Abstract
Adolescence is a period of ongoing brain maturation characterized by hierarchical changes in the functional and structural networks. For this reason, the young brain is particularly vulnerable to the toxic effects of alcohol. Nowadays, binge drinking is a pattern of alcohol consumption increasingly prevalent among adolescents. The aim of the present study is to evaluate the evolution of the functional and anatomical connectivity of the Default Mode Network (DMN) in young binge drinkers along two years. Magnetoencephalography signal during eyes closed resting state as well as Diffusion Tensor Imaging (DTI) were acquired twice within a 2-year interval from 39 undergraduate students (22 controls, 17 binge drinkers) with neither personal nor family history of alcoholism. The group comparison showed that, after maintaining a binge drinking pattern along at least two years, binge drinkers displayed an increased brain connectivity of the DMN in comparison with the control group. On the other hand, the structural connectivity did not show significant differences neither between groups nor over the time. These findings point out that a continued pattern of binge drinking leads to functional alterations in the normal brain maturation process, even before anatomical changes can be detected.
Similar content being viewed by others
Introduction
In the last decades, binge drinking (BD) alcohol consumption has taken social relevance given its high prevalence in adolescence1,2. This consumption pattern is characterized by the intake of large quantities of alcohol in a short interval of time, followed by periods of abstinence3. Generally, BD has been defined as the intake of 5 or more drinks (4 or more for females) on one occasion within a 2 hour interval (which corresponds to a blood alcohol concentration (BAC) of around 0.08% or above), at least once in the last two weeks or the last month3.
Adolescence is a period of ongoing neurodevelopment that is characterized by ordered changes, such as synaptic pruning and myelination4, that lead to functional5 and structural6 networks maturation. Understanding the neurophysiology consequences of BD during youth is essential since an undeveloped brain is more vulnerable to alcohol-induced damage than adult brain7. In this sense, animal studies have pointed out that a BD pattern induces more brain damage in adolescent than in adult rats8 as well as a higher degree of cognitive impairment9. In human BD population, cognitive deficits have been reported especially regarding executive functions10,11,12. Functional neuroimaging studies demonstrated differences in the BD’s brain dynamics during cognitive task performance13,14,15,16. From a structural point of view, alterations were also found white17,18 and gray matter19,20 associated with BD.
Despite the growing interest that this pattern of consumption generates in the scientific community, the extent in which BD affects the maturation of the functional and structural networks over time is an issue scarcely studied. Functional connectivity (FC) quantifies the connections between different brain regions based on temporal correlation21,22. To our knowledge, the only study that has analyzed FC in BDs is a previous study of our research group23. In this study we examined FC assessed with magnetoencephalograpy (MEG) during resting state and we found a decrease in alpha band as well as an increase of FC in delta, theta and beta bands in young BDs compared to an aged-matched control group. Currently, the FC studies are focused in the characterization of brain networks, where the default mode network (DMN) is one of the most assessed. This network is highly active during an idle state, when the brain is not involved in an externally imposed goal-directed activity and it deactivates during task performance. The DMN includes brain regions such as the precuneus, posterior and anterior cingulate, middle prefrontal cortex and the inferior parietal cortex24,25,26. Although the DMN has garnered increasing attention during the last decade, no study has described it in BDs so far.
Alternatively, structural information about brain connectivity can be provided by diffusion tensor image (DTI) as it enables modeling of the white matter connections that support structural networks. Using this technique, weighted connectivity measures estimate the number of tracts connecting two regions and the integrity of anatomical connections respectively. For instance, fractional anisotropy, which is an estimation of white matter integrity, has been shown to be reduced in BD population along several white matter pathways18,27.
However, although both functional and structural connectivity studies have found differences in BD subjects, there are no follow-up studies showing the progression of the brain connectivity damage caused by BD. Accordingly, the present study is framed as a two years follow-up study and seeks to provide new evidence about how the maintenance of this drinking pattern in mild-long term may affect brain connectivity.
