ti8C12 metallo-carbohedrene composed of only Ti atoms and C2 dimers30. Similarly, our previous studies of calcium metal cabides also support the C2 dimers as preferable composition fragments31,32. The composition of dinitrogen could also be seen in the recently reported carbon nitride materials33,34. These studies shed light on the possibilities in synthesizing the dinitrogen composed penta-BN2 nanostructure. The formation energies of B atom and dinitrogen compositions of penta-BN2 are calculated to be 10.77 and 0.04 eV, respectively, showing exothermic reaction properties. Besides, we have vertically displaced a single B atom or dinitrogen from the planar structure step by step. At each step, by freezing the vertical distance between the displaced fragment and the nanosheet, the total energy has been calculated after structural optimization. The energy barriers are found to be 4.40 and 2.04 eV to remove a single B atom and dinitrogen, respectively, supporting the structural stability of penta-BN2. Also, we have estimated the possibility in synthesizing it by the reaction channel through introducing atomic boron species into the source of nitrogen molecules such as the liquid nitrogen. Actually, a similar fabrication method was previously applied to successfully synthesize transition metal nitrides by compressing metal and N2 molecules at high pressure35,36. A boron atom could bind three N2 molecules in maximum through exothermic reaction to form B(N2)3 complex, which could then meet each other by overcoming 0.68 eV energy barrier to produce the pentagonal building block of penta-BN2 nanostructure, indicating the possibility for its experimental fabrication.
The bandstructure of penta-BN2 presented in Fig. 6b shows conducting properties. Its work function is calculated to be 3.3 eV being comparable to those of simple metals such as the 3.68 eV of Mg and the 4.28 eV of Al. When supporting on substrate, the penta-BN2 might donate electrons, which would in turn affect its electronic properties. After ionizing one electron per dinitrogen, we have again calculated the electronic properties, which suggest the semiconducting properties as shown in Fig. 6c. Due to the fact that the dinitrogens are located on the outer sides to be naked in the quasi-planar nanostructure, they may be capped by functional groups in fabrication, which may affect the structural stability. In our studies, the hydroxyl group is adopted to check the adsorption effects, which may gain presence in experimental studies37,38. By adsorbing hydroxyl groups on the penta-BN2 nanostructure, we have carried full optimization of geometrical structure. Then, the optimized structure has been forwarded to carry out MD simulations which suggest the thermal stability up to 1400 K (>1000 K of the melting temperature of free-standing penta-BN2), hinting enhanced stability. Also, for the optimized structure fully covered by hydroxyl groups, we have calculated its bandstructure and shown in Fig. 6d. For the adsorption configuration, the Bader charge analysis does not show obvious charge donation from dinitrogen to hydroxyl group. The interatomic distance is 1.45 Å between the O and its nearest N, which is only 6% longer than the sum of atomic radii of O and N atoms. In our charge density study, the charge accumulation could be clearly seen between hydroxyl radical and dinitrogen. Furthermore, we have estimated the localization of electrons of the hydroxyl group adsorbed penta-BN2 by using the electron localization function (ELF) analysis39,40,41, which was introduced in quantum chemistry to measure the parallel spin correlation by defining conditional probability of finding an electron in the neighborhood of another electron with the same spin. ELF is defined as



where  is the Kohn-Sham orbital, and ρ is the local density. The ELF data of 0.7 between the dinitrogen and the adsorbed hydroxyl radical supports weak localization of electrons along H-O bond. Therefore, the bonding between dinitrogen and hydroxyl radical should be covalent-like. One of the electrons of the highest occupied molecular orbital of the dinitrogen would be bound in the covalent bond, making the conducting property transition from conducting to semiconducting as shown in Fig. 6.
is the Kohn-Sham orbital, and ρ is the local density. The ELF data of 0.7 between the dinitrogen and the adsorbed hydroxyl radical supports weak localization of electrons along H-O bond. Therefore, the bonding between dinitrogen and hydroxyl radical should be covalent-like. One of the electrons of the highest occupied molecular orbital of the dinitrogen would be bound in the covalent bond, making the conducting property transition from conducting to semiconducting as shown in Fig. 6.
