Abstract
To explore an effective noninvasive tool for monitoring liver fibrosis of children with biliary atresia (BA) is important but evidences are limited. This study is to investigate the predictive accuracy of supersonic shearwave elastography (SSWE) in liver fibrosis for postoperative patients with BA and to compare it with aspartate aminotransferase to platelet ratio index (APRI) and fibrosis-4 (FIB-4). 24 patients with BA received SSWE and laboratory tests before scheduled for liver biopsy. Spearman rank coefficient and receiver operating characteristic (ROC) were used to analyze data. Metavir scores were F0 in 3, F1 in 2, F2 in 4, F3 in 7 and F4 in 8 patients. FIB-4 failed to correlate with fibrosis stage. The areas under the ROC curves of SSWE, APRI and their combination were 0.79, 0.65 and 0.78 for significant fibrosis, 0.81, 0.64 and 0.76 for advanced fibrosis, 0.82, 0.56 and 0.84 for cirrhosis. SSWE values at biopsy was correlated with platelet count (r = −0.426, P = 0.038), serum albumin (r = −0.670, P < 0.001), total bilirubin (r = 0.419, P = 0.041) and direct bilirubin levels (r = 0.518, P = 0.010) measured at 6 months after liver biopsy. Our results indicate that SSWE is a more promising tool to assess liver fibrosis than APRI and FIB-4 in children with BA.
Similar content being viewed by others
Introduction
Biliary atresia (BA), characterized by progressive fibro-obliteration of the bile ducts, is a common infantile cholestatic disease with high morbidity and mortality1. Kasai portoenterostomy (KPE) remains the initial strategy to restore bile flow2. However, despite of timely KPE, the progressive fibrosis develops in almost all patients3, which results in liver cirrhosis and requires subsequent liver transplantation (LT)4. The degree of liver fibrosis after KPE contributes to be the main prognostic factors for the survival of patients5. Therefore, close monitoring of fibrosis is critical for identifying high-risk patients and referring them to LT.
Liver biopsy is the criterion standard for evaluating liver fibrosis. However, as an invasive procedure, it has many limitations including complications, discomfort, inter-observer variations and sampling errors6,7. Given this situation, various non-invasive methods have rapidly emerged as alternatives to liver biopsy, such as quantitative elastography and serum fibrosis biomarkers.
Several quantitative elastography technologies such as transient elastography (TE), acoustic radiation force impulse (ARFI), and supersonic shearwave elastography (SSWE), have been used to evaluate liver fibrosis in pediatric patients and showed good correlation (ρ = 0.53–0.63) between liver fibrosis and elastographic value8,9,10,11. However, TE has many pitfalls such as the inability to choose different locations for the region of interest (ROI) and to avoid other structures such as liver vessels and bile ducts12, and has also been reported to have more technical failures in young children13,14. APRI, also named as point shear wave elastography, has the limitation in being unable to provide a real-time quantitative map of liver tissue stiffness15. SSWE, a newly developed elastography technology, has three advantages over TE. First, SSWE is integrated into a conventional diagnostic ultrasound (US) system and therefore can make use of real-time gray scale mode imaging for the assessment of morphologic changes or avoiding big vessels16. Second, improved separation of fibrosis stages due to the use of shear waves with greater bandwidths has improved its discriminative power in staging fibrosis17. Third, SSWE enables a real-time stiffnessanalysis with a real-time map of the elasticity in a region18, which showed benefit over TE and also ARFI. Previous adult studies have showed that it was a promising diagnostic tool in predicting cirrhosis19,20,21. However, to the best of our knowledge, there were very few studies regarding the performance of SSWE in liver fibrosis for children until now15,22. The aspartate aminotransferase (AST) to platelet (PLT) ratio index (APRI) and fibrosis-4 (FIB-4) score calculated from age, AST, alanine amino-transferase (ALT) and PLT, are both, inexpensive, easily available and non-invasive indices. They have been reported to be a surrogate marker of fibrosis and cirrhosis in adults with hepatitis C, hepatitis B, alcoholic hepatitis and nonalcoholic fatty liver23,24,25. However, their diagnostic performance for liver fibrosis in pediatric patients with different liver diseases including BA have exhibited variations among different studies (area under the receiver operating characteristic curves (AUROCs): 0.54–0.97)26,27,28.