Results
Functional Connectivity
Results of the FC analysis are summarized in Table 1 and Figs 1 and 2. We did not found any significant difference between groups in the intra-ROI (intra-Region of Interest) analysis. In the inter-ROI analysis, we found several significant differences (p < 0.05) between groups in the delta, theta and beta frequency bands. In all cases, the BD group showed an increased FC ratio when compared to the CN group. Besides from the group’s comparison, the FC ratios in the BD group were always significantly higher than 1, which implied an increment of the FC along time. By contrast, the CN group showed a stable or decrement FC ratio along time (FC ratio <1 in 8 links and non-different from 1 in 3 links). Regarding the ANCOVA´s covariate, sex was not significant in any of the connectivity links.
The significant FC network basically pointed out the existence of two kinds of FC patterns: 1) a frontal-parietal pattern, which involved mainly the FMC and its communication with both IPL and the Pc and 2) a parietal pattern, which consisted of alterations in the communications between the rIPL and most of the DMN ROIs. The frontal-parietal pattern was found in delta, theta and beta frequency bands, whereas the parietal one emerged in theta and beta frequency bands.
Finally, the classification analysis pointed out that it is possible to distinguish between groups with a minimum and maximum of accuracy of 74% and 90% respectively (see Table 2).
Structural Connectivity
As a result of repeated measures ANCOVA performed with DTI data, we did not find any group differences neither in the pre nor in the post condition. Likewise, no intragroup differences in the BD group were found across time.
Discussion
This is the first study assessing functional connectivity (FC) along with structural connectivity (SC) in young BD subjects who maintained a pattern of intensive alcohol consumption for more than two years. The results of the present study showed that the FC of the DMN, as assessed with MEG, increased over time in young subjects with a BD pattern compared to the control group. Namely, the BD group showed a significant enhanced FC ratio in several links among DMN ROIs. On those significant ROIs, the FC ratios were always significantly higher than 1 in the BD group, whereas the FC ratios in the control group remained stable or lower than 1. On the other hand, the SC, as assessed by fractional anisotropy, medial diffusivity, radial diffusivity and axial diffusivity did not show significant differences neither between groups nor over time.
To the best of our knowledge, only one study has examined the brain FC in young binge drinkers during resting state. That previous study, carried out by our research group23, was conducted when the age of the subjects was 18–19 and we found that the BD group already showed an increased FC in the delta, theta and beta frequency bands in frontal areas, as well as a decreased FC in the alpha band. In the present follow-up study, we have analyzed the rate of change of each group over two years and noticed that the BD group has increased its DMN FC over time in delta, theta and beta frequency bands. By contrast, a decrease of FC of some links of the DMN was observed in the control group. Given the longitudinal nature of the current study, it is important to consider this decrement of FC in the control group in the context of typical adolescent neural maturation. During brain maturation, adolescents exhibit less activation over time, as neural networks become more developed and efficient28. As it can be seen in the present results, the typical pattern of neural maturation occur among adolescents who remained nondrinkers, but in the case of young people who continued drinking over those two years, the opposite pattern occurred. These results suggest that alcohol consumption may alter the typical neural development and may produce a developmental delay, hypotheses reported by different studies on binge drinking20,29,30,31,32.
In this line, this relative brain immaturity might be related to the effects of alcohol on brain receptors. It is well established that moderate to heavy alcohol intake disrupts the normal functioning of brain receptors, mainly N-methyl D-aspartate (NMDA) and gamma-aminobutyric acid-A (GABA-A)33,34. Given that glutamate-sensitive NMDA receptors has a central role in the synaptic pruning needed to remove and strengthen brain connections35, it is possible that a BD pattern during adolescence may interfere with the cortical networks refinement mediated via NMDA receptors. In accordance with this argument, several studies with structural MRI have reported greater grey matter volume in BDs compared to controls in cortical30,31 and subcortical regions20. On the basis of these findings, it has been proposed that this enlarged cortical and subcortical volume would be cause by the reduced pruning resulting from excessive alcohol consumption. Together, these impairments in brain development could explain the opposite pattern of change in the FC of BDs as compared to controls (increase vs. decrease of the DMN FC) observed in the present study.