Besides the effects of charge states and functional group adsorption, the oxidization of penta-BN2 nanostructure also needs to be discussed considering the fabrication and application in the presence of oxygen. A supercell of 5 × 5 has been employed for studying the adsorption and dissociation of a single O2 molecule. The adsorption configurations as shown in Fig. 7 are fully relaxed. The oxygen molecule could parallel cap upon N-N dinitrogen. The O-O bond length would be slightly elongated by 0.5% and the length of the underlying N-N bond would be reduced by 0.7%. The charge gain of O2 is 0.1 electrons and the charge depletion of the underlying dinitrogen is also 0.1 electrons. Actually, in our studies, we have also calculated the adsorption of H2, N2, F2, NO, and CO gas molecues. The slight charge transfer only happens in the O2 adsorption. This may be attributed to the fact that only O2 molecule has the lowest unoccupied molecular orbital to be slightly lower in binding energy than the valence band maximum of penta-BN2. In the process of band alignment, nearly neglected charge is transferred from dinitrogen to O2 molecule. However, the interatomic distance between O and its nearest N is 2.22 Å being of 63% larger than their atomic radius sum42, excluding the possibility of obvious orbital overlap between them. Besides the weak overlap indicated by the weak charge transfer, the calculated binding energy of ~0.2 eV for O2 adsorption may also include the Coulomb energy of the charged dioxygen felt in the local electric field surrounding dinitrogen. The charge accumulation on dinitrogen as presented in Table 1 would induce a local electric field to affect the adsorption of charged or polarized molecules, being like the case of H2 adsorption on C60(OM)12 (M = Li and Na) clusters38. We have used the climbing image nudged elastic band method to study O2 dissociation43,44,45,46, whose activation energy is calculated to be >2.6 eV to hinder the oxidization of penta-BN2. In our studies, we have also investigated the structural stability in the conditions of oxygen molecules. After putting one O2 molecule upon each dinitrogen, the first-principles molecular dynamics simulation has been carried out at 300 K by using the 4 × 4 supercell. The O2 would however start to leave the penta-BN2 at ~1 ps in our MD simulation. Based on these studies, we would like to conclude that the oxidization of penta-BN2 sheet structure is not easy.
In summary, the new two-dimensional nanostructures of BxNy with Cairo pentagonal tiling geometry have been carefully investigated. Only penta-BN and BN2 are found to be kinetically stable which do not have imaginary modes in the calculated phonon spectra. Besides, we have also discussed their stabilities from the sides of: (1) the formation energies of structural composition fragments; (2) the thermal stabilities indicated by the melting temperatures found in our molecular dynamics simulations; and (3) the mechanical stabilities to sustain mechanical strain. The penta-BN2 composed of only atomic boron and dinitrogen species has superior stability than penta-BN, which may be synthesized by introducing atomic boron atoms into the source of nitrogen molecules such as the liquid nitrogen. Also, our studies on the oxygen molecule adsorption and its dissociation suggest the stability of penta-BN2 sheet against oxidization. Its stability has also been evaluated against hydroxyl group adsorption. The bandstructure study of penta-BN2 sheet shows conducting properties. Due to the charge accumulation, the dinitrogen may tend to donate electrons when the sheet structure of penta-BN2 is supported on substrate. By ionizing one electron per dinitrogen, the charged penta-BN2 would be changed to be semiconducting. For the hydroxyl group adsorbed penta-BN2, each hydroxyl group could bind one electron of each dinitrogen in the covalent-like bond, which could also make the metal-semiconductor transition. Here, we would like also to mention Yagmurcukardes et al.’s studies of pentagonal B2N4 and B4N2 on the bandstructures and mechanical properties (for example, the stiffness)47. In comparison, we have investigated all the possible pentagonal monolayer structure candidates by changing composition species in the primitive unit cell. Only penta-BN and BN2 are found to be dynamically stable. As for the penta-BN2 which was also previously investigated by Yagmurcukardes and coworkers47, more detailed studies on its structural stability have been performed, for example, the thermal stability estimated by first-principles molecular dynamics simulations, the thermodynamic stability evaluated by formation energy analysis. Besides, considering the potential usages, we have also carefully studied its stability against oxidization and functional group adsorption. The effects of substrate and functional group adsorption on its transport properties are also discussed in our studies. Furthermore, in order to facilitate experimental fabrication, we have also carried out synthesizing reaction path analysis. So, we would like to conclude that our studies contribute to give more comprehensive theoretical results on the possible pentagonal boron nitride monolayer materials, including detailed stability analyses, effects of oxidization and functional group adsorption, transport property modifications, and potential synthesizing reaction path investigations.