This study aimed at evaluating the performance of SSWE to predict fibrosis stage in postoperative Chinese children with BA by comparing it with APRI and FIB-4, and to preliminarily investigate the correlation of SSWE with biochemical parameters of liver function at 6 months after liver biopsy.
Materials and Methods
Patients
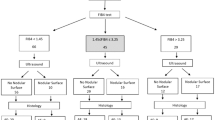
This retrospective study based on the prospectively collected data was approved by the ethics committee of the First Affiliated Hospital of Sun Yat-sen University, and written informed parental consent was obtained from each patient. Our study was carried out in accordance with the approved guidelines. Between August 2012 and November 2015, 24 patients with BA followed at the First Affiliated Hospital of Sun Yat-sen University and scheduled for a liver biopsy were prospectively enrolled. All these patients underwent KPE before enrollment. SSWE examination and serum biochemical tests were offered to all the included patients almost in the same day or within 1 week of liver biopsy. Exclusion criteria were as follows: (1) failure of SSWE examination; (2) recent other diseases such as acute febrile illnesses or skin rashes, which could affect biochemical parameters (i.e. AST levels and PLT counts) other than BA; (3) co-infection with hepatitis B, hepatitis C, hepatitis D or HIV; (4) inadequate liver biopsies defined as having fewer than six portal tracts under the microscope; (5) no follow-up data. No patients were excluded in this study.
Examination Protocols
Clinical and laboratory test
Demographic data including age and sex were recorded at the time of liver biopsy. During follow-up, liver related events such as prolonged jaundice, ascites, gastrointestinal bleeding, hepatic encephalopathy, liver transplantation and death were also recorded. Laboratory examinations were collected venously as part of routine clinical care throughout the follow-up. Data including AST, ALT, serum albumin (ALB), serum total bilirubin (TBIL), serum direct bilirubin (DBIL), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), and PLT count were obtained. Laboratory data obtained within 1 week of liver biopsy and at 6 months after liver biopsy were used for analysis in this study. The diagnosis of gastrointestinal bleeding was confirmed by endoscopic finding that bleeding developed from the varicose veins in the distal esophagus or gastric fundus29. Ascites was diagnosed when fluid was found in peritoneal cavity by imaging tests such as ultrasonography or computed tomography30. Jaundice (serum bilirubin ≥ 5 mg/dl [85 mmol/l]) was defined based on the guidelines of Asian Pacific Association for the study of liver31. APRI and FIB-4 were calculated according to the following formulas: APRI = (AST/upper limit of normal AST × 100)/ Platelet Count [109/L], FIB-4 = (Age [years] × AST [U/L])/ (Platelets [109/L] × ( [U/L])). The upper normal range of AST were 37 IU/L.
[U/L])). The upper normal range of AST were 37 IU/L.
SWE examination
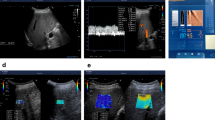
An AixPlorer scanner (Supersonic, Paris, France) incorporating a SC6-1curvilinear transducer (1–6 MHz) was used to perform the SSWE examinations. The infants were remained still by sedation or by holding their breath. Segments V or VI were selected as the target areas for measurement. All SSWE examinations were performed by a single sonographer (Luyao Zhou, 6 years of experience for US and 3 years of experience for elastography, respectively) with an intercostal or a subcostal transducer position. If possible, SSWE was performed during a short breath hold (3 seconds). Otherwise, the acquisitions were made during one normal, gentle breathing cycle. The transducer was kept perpendicular to the skin and no press was given during the SSWE examination. Three measurements were made and recorded at the same segment for every infant.