On the other hand, fMRI studies have also found increased brain activity in the BD subjects during the performance of different cognitive tasks such as verbal learning36, working memory37 and decision making13. This increased brain activity in the BD group, despite having the same behavioral performance, has been interpreted as a compensatory mechanism that allows maintaining an equivalent cognitive performance level. The results of these fMRI studies are no directly comparable with those obtained in the present work, since the BOLD activity obtained with fMRI is different from the FC obtained with MEG and also the brain networks involved in performing a cognitive task are different from the DMN. However, our results can be added to the existing studies which have found brain hyperactivity in BD population. In a recent study, Wetherill et al.38 found, in a longitudinal study, that future heavy drinkers showed higher brain activation during response inhibition than nondrinkers after transitioning into heavy drinkers. Similarly, previous EEG studies from our research group showed that BDs, as compared to controls, displayed larger amplitudes in several components of the event-related potentials (which was interpreted as greater neural activity involved in tasks performance) and these differences became to be greater after two years maintaining the BD pattern14,39. Taken together, these findings are indicating that heavy alcohol consumption may lead to alterations in brain functioning in terms of increasing brain activity both during task performance38,39 and also during resting state (DMN), as the present study shows.
As mentioned above, in the present study we tested both functional and structural connectivity. Regarding structural networks, we did not find any group differences neither in the pre nor in the post evaluation. Likewise, no intragroup differences in the BD group were found across time. In our view, the brain changes that occur as a result of maintaining BD alcohol consumption are measurable with electrophysiological FC techniques, but maybe the underlying structural changes are not detectable in a structural level.
Finally, the anomalous DMN FC might be considered as a marker of posterior structural and cognitive impairments linked to the maintenance of BD. In this sense, hypersynchronization has been also seen as a biomarker of brain damage in several neurological conditions such as traumatic brain injury40 or in early stages of dementia41. In a review paper, Bryer et al.42 developed a model that explains how this brain overactivation reflects brain excitability causing network malfunctioning. In fact, in a sample of mild cognitive impairment patients, higher synchronization was a predictor factor of conversion to Alzheimer disease43 and also has been associated with a random network organization44.
A number of limitations should be noted; first, the lack of differences between the groups in the anatomical connectivity may be due to the two years’ follow-up time limitation of the study, therefore more longitudinal measures would be needed. Second, although the sample size of the study was considerable for a follow-up study of these characteristics, a larger sample would increase the reliability of the results and would make possible the assessing of the influence of other factors such as the sex. And finally, the cross-sectional nature of this type of studies makes difficult to draw a conclusion about causal relationship between the physiological differences and the alcohol consumption. So, it cannot be excluded that the differences between groups are previous to the consumption, but in any case, we are able to demonstrate reliable differences of the evolution of the FC between BD group and control subjects.
In conclusion, a continued pattern of BD over at least two years appear to lead to hypersynchronized DMN as compared with the non BD group. This could be taken as a biomarker of potential brain damage caused by alcohol consumption without a clear evidence of deficits on structural connectivity. Understanding the effects of the BD pattern on FC has important implications for the etiology and prevention of future alcohol dependence. Future follow-up studies should explore whether functional networks associated with specific cognitive tasks are as well affected by BD alcohol consumption.