Methods
Our first-principles calculations were performed within the framework of density functional theory with a plane wave basis set as implemented in the Vienna ab initio simulation package (VASP)48,49. The cutoff energy for plane-wave basis set was chosen to be 400 eV. The projector augmented-wave (PAW) method was used49. The exchange and correlation energy was described by the generalized gradient approximation with Perdew, Burke, and Ernzerhof (PBE) parameterization50. The nanostructure of penta-BxNy was placed in xy plane of the supercell with a 15 Å vacuum in z direction, which is large enough to ignore the effects from its neighboring images. The Monkhorst-Pack k-mesh of 15 × 15 × 1 was applied to sample k points in the first Brillouin zone for integrating electronic properties51. All the atoms were fully relaxed with force converge up to 0.02 eV/Å. The calculated total energy was converged to 10−5 eV. The first-principles molecular dynamics (MD) simulations lasted for 6 ps with time step of 1 fs. The MD simulations were preformed starting from the temperature of 300 K. Due to the intensive computing loading of MD simulations, the melting temperature was only estimated with the precision of 50 K. Our Bader charge analysis was carried out by using the code developed by Henkelman et al.24,52,53. Phonon properties were calculated with the finite displacement method as implemented in Phonopy54. In calculating the phonon spectra, the energy convergence criteria were set to 10−8 eV for total energy and 0.1 meV/Å for Hellmann-Feynman force.
Additional Information
How to cite this article: Li, J. et al. Penta-BxNy sheet: a density functional theory study of two-dimensional material. Sci. Rep. 6, 31840; doi: 10.1038/srep31840 (2016).
References
Novoselov, K. S. et al. Electric Field Effect in Atomically Thin Carbon Films. Science 306, 666 (2004).
Das, S., Kim, M., Lee, J.-W. & Choi, W. Synthesis, Properties, and Applications of 2-D Materials: A Comprehensive Review. Crit. Rev. Solid State Mater. Sci. 39, 231 (2014).
Wang, F. et al. Synthesis, properties and applications of 2D non-graphene materials. Nanotechnology 26, 292001 (2015).
Naguib, M. & Gogotsi, Y. Synthesis of two-dimensional materials by selective extraction. Acc. Chem. Res. 48, 128 (2015).
Gupta, A., Sakthivel T. & Seal. S. Recent development in 2D materials beyond graphene. Prog. Mater. Sci. 73, 44 (2015).
Novoselov, K. S. et al. Two-dimensional gas of massless Dirac fermions in graphene. Nature 438, 197 (2005).
Wang, Z. et al. Phagraphene: A low-energy graphene allotrope composed of 5-6-7 carbon rings with distorted Dirac cones. Nano Lett. 15, 6182 (2015).
Xu, L.-C. et al. Two dimensional Dirac carbon allotropes from graphene. Nanoscale 6, 113 (2014).
Malko, D. Neiss, C. Viñes, F. & Görling, A. Competition for Graphene: Graphynes with Direction-Dependent Dirac Cones. Phys. Rev. Lett. 108, 086804 (2012).
Liu, Y., Wang, G., Huang, Q., Guo, L. & Chen, X. Structural and Electronic Properties of T Graphene: A Two-Dimensional Carbon Allotrope with Tetrarings. Phys. Rev. Lett. 108, 225505 (2012).
Enyashin, A. N. & Ivanovskii, A. L. Graphene allotropes. Phys. Status Solidi B 248, 1879 (2011).