SSWE was performed in dual mode (ie, elastograms displayed alongside gray-scale sonograms in real time). The operator chose the best static SSWE display images onto which a rectangular electronic region of interest (2.0 cm × 2.5 cm) and a circular region of interest (placed within the center of the rectangular region of interest, with diameter range from 1.5 cm to 2.5 cm) were positioned within 1.0 cm−3.0 cm from the capsular surface of the liver for analysis (Fig. 1). Once the optimal sizes of the regions of interest were chosen, they were fixed for subsequent measurements in each subject. Special attention was paid to avoid any focal lesions, vessels, biliary tracts, or artifacts from nearby lung gas. A successful SSWE was defined as the ROI box could be filled with color over 90% of the box. From the circular region of interest, the mean, standard deviation, and minimum and maximum kilopascal values were recorded. The average values from the three readings in each infant were used for subsequent statistical analyses.
Liver Histopathology
Under the guidance of US, liver biopsy was performed percutaneously in the right liver lobe using an inter-costal approach with an 18-gauge needle (Bard, USA). A minimum length of 20 mm for biopsy specimens must be guaranteed. Then, all specimens were fixed in formalin and embedded in paraffin. Following staining with haematoxylin-eosin (H&E) and Masson trichrome, each specimen was evaluated by two independent and blinded pathologists with more than 10 years of experience in liver pathology. Only the adequate samples defined by showing at least six portal tracts were included for further analysis32. Disagreement was resolved by the consultation of a third pathologist. Liver fibrosis and necroinflammatory activity was assessed using the Metavir classification as follows31,33: F0, no fibrosis; F1, portal fibrosis with no septa; F2, portal fibrosis with rare fibrous septa; F3, bridging fibrosis with many fibrous septa; F4,cirrhosis. Activity was staged as follows: A0, none; A1, mild; A2, moderate; A3, severe.
Statistical Analysis
The normal distribution test was conducted in the variables using Shapiro-Wilk test.
Normally distributed variables were represented as mean ± SD while non-normally distributed variables such as skew variables were represented as median and inter-quartile range (IQR). Differences between two groups were compared by the t test for normally distributed variables, Wilcoxon rank test for skewed variables and χ2 test for categorical variables. Spearman’s rank coefficient test was performed to evaluate the correlation between variables. The Kruskal–Wallis test was used to detect the variation of SSWE values and APRI among different fibrosis stages. The diagnostic performance of SSWE, APRI and their combination to predict liver fibrosis severity (F0–1 vs. F2–4: F ≥ 2; F0-2 vs. F3–4: F ≥ 3; F0–3 vs. F4: F = 4) was estimated by the area under the receiver operating characteristic (ROC) curve. The optimal cut-off values were determined based on the largest Youden index. And the differences of AUROCs between different parameters were presented as P values estimated by Z tests. Statistical significance was considered as a two-sided P value of less than 0.05. All the analyses was performed by the SPSS 20.0 (SPSS Inc., Chicago, IL,USA), SAS 9.2 (SAS Institute, Inc, Cary, NC) and Sigmaplot 10.0. Ink (Systat Software, Inc.).
Results
Subject Characteristics
A total of 24 patients met the inclusion criteria for our study. Biochemical tests, SSWE examinations and liver biopsy were performed within one week of each other. In terms of fibrosis stage, there were 3 (12.5%) patients for F0, 2(8.3%) for F1, 4 (16.7%) for F2, 7 (29.2%) for F3, 8 (33.3%) for F4. The demographics, biochemical results, ultrasonic findings, histological features and follow-up data of patients by fibrosis level are summarized in Table 1. The mean age at liver biopsy was 6.6 years with 13 (54.2%) male patients. Patients with more advanced fibrosis stage were found to have significantly higher serum AST levels, GGT, DBIL levels and lower serum ALB level (P = 0.046; P = 0.049; P = 0.025; P = 0.044). SSWE values ranged from 6.2 to 60.6 (median, 13.2 kPa). On serum fibrosis biomarkers, the median value of APRI and FIB-4 was 1.3 (0.34–12.5) and 0.31(0.03–3.5) with the corresponding wide ranges. During the follow up from liver biospy, no liver-related death or gastrointestinal bleeding was detected. There were two patients in need of liver transplantation (one with F4 has underwent surgery and one with F2 is on the waiting list). Three patients showed with ascites(1 with F4, 1 with F3 and 1 with F2).