Methods
Participants
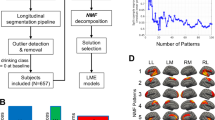
Thirty-nine undergraduate students of the Complutense University of Madrid (Madrid, Spain) participated in the study. Twenty two were classified as controls (12 females) and 17 as BDs (8 females). The procedure for subject selection is fully described in Correas et al.23. The participants were evaluated twice within a 2-year interval (at 18–19 and 20–21 years old) and the number of months between evaluations of each group didn’t differ (control group = 22.86 ± 0.89; BD group = 23.26 ± 0.94). The demographic data of each group is shown in Table 2.
Participants were divided into BD and control group according to a questionnaire and a semi-structured interview inquiring about alcohol and other drug consumptions habits. Participants were asked to cover a record of daily consumption indicating what they drank, the quantity and for how long (hours). The BAC was calculated based on the information of the last dinking episode. We considered the BAC as a rough index representing the BD level of each subject. Participants reaching a BAC of 0.08% or above at least once during the last month were classified as BD. On the other hand, the control group consisted of students who never achieved that BAC. Participants were asked to refrain from alcohol consumption for, at least, 24 hours prior to MEG recordings. Subjects were submitted to a breathalyzer test and the assessment was only performed after verifying a 0.00% breath alcohol level. All volunteers provided written informed consent prior to assessment. The study was approved by the Ethics Committee of the Complutense University of Madrid, Spain and the procedure was performed in accordance with approved guidelines and regulations. Principles of the Declaration of Helsinki were followed.
MEG Acquisition
Four minutes of MEG signal were acquired (1000 Hz sampling rate and online band pass filter at 0.1–330 Hz) during eyes-closed resting state using a 306-channel (102 magnetometers and 204 gradiometers) system (Elekta©, VectorView). In this study only magnetometers (102 channels) information was submitted to source and statistical analyses. The system was housed in a magnetically shielded room (VacuumSchmelze GmbH, Hanua, Germany). The head movement was monitored by means of four head-position indicator coils attached to the scalp. Ocular movements were tracked with two bipolar electrodes.
MEG Analysis
Preprocessing
The raw recording data were at first submitted to Maxfilter software (v 2.2, Elekta Neuromag) to remove external noise with the temporal extension of the signal space separation method with movement compensation45. In this study, we used only magnetometers data in order to avoid mixing MEG sensors with different sensitivities or resorting to scaling. Accordingly, all of the magnetometers’ resting state signals were automatically scanned for ocular, muscle and jump artifacts with Fieldtrip package46 and were visually confirmed by a MEG expert. The artifact-free data were segmented in continuous 4 seconds fragments (trials). At least 15 clean trials were obtained from all participants and preserved for further analyses. The number of surviving trials did not differ significantly between groups. To calculate the source´s reconstruction, the time series were filtered in the following frequency bands: delta (2–3.9 Hz), theta (4.1–7.9 Hz) alpha (8.1–11.9 Hz) and beta (12.1–29.9 Hz). The filtering was performed with a finite impulse response filter of order 1500. This filter was applied using a two-pass procedure over the whole four-minute registers, in order to avoid phase distortion and edge effects.
Headmodels & Beamforming
A regular grid of 2455 nodes with 1 cm spacing was created in the Montreal Neurological Institute (MNI) template brain. This set of nodes was transformed to each participant´s space using a non-linear normalization between the native T1 image and a standard T1 in MNI template space. The forward model was solved with the realistic single-shell model introduced by Nolte47.
Source reconstruction was performed with a Linearly Constrained Minimum Variance Beamformer48. For each subject, the covariance matrix was first averaged over all trials to compute the spatial filter´s coefficients and these coefficients were applied to individual trials, obtaining a time series per segment and the source location.
Atlas Based Analysis
The FC analysis was performed using atlas-based ROIs. For the subsequent analysis, we specifically focused on FC in the DMN. We set DMN-related ROIs in the precuneus (Pc), posterior cingulate cortex (PCC), anterior cingulate cortex (ACC), frontal medial cortex (FMC) and bilateral inferior parietal lobe (IPL) by referring to the Harvard-Oxford probabilistic49. In total, 156 nodes were included in this study as they were located within the 6 ROIs.