Maruyama, M. & Okada, S. Two-dimensional sp2 carbon network of fused pentagons: All carbon ferromagnetic sheet. Appl. Phys. Express 6, 095101 (2013).
Li, Y., Xu, L., Liu, H. & Li, Y. Graphdiyne and graphyne: From theoretical predictions to practical construction. Chem. Soc. Rev. 43, 2572 (2014).
Terrones, H. et al. New metallic allotropes of planar and tubular carbon. Phys. Rev. Lett. 84, 1716 (2000).
Xiu, S. L. et al. An effective method of tuning conducting properties: First-principles studies on electronic structures of graphene nanomeshes. Carbon 79, 646 (2014).
Jia, T.-T. et al. Dirac cone move and bandgap on/off switching of graphene superlattice. Sci. Rep. 6, 18869 (2016).
Banhart, F., Kotakoski, J. & Krasheninnikov, A. V. Structural defects in graphene. ACS Nano 5, 25 (2011).
Tan, Y.-Z., Xie, S.-Y., Huang, R.-B. & Zheng, L.-S. The stabilization of fused-pentagon fullerene molecules. Nat. Chem. 1, 450 (2009).
Zhang, S. et al. Penta-graphene: A new carbon allotrope. Proc. Natl. Acad. Sci. USA 112, 2372 (2015).
Zhang, S., Zhou, J., Wang, Q. & Jena, P. Beyond graphitic carbon nitride: nitrogen-rich penta-CN2 sheet. J. Phys. Chem. C 120, 3993 (2016).
Ding, Y. & Wang Y. Hydrogen-induced stabilization and tunable electronic structures of penta-silicene: a computational study. J. Mater. Chem. C 3, 11341 (2015).
Li, F., Tu, K., Zhang, H. & Chen, Z. Flexible structural and electronic properties of a pentagonal B2C monolayer via external strain: a computational investigation. Phys. Chem. Chem. Phys. 17, 24151 (2015).
Bader, R. F. Atoms in Molecules–A Quantum Theory. (Oxford University Press, 1990).
Tang, W., Sanville, E. & Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys.: Condens. Matter 21, 084204 (2009).
Marianetti, C. A. & Yevick, H. G. Failure mechanisms of graphene under tension. Phys. Rev. Lett. 105, 245502 (2010).
Andrew, R. C., Mapasha, R. E., Ukpong, A. M. & Chetty, N. Mechanical properties of graphene and boronitrene. Phys. Rev. B 85, 125428 (2012).
Ding, Y. & Wang, Y. Density functional theory study of the silicene-like SiX and XSi3 (X = B, C, N, Al, P) honeycomb lattices: the various buckled structures and versatile electronic properties. J. Phys. Chem. C 117, 18266 (2013).
Kudin, K. N., Scuseria, G. E. & Yakobson, B. I. C2F, BN and C nanoshell elasticity from ab initio computations. Phys. Rev. B 64, 235406 (2001).
Greaves, G. N., Greer, A. L., Lakes, R. S. & Rouxel, T. Poisson’s ratio and modern materials. Nat. Mater. 10, 823 (2011).
Guo, B. C., Kerns, K. P. & Castleman, A. W. Ti8C12 +-Metallo-Carbohedrenes: a new class of molecular clusters? Science 255, 1411 (1992).
Chen, G., Peng, Q. & Kawazoe, Y. Structural and electronic properties of neutral and charged Ca8C12 metal carbides. Chem. Phys. Lett. 507, 260 (2011).
Chen, G., Peng, Q. & Kawazoe, Y. First-principles study on Ca8Cn (n ≤ 12) and CamC12 (m ≤ 8) metal carbides. Phys. Lett. A 375, 994 (2011).
Li, Q. et al. A novel low compressible and superhard carbon nitride: body-centered tetragonal CN2 . Phys. Chem. Chem. Phys. 14, 13081 (2012).
Zhang, S., Zhou, J., Wang, Q. & Jena, P. Beyond graphitic carbon nitride: nitrogen-rich penta-CN2 sheet. J. Phys. Chem. C 120, 3993 (2016).