Performance of APRI, FIB-4 and SSWE in Predicting Liver Fibrosis Severity
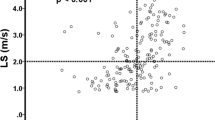
APRI scores had a positive correlation with fibrosis stage (r = 0.583, P < 0.001) (Supplementary Figure A) whereas FIB-4 scores had a very weak correlation (r = 0.075, P = 0.001). APRI scores showed a significant difference between different fibrosis stages (P = 0.035; Fig. 2A) whereas FIB-4 scores did not. Thus, we moved to further explore the performance of APRI in predicting fibrosis severity. A significant difference was detected between consecutive fibrosis stages except F3 and F4 (F0 vs. F1: P < 0.001; F1 vs. F2: P < 0.001; F2 vs. F3: P = 0.009; F3 vs. F4: P = 0.323). Moreover, the AUROCs of APRI were 0.65 (95% CI: 0.35–0.96), 0.64 (95% CI: 0.41–0.88), and 0.56 (95% CI: 0.31–0.80) respectively for predicting significant (≥F2), advanced fibrosis stage (≥F3), and cirrhosis (F4) (Table 2, Fig. 3). With the corresponding cut-off values of 0.70, 0.93 and 1.00 in the above three situations, the performance of APRI in predicting fibrosis severity was estimated (Table 2). SSWE values showed a positive correlation with fibrosis stage (r = 0.762, P < 0.001) (Supplementary Figure B) and exhibited significant difference among different fibrosis stages (P = 0.004; Fig. 2B). There was significant difference between consecutive fibrosis stages except F2 and F3 (F0 vs. F1: P < 0.001; F1 vs. F2: P < 0.001; F2 vs. F3: P = 0.450; F3 vs. F4: P = 0.001). Compared to APRI, SSWE had larger AUROCs in predicting significant fibrosis (0.79, 95% CI: 0.54–1.00), advanced fibrosis (0.81, 95% CI: 0.63–0.99) and cirrhosis (0.82, 95% CI: 0.58–1.00) with the corresponding cut-off values of 9.4, 10.8 and 24.4 kPa (Table 2, Fig. 3). However, the difference between SSWE and APRI in predicting significant fibrosis was not significant (P = 0.65) whereas the differences in predicting advanced fibrosis and cirrhosis were statistically significant (both P < 0.001). The details on the diagnostic accuracy of SSWE are presented in Table 2.
Moreover, SSWE demonstrated a positive correlation with APRI with significant difference (r = 0.543, P = 0.006). Combining SSWE with APRI did not improve the predictive power in significant or advanced fibrosis over SSWE alone, with the AUROC being 0.78 (95% CI: 0.55–1.00) for significant fibrosis and 0.76 (95% CI: 0.55–0.97) for advanced fibrosis, respectively. However, the combination of SSWE and APRI could slightly improve the diagnostic accuracy for cirrhosis, with the AUROC being 0.84 (95% CI: 0.63–1.00) (Table 2). However, the difference between combination and SSWE was not statistically significant (P = 0.33).