Functional Connectivity: Phase Locking Value
The FC was measured by means of phase-locking value (PLV)48 in each frequency band and was calculated per each trial as explained in23. Finally, the results were averaged across trials ending up with symmetrical 156 × 156 nodes connectivity matrices per subjects, phase (pre/post) and frequency band. In order to assess changes over time, we assess the ratio of change between the two phases of the longitudinal study by dividing the value of each connectivity node of the second evaluation between the first evaluation. Finally, to address whether volume conduction could be causing these differences, we have calculated the correlation between beamformer weights in both groups in order to have an estimate of volume conduction50. Beamformer weights did not differ between groups in any frequency band, which makes it unlikely that the functional connectivity differences were caused by volume conduction.
Statistical Analysis
Clusters of connections, which showed statistically significant group differences (BD subjects vs. control subjects), were explored relying on the cluster-based permutation test51 for each frequency band. The methodology was composed by two steps: 1) an intra-ROI analysis that computed the local connectivity within each ROI; and 2) inter ROI FC that evaluated the inter-regional FC among each pair of ROIS of the DMN. In both cases, the procedure was essentially the same. In the intra-ROI analysis, we assessed the FC of all the nodes contained within a ROI; whereas in the inter-ROI the analysis we focused in the FC between the nodes located in the corresponding two bilateral ROIs.
The procedure started by assessing the FC difference between groups for each pair of nodes by means of ANCOVA with sex as a covariate. The significance of the links was assessed using a non-parametric randomization (5000 permutations) testing52, Only those links with p-values below 0.05 were kept and included in the following steps of the analysis. Then, we aimed to extract a robust significant network, so-called network motifs in graph theory53. These networks consisted of several consecutive significant links, which systematically showed a diminished or enhanced FC in the BD group compared with the CN group. We considered a significant motif only when: 1) at least the 25% of the nodes which composed the ROI were involved, 2) at least the 10% of the links among them had significant FC differences between groups and 3) the motifs should be connected, i.e. there exists a path between each pair of nodes in the motif 54. The first two conditions set the minimum dimensions of the motif and the third one fixed a constraint in the morphology, dismissing the insulated links. Then, we submitted all the FC values of the links that composed the significant motifs to a cluster-based (in this case motif-based) non-parametric test (5000 permutations)55,56 to control the multiple comparisons problem. At this point, we wanted to offer a value which would characterize the network of each significant cluster. Thus, we calculate for each significant motif their corresponding degree53, i.e. the average PLV-ratio across all links. Then we performed an ANCOVA with sex as covariate between groups, which was corrected by multiplying the p value by 5, to further account for the family-wise error for the 5 frequency bands and we obtained the corresponding effect sizes (Cohen’s d). In addition, we applied a classification analysis using a logistic regression analysis with the leave-one-out cross-validation procedure43. Finally, we performed a one-sample t-test to determine whether the value of the ratio of each group differs from a distribution with mean 1.
MRI Acquisition
MRI was collected from a General Electric 1.5 Tesla using an eight-channel head coil. The imaging protocol consisted of: 3D T1-weighted high-resolution images using a Fast Spoiled Gradient Echo sequence [TR/TE/TI = 11.2/4.2/450 ms; flip-angle = 12°; FoV = 250 mm; acquisition matrix = 256 × 256; slice thickness = 1 mm] and Diffusion weighted images (DWI) using a single-shot echo planar imaging sequence [TR/TE = 12000/96.1 ms; FoV = 307 mm; acquisition matrix = 128 × 128; slice thickness = 2.4 mm; NEX = 3]. DWI was acquired along 25 non-coplanar directions with a b-value of 900 s/mm2 and 1 image with no diffusion sensitization, i.e. b0 image.