Gregoryanz, E. et al. Synthesis and characterization of a binary noble metal nitride. Nat. Mater. 3, 294 (2004).
Crowhurst, J. C. et al. Synthesis and characterization of the nitrides of platinum and iridium. Science 311, 1275 (2006).
Feng, G. et al. Accelerated crystallization of zeolites via hydroxyl free radicals. Science 351, 1188 (2016).
Peng, Q., Chen, G., Mizuseki, H. & Kawazoe, Y. Hydrogen storage capacity of C60(OM)12 (M = Li and Na) clusters. J. Chem. Phys. 131, 214505 (2009).
Silvi, B. & Savin, A. Classification of chemical bonds based on topological analysis of electron localization functions. Nature 371, 683 (1994).
Becke, A. D. & Edgecombe, K. E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 92, 5397 (1990).
Chen, G., Liu, Z. F. & Gong, X. G. Ab initio study on structural and electronic properties of BanOm clusters. J. Chem. Phys. 120, 8020 (2004).
Kittel, C. Introduction to Solid State Physics. (John Wiley & Sons, 1996).
Chen, S. P., Chen, G., Gong, X. G. & Liu, Z. F. Oxidation of carbon nanotubes by singlet O2 . Phys. Rev. Lett. 90, 086403 (2003).
Chen, G. et al. Improved stability and catalytic properties of Au16 cluster suppprted on graphane. J. Phys. Chem. C 115, 20168 (2011).
Mills, G., Jónsson, H. & Schenter, G. K. Reversible work transition state theory: application to dissociative adsorption of hydrogen. Surf. Sci. 324, 305 (1995).
Henkelman, G. & Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978 (2000).
Yagmurcukardes, M. et al. Pentagonal monolayer crystals for carbon, boron nitride, and silver azide. J. Appl. Phys. 118, 104303 (2015).
Kress, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Kress, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188 (1976).
Henkelman, G., Arnaldsson, A. & Jónsson, H. A Fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 36, 354 (2006).
Sanville, E., Steven, D. K., Smith, R. & Henkelman, G. Improved grid-based algorithm for bader charge allocation. J. Comput. Chem. 28, 899 (2007).
Togo, A., Oba, F. & Tanaka, I. First-principles calculations of the ferroelastic transition between rutile-type and CaCl2-type SiO2 at high pressures. Phys. Rev. B 78, 134106 (2008).
Acknowledgements
The authors gratefully acknowledge the computing resources from the University of Jinan. This work was jointly supported by the funds from Shandong Province (Grant No. TSHW20101004) and the National Natural Science Foundation of China (NSFC) (Grant No. 11374128). Prof. Peng Zhao, Dr. Hongbo Wang, and Dr. Jinxiang Liu are highly appreciated for carefully reading through our manuscript.
Author information
Authors and Affiliations
Contributions
G.C. conceived the idea. J.L. performed the calculations. The data analyses were performed by J.L., X.F. and G.C. Y.W. helped discussing. This manuscript was written by G.C. All authors reviewed this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, J., Fan, X., Wei, Y. et al. Penta-BxNy sheet: a density functional theory study of two-dimensional material. Sci Rep 6, 31840 (2016). https://doi.org/10.1038/srep31840
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep31840
This article is cited by
-
Prediction of novel penta-\({\text{Si}}_{3} {\text{P}}_{2} {\text{X}}\) (X = S, Se) monolayers as photocatalytic materials for water splitting by first principles calculations
Indian Journal of Physics (2024)
-
On the mechanical, electronic, and optical properties of the boron nitride analog for the recently synthesized biphenylene network: a DFT study
Journal of Molecular Modeling (2023)
-
CO2 capture and separation on the penta-BN2 monolayer with the assistance of charge/electric field
Journal of Materials Science (2021)
-
Two-dimensional Penta-BP5 Sheets: High-stability, Strain-tunable Electronic Structure and Excellent Mechanical Properties
Scientific Reports (2017)
-
Half-metallicity and ferromagnetism in penta-AlN2 nanostructure
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.