Discordance between SSWE, APRI and Fibrosis Stage
Based on the above cut-off values, the predicted fibrosis stage by SSWE and APRI and the actual fibrosis level was compared. APRI was in agreement with fibrosis staging 50% (12/24) of the time. Overall, APRI overestimated fibrosis stage 29.2% of the time and underestimated fibrosis 20.8% of the time. For SSWE, more patients (15, 62.5%) were diagnosed with agreement to actual fibrosis stage than APRI. SSWE overestimated fibrosis stage in 12.5% of study subjects and underestimated fibrosis in 25.0% of study subjects. More specifically, about 25.0% (6 of 24) of the patients had discordance of one stage between SSWE value and Metavir score. About 12.5% (3 of 24) of the patients had discordance of two stages or more between SSWE value and Metavir score. For the six underestimated cases by SSWE, there were two patients with SSWE values less than 7.0 but turned out to be in F3 stage by histology. These patients have low APRI, normal PLT count, normal serum bilirubin level and albumin level.
There were two patients with SSWE values of >10.8 kPa but without advanced fibrosis or cirrhosis on liver biopsy. To look further into these patients, we found that they had other biochemical and ultrasonic manifestations suggesting of advanced fibrosis or cirrhosis, such as APRI ≥ 1.0, low PLT count, high serum AST level and splenomegaly (Table 3).
Correlation between SSWE and Liver Function Biomarkers after 6 months from biopsy
In order to preliminarily investigate the role of SSWE in predicting patients’ liver function in future day, we analyzed the correlation between our SSWE results and liver function biomarkers after 6 months from liver biopsy. It showed that SSWE had significant negative correlation with PLT count (r = −0.426, P = 0.038) and serum ALB level (r = −0.670, P < 0.001). Besides, SSWE values appeared to be positively correlated with serum TBIL (r = 0.419, P = 0.041) and DBIL levels (r = 0.518, P = 0.010) at 6 months after liver biopsy.
Discussion
Our study revealed that SSWE examination had a promising diagnostic accuracy to predict liver fibrosis stage, which is better than APRI. SSWE values had significant positive correlations with liver fibrosis severity in patients after kasai surgery. Moreover, SSWE values were correlated with biological parameters of liver function at 6 months after liver biopsy.
Liver stiffness measurement has become a reliable tool to assess liver fibrosis in adult patients with chronic liver diseases19,20,21,34,35, however, the evaluation of its use in children is limited. Until now, there are a few studies on assessing the predictive power of Fibroscan, TE, ARFI in fibrosis stage for children with various liver diseases7,8,9. To the best of our knowledge, this is the first study to investigate the performance of SSWE in predicting liver fibrosis for postoperative children with BA and to compare with those of APRI and FIB-4. The reported cutoff values of TE for predicting advanced fibrosis and cirrhosis in previous literatures varied, ranging from 7.9 to 11 and 11.0 to 25.8 kPa, respectively36,37,38,39. Besides, a study on the performance of SSWE in the evaluation of liver fibrosis for children reported a cut-off value of 10.4 kPa for predicting significant fibrosis15. In our study, the cut-off values for diagnosis of significant fibrosis, advanced fibrosis and cirrhosis were 9.4, 10.8 and 24.4 kPa, which were within the ranges presented in the previous studies. However, we should note that the cutoff values for diagnosing liver fibrosis differ depending on the method we used. Compared to the results of Fibroscan in previous pediatric BA studies (AUROC of 0.87–0.88 for F = 4)26,40, SSWE yielded similar performance characteristics with AUROC of 0.82 in our study. Shin NY et al. reported the AUROCs of 0.86 and 0.96 respectively in predicting severe fibrosis and cirrhosis for TE in 47 infants with BA7, which was slightly better than those of our study. This might be probably due to the relatively poorer discriminative power of our small sample size. For ARFI, Shima H et al. only suggested a correlation between ARFI and fibrosis stage in 8 patients with BA and there was no data regarding AUROC of ARFI in predicting fibrosis in BA patients8. Since there was limited data on the efficacy of SSWE in liver fibrosis for pediatric patients, we tried to compare them with those of adult studies. Two adult studies reported the AUROCs of 0.88 to 0.90 and 0.90 to 0.93 for SSWE in predicting significant fibrosis and severe fibrosis, respectively41,42. Compared to these, the diagnostic accuracy of SSWE was slightly poorer in our study. The underlying explanations might be as follows. First, adults could corporate better than