MRI Analysis
DWI Analysis
DWIs were corrected for motion and eddy currents using EDDY-FSL, which performs linear affine registration of the volumes to the reference b0 volume. The resulting rotations were used to realign the gradient directions matrix. Non-brain tissue was removed from DWI using BET-FSL and diffusion tensor images (DTIs) were obtained using a linear least-squares approach as implemented in FSL-FDT. From that several scalar images representing the shape of the diffusion tensor were obtained: fractional anisotropy, mean diffusivity, radial diffusivity and axial diffusivity.
Rigid transformations were performed between b0 and T1-weighted images. In addition, linear affine transformations followed by nonlinear local deformations using FSL-FNIRT were applied to normalize brain-extracted T1-weighted images into the MNI brain template of 1 mm3 isotropic resolution. These three transformations were concatenated in one single transformation in order to reduce the number of interpolations and were applied inversely to transform the JHU white matter tractography atlas57 into the diffusion subject-specific space. Average values for the different DTI metrics were obtained for each of the masks included in the JHU atlas.
Statistical Analysis
A repeated measures ANCOVA with sex as a covariate was performed with fractional anisotropy, mean diffusivity, radial diffusivity and axial diffusivity data.
Additional Information
How to cite this article: Correas, A. et al. Functional and structural brain connectivity of young binge drinkers: a follow-up study. Sci. Rep. 6, 31293; doi: 10.1038/srep31293 (2016).
References
Archie, S., Zangeneh Kazemi, A. & Akhtar-Danesh, N. Concurrent binge drinking and depression among Canadian youth: prevalence, patterns and suicidality. Alcohol 46, 165–172 (2012).
Johnston, L. Monitoring the Future: National Results on Adolescent Drug Use: Overview of Key Findings. (2011).
Courtney, K. E. & Polich, J. Binge Drinking in Young Adults: Data, Definitions and Determinants. Psychol bull 135, 142–156 (2009).
Guerri, C. & Pascual, M. Mechanisms involved in the neurotoxic, cognitive and neurobehavioral effects of alcohol consumption during adolescence. Alcohol 44, 15–26 (2010).
Sherman, L. E. et al. Development of the default mode and central executive networks across early adolescence: a longitudinal study. Dev. Cogn. Neurosci. 10, 148–159 (2014).
Simmonds, D. J., Hallquist, M. N., Asato, M. & Luna, B. Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. Neuroimage 92, 356–368 (2014).
Crews, F., He, J. & Hodge, C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol. Biochem. Behav. 86, 189–199 (2007).
Crews, F. T., Braun, C. J., Hoplight, B., Switzer, R. C. & Knapp, D. J. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol. Clin. Exp. Res. 24, 1712–1723 (2000).
Barron, S. et al. Adolescent vulnerabilities to chronic alcohol or nicotine exposure: findings from rodent models. Alcohol. Clin. Exp. Res. 29, 1720–1725 (2005).
Goudriaan, A. E., Grekin, E. R. & Sher, K. J. Decision making and response inhibition as predictors of heavy alcohol use: a prospective study. Alcohol. Clin. Exp. Res. 35, 1050–1057 (2011).
García-Moreno, L. M., Expósito, J., Sanhueza, C. & Angulo, M. T. [Prefrontal activity and weekend alcoholism in the young]. Adicciones 20, 271–279 (2008).
Scaife, J. C. & Duka, T. Behavioural measures of frontal lobe function in a population of young social drinkers with binge drinking pattern. Pharmacol. Biochem. Behav. 93, 354–362 (2009).
Xiao, L. et al. Abnormal affective decision making revealed in adolescent binge drinkers using a functional magnetic resonance imaging study. Psychol. Addict. Behav. 27, 443–454 (2013).
López-Caneda, E. et al. Hyperactivation of right inferior frontal cortex in young binge drinkers during response inhibition: a follow-up study. Addiction 107, 1796–1808 (2012).