children with complete breath holding during the SSWE examination and the influence of respiration to SSWE results could not be completely reduced by sedation for pediatric patients. Second, the serum levels of bilirubin especially the DBIL could affect the SSWE values, and they were relatively not high for patients in adult studies but might be abnormally high for some children in our study. It was reported that bile duct obstruction with increased level of bilirubin could influence the evaluation of liver stiffness by over-diagnosis of liver cirrhosis43. The mechanisms behind the high stiffness in cholestasis remained unclear but might be probably associated with tissue swelling, inflammation, edema, and increased intracellular pressure43. Therefore, the threshold values of SSWE for different fibrosis stages were relatively higher in our study than those in adult studies. Third, the age range of our patients might be a little wide resulting in poor homogeneity of the study subjects. However, it was reported that there was no significant difference in SSWE values among different age groups22 which meant that age might not influence the SSWE results to a dominant level. Fourth, it remained uncertain whether there existed differences between children and adults, or between BA and other kinds of liver diseases for the performance of SSWE in predicting fibrosis. This required further studies to elucidate. In addition to its promising efficacy in predicting fibrosis, SSWE has technical and operating advantages over TE and ARFI. Unlike TE, SSWE can be performed with conventional US probes during an abdominal ultrasound scan as it was integrated into a conventional US system, which was much more convenient. Moreover, SSWE can locate ROI precisely with avoiding large vessels and bile ducts and real-time quantitative analysis of liver stiffness. Thus, SSWE, as a non-invasive, convenient, readily available and non-expensive technique, has shown encouraging predictive accuracy in liver fibrosis, which was critical for identifying high-risk patients and disease monitoring. This importance was especially significant for BA children after KPE who required close surveillance and hence were with more demanding on non-invasive tests due to frequent reexaminations.
APRI and FIB-4, as the noninvasive, readily available biomarkers, have performed well in predicting fibrosis in various adult liver diseases with AUROCs of more than 0.8023,24,25. Previous studies were consistent in reporting that APRI was superior to FIB-4 in predicting fibrosis stage for pediatric liver diseases28,44. As in our study, FIB-4 completely failed to be the reliable fibrosis biomarker (AUROC < 0.50). However, controversy remained regarding the diagnostic accuracy of APRI in liver fibrosis for children. On one hand, Leung DH et al. reported that APRI exhibited a high AUROC (0.81) in predicting advanced liver fibrosis in children with cystic fibrosis liver disease44. Kim SY et al. also suggested that APRI was a promising surrogate of advanced fibrosis (AUROC = 0.92) and cirrhosis (AUROC = 0.91) in children with BA45. On the other hand, a study from China reported a suboptimal performance of APRI in predicting cirrhosis (AUROC = 0.54) for children with BA40. A multicenter data from U.S.A also demonstrated that APRI had poor diagnostic accuracy for significant (AUROC = 0.67) or advanced fibrosis (AUROC = 0.63) in children with nonalcoholic fatty liver disease28. In our study, APRI proved to be a poor predictor for liver fibrosis in children with BA (AUROC = 0.65, 0.64, 0.56 for F ≥ 2, F ≥ 3, F = 4, respectively), which was consistent with the above two studies. Therefore, although APRI show extremely promising results in adult studies, the results could not be confirmed in BA children after KPE based on the data in this and previous studies. Besides, SSWE outperformed APRI in predicting liver fibrosis in our study. APRI did not seem to improve the diagnostic accuracy and reliability of SSWE in evaluating fibrosis stages. This might be explained by the fact that SSWE could reflect fibrosis severity with application of local mechanical compression on liver tissue using focused ultrasonography and acquiring strain images whereas the parameters used in the APRI such as AST and ALT are increased because of cholestasis itself or liver disease, in which their power in evaluating fibrosis stages is very limited. Since SSWE could reflect a patient’s liver fibrosis stage to some extent whereas laboratory tests could not, SSWE has the advantage over them as the tool to predict prognosis and to assist with designing future treatment plan. Moreover, SSWE is cheaper than laboratory tests. In such a limited resource world, SSWE may be cost-effective.
Furthermore, the effective measurement of liver fibrosis may help predict the liver function during follow-up. Thus, we turned to investigate the correlation of SSWE with the biochemical parameters of liver function at 6 months after SSWE. Results showed that SSWE were negatively correlated with PLT and ALB which reflected the extent of portal hypertension and synthesis function of liver. SSWE appeared to be moderately correlated with bilirubin (TBIL or DBIL) which was the recognized factor associated with prognosis of patients with BA. Thus, these results showed that SSWE might probably serve as the predictor of the prognosis of liver fibrosis during follow-up, which was consistent with the previous study suggesting that liver stiffness measurement values of 3 months after KPE can be used to predict the development of liver related events46. However, future studies with large sample size are needed to validate the above results.
In our study, there was two patients’ fibrosis stages over-diagnosed by SSWE. However, careful analysis of these cases suggested that they both had laboratory features suggestive of portal hypertension or actual cirrhosis. This indicates that the gold standard of liver biopsy is not always reliable due to the sampling error and the subjective nature of the reading. We should evaluate a patient’s fibrosis stage based on pathologic results together with clinical, laboratory and ultrasonic features.
There are a number of limitations to our study. First, the sample size is small without even distribution of patients in different fibrosis stage. The cut-off value chosen here is a relatively limited result based on the data of 24 patients. These factors make the statistical power of this study low. Second, it is a retrospective study with all its inherent defects. Third, our study excluded the patients with failure in performing SSWE examinations, in which we could not investigate the applicability of SSWE examinations in children with BA. In conclusion, SSWE might be a reliable and noninvasive tool for assessing liver fibrosis in postoperative children with BA whereas APRI showed poor predictability for fibrosis stage. However, due to the relatively low statistical power of this study, the findings here need to be validated in large prospective studies with long follow-up time in the future.
Additional Information
How to cite this article: Chen, S. et al. Supersonic shearwave elastography in the assessment of liver fibrosis for postoperative patients with biliary atresia. Sci. Rep. 6, 31057; doi: 10.1038/srep31057 (2016).
References
Hartley, J. L., Davenport, M. & Kelly, D. A. Biliary atresia. Lancet. 374, 1704e13 (2009).
Davenport, M. et al. Seamless management of biliary atresia in England and Wales (1999e2002). Lancet. 363, 1354e7 (2004).
Hadzic, N. et al. Long term survival following Kasai portoenterostomy: is chronic liver disease inevitable? J Pediatr Gastroenterol Nutr. 37, 430–433(2003).
Ohi, R. Surgical treatment of biliary atresia in the liver transplantation era. Surg Today 28, 1229–1232 (1998).
Chardot, C. et al. Epidemiology of biliary atresia in France: a national study 1986–96. J Hepatol. 31, 1006–1013 (1999).
Bravo, A. A., Sheth, S. G. & Chopra, S. Liver biopsy. N Engl J Med. 344, 495–500 (2001).
Bedossa, P., Darge, ´re D. & Paradis, V. Sampling variability of liver biopsy in chronic hepatitis C. Hepatology 38, 1449–1457 (2003).
Shin, N. Y. et al. Transient elastography and sonography for prediction of liver fibrosis in infants with biliary atresia. J Ultrasound Med. 33, 853–864 (2014).
Shima, H. et al. Noninvasive acoustic radiation force impulse (ARFI) elastography for assessing the severity of fibrosis in the post-operative patients with biliary atresia. Pediatr Surg Int. 28, 869–872 (2012).
Matos, H., Trindade, A. & Noruegas, M. J. Acoustic radiation force impulse imaging in paediatric patients: normal liver values. J Pediatr Gastroenterol Nutr. 59, 684–688 (2014).