Smith, J. L. & Mattick, R. P. Evidence of deficits in behavioural inhibition and performance monitoring in young female heavy drinkers. Drug Alcohol Depend, 10.1016/j.drugalcdep.2013.06.020 (2013).
Crego, A. et al. Increased amplitude of P3 event-related potential in young binge drinkers. Alcohol 46, 415–425 (2012).
Smith, K. W. et al. Altered white matter integrity in whole brain and segments of corpus callosum, in young social drinkers with binge drinking pattern. Addict. Biol, 10.1111/adb.12332 (2015).
McQueeny, T. et al. Altered white matter integrity in adolescent binge drinkers. Alcohol. Clin. Exp. Res. 33, 1278–1285 (2009).
Lisdahl, K. M., Thayer, R., Squeglia, L. M., McQueeny, T. M. & Tapert, S. F. Recent binge drinking predicts smaller cerebellar volumes in adolescents. Psychiatry Res. 211, 17–23 (2013).
Howell, N. A. et al. Increased ventral striatal volume in college-aged binge drinkers. PLoS One 8, e74164 (2013).
Varela, F., Lachaux, J. P., Rodriguez, E. & Martinerie, J. The brainweb: phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2, 229–239 (2001).
Bardin, J. Making connections. News Featur. 8–10 (2012).
Correas, A. et al. Exploratory Analysis of Power Spectrum and Functional Connectivity During Resting State in Young Binge Drinkers: A MEG Study. Int. J. Neural Syst. 25, 1550008 (2015).
Buckner, R. L., Andrews-Hanna, J. R. & Schacter, D. L. The brain’s default network: anatomy, function and relevance to disease. Ann. N. Y. Acad. Sci. 1124, 1–38 (2008).
Greicius, M. D., Krasnow, B., Reiss, A. L. & Menon, V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 100, 253–258 (2003).
Rosazza, C. & Minati, L. Resting-state brain networks: literature review and clinical applications. Neurol. Sci. 32, 773–785 (2011).
Jacobus, J. et al. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol. Teratol. 31, 349–355 (2009).
Luna, B., Padmanabhan, A. & O’Hearn, K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 72, 101–113 (2010).
Jacobus, J. & Tapert, S. F. Neurotoxic effects of alcohol in adolescence. Annu. Rev. Clin. Psychol. 9, 703–721 (2013).
Squeglia, L. M. et al. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology (Berl). 220, 529–539 (2012).
Doallo, S. et al. Larger mid-dorsolateral prefrontal gray matter volume in young binge drinkers revealed by voxel-based morphometry. PLoS One 9, e96380 (2014).
López-Caneda, E. et al. [Neurocognitive anomalies associated with the binge drinking pattern of alcohol consumption in adolescents and young people: a review]. Adicciones 26, 334–359 (2014).
Tsai, G. & Coyle, J. T. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu. Rev. Med. 49, 173–184 (1998).
Ward, R. J., Lallemand, F. & de Witte, P. Biochemical and neurotransmitter changes implicated in alcohol-induced brain damage in chronic or ‘binge drinking’ alcohol abuse. Alcohol Alcohol 44, 128–135 (2009).
Stoneham, E. T., Sanders, E. M., Sanyal, M. & Dumas, T. C. Rules of engagement: factors that regulate activity-dependent synaptic plasticity during neural network development. Biol. Bull. 219, 81–99 (2010).
Schweinsburg, A. D., McQueeny, T., Nagel, B. J., Eyler, L. T. & Tapert, S. F. A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol 44, 111–117 (2010).
Squeglia, L. M., Schweinsburg, A. D., Pulido, C. & Tapert, S. F. Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcohol. Clin. Exp. Res. 35, 1831–1841 (2011).
Wetherill, R. R., Squeglia, L. M., Yang, T. T. & Tapert, S. F. A longitudinal examination of adolescent response inhibition: neural differences before and after the initiation of heavy drinking. Psychopharmacology (Berl). 230, 663–671 (2013).
López-Caneda, E. et al. Effects of a persistent binge drinking pattern of alcohol consumption in young people: a follow-up study using event-related potentials. Alcohol Alcohol 48, 464–471 (2013).
Castellanos, N. P. et al. Principles of recovery from traumatic brain injury: reorganization of functional networks. Neuroimage 55, 1189–1199 (2011).
Bajo, R. et al. Functional connectivity in mild cognitive impairment during a memory task: implications for the disconnection hypothesis. J. Alzheimers. Dis. 22, 183–193 (2010).
Bryer, E. J., Medaglia, J. D., Rostami, S. & Hillary, F. G. Neural recruitment after mild traumatic brain injury is task dependent: a meta-analysis. J. Int. Neuropsychol. Soc. 19, 751–762 (2013).
Lopez, M. E. et al. Alpha-Band Hypersynchronization in Progressive Mild Cognitive Impairment: A Magnetoencephalography Study. J. Neurosci. 34, 14551–14559 (2014).
Buldú, J. M. et al. Reorganization of functional networks in mild cognitive impairment. PLoS One 6, e19584 (2011).
Taulu, S. & Simola, J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 51, 1759–1768 (2006).
Oostenveld, R., Fries, P., Maris, E. & Schoffelen, J.-M. FieldTrip: Open source software for advanced analysis of MEG, EEG and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 156869 (2011).
Nolte, G. The magnetic lead field theorem in the quasi-static approximation and its use for magnetoencephalography forward calculation in realistic volume conductors. Phys. Med. Biol. 48, 3637–3652 (2003).
Van Veen, B. D., van Drongelen, W., Yuchtman, M. & Suzuki, A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 44, 867–880 (1997).
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006).
Brookes, M. J. et al. Measuring functional connectivity using MEG: Methodology and comparison with fcMRI. Neuroimage 56, 1082–1104 (2011).
Maris, E. & Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164, 177–190 (2007).
Ernst, M. D. Permutation Methods: A Basis for Exact Inference. Stat. Sci. 19, 676–685 (2004).
Stam, C. J. Modern network science of neurological disorders. Nat. Rev. Neurosci. 15, 683–695 (2014).
Stam, C. J. et al. The trees and the forest: Characterization of complex brain networks with minimum spanning trees. Int. J. Psychophysiol. 92, 129–138 (2014).
Garcés, P. et al. Brain-wide slowing of spontaneous alpha rhythms in mild cognitive impairment. Front. Aging Neurosci. 5, 100 (2013).
Cuesta, P. et al. Influence of the APOE ε4 Allele and Mild Cognitive Impairment Diagnosis in the Disruption of the MEG Resting State Functional Connectivity in Sources Space. J. Alzheimers. Dis. 10.3233/JAD-141872 (2014).
Hua, K. et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 39, 336–347 (2008).
Acknowledgements
This study was supported by the projects SPI/2010 and SPI/2010/051 from Spanish Ministry of Health and Social Politics (National Plan on Drugs). Funding was also provided from the SFRH/BPD/109750/2015 Postdoctoral Fellowship of the Portuguese Foundation for Science and Technology as well as the Research Center on Psychology (UID/PSI/01662/2013).
Author information
Authors and Affiliations
Contributions
A.C. wrote the main manuscript text and prepared figures. C.P. collaborated with the MEG data analysis. L.-C.E. collaborated with the writing of the manuscript. R.H.S. collaborated with the experimental design. L.M. G.-M. collaborated collecting the sample. P.-P.J.A. collaborated with the DTI data analysis. C.F. collaborated supervising the experiment. M.F. collaborated supervising the experiment. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Correas, A., Cuesta, P., López-Caneda, E. et al. Functional and structural brain connectivity of young binge drinkers: a follow-up study. Sci Rep 6, 31293 (2016). https://doi.org/10.1038/srep31293
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep31293
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.