Leschied, J. R. et al. Shear wave elastography helps differentiate biliary atresia from other neonatal/infantile liver diseases. Pediatr Radiol. 45, 366–75 (2015).
Lupsor, M. et al. Performance of a new elastographic method (ARFI technology) compared to unidimensional transient elastography in the noninvasive assessment of chronic hepatitis C. Preliminary results. J Gastrointest Liver Dis. 18, 303–310 (2009).
Goldschmidt, I. et al. Application and limitations of transient liver elastography in children. J Pediatr Gastroenterol Nutr. 57, 109–13 (2013).
Engelmann, G. et al. Feasibility study and control values of transient elastography in healthy children. Eur J Pediatr. 171, 353–60 (2012).
Tutar, O. et al. Shear wave elastography in the evaluation of liver fibrosis in children. J Pediatr Gastroenterol Nutr. 58, 750–755 (2014).
Friedrich-Rust, M. et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 252, 595–604 (2009).
Bavu, E. et al. Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: a clinical study on 113 hepatitis C virus patients. Ultrasound Med Biol. 37, 1361–1373 (2011).
Bercoff, J., Tanter, M. & Fink, M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE T Ultrason Ferr. 51, 396–409 (2004).
Leung, V. Y. et al. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology 269, 910–918 (2013).
Ferraioli, G. et al. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology 56, 2125–2133 (2012).
Zheng, J. et al. Two-dimensional shear-wave elastography and conventional US: the optimal evaluation of liver fibrosis and cirrhosis. Radiology 275, 290–300 (2015).
Franchi-Abella, S. et al. Feasibility and Diagnostic Accuracy of Supersonic Shear-Wave Elastography for the Assessment of Liver Stiffness and Liver Fibrosis in Children: A Pilot Study of 96 Patients. Radiology 278, 554–562 (2016).
Lin, Z. H. et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 53, 726–736 (2011).
Wai, C. T. et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38, 518–526 (2003).
Li, Y., Chen, Y. & Zhao, Y. The diagnostic value of the FIB-4 index for staging hepatitis B-related fibrosis: a meta-analysis. PLoS One 9, e105728 (2014).
de Lédinghen, V. et al. Liver stiffness measurement in children using FibroScan: feasibility study and comparison with Fibrotest, aspartatetransaminase to platelets ratio index, and liver biopsy. J Pediatr Gastroenterol Nutr. 45, 443–450 (2007).
Flores-Caldero, 'n J. et al. Non-invasive markers of liver fibrosis in chronic liver disease in a group of mexican children. A multicenter study. Ann Hepatol. 11, 364–368 (2012).
Mansoor, S. et al. The evaluation of hepatic fibrosis scores in children with nonalcoholic fatty liver disease. Dig Dis Sci. 60, 1440–1447 (2015).
Patch, D. & Dagher, L. Acute variceal bleeding: general management. World J Gastroenterol 7, 466–475 (2001).
Moore, K. P. & Aithal, G. P. Guidelines on the management of ascites in cirrhosis. Gut 55 Suppl 6, vi1–12 (2006).
Sarin, S. K. et al. Acute-on- chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 3, 269–282 (2009).
Huang, Y. et al. Assessment of liver fibrosis in chronic hepatitis B using acoustic structure quantification: quantitative morphological ultrasound. Eur Radiol (2015) Oct 20. [Epub ahead of print].
Goodman, Z. D. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 47, 598–607 (2007).
Xu, X. et al. Performance of transient elastography assessing fibrosis of single hepatitis B virus infection: a systematic review and meta-analysis of a diagnostic test. Hepatol Int. 9, 558–566 (2015).
Crespo, G. et al. ARFI, FibroScan, ELF, and their combinations in the assessment of liver fibrosis: a prospective study. J Hepatol. 57, 281–287 (2012).
Corpechot, C. et al. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology 43, 1118–1124 (2006).
Wong, V. W. et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 51, 454–462 (2010).
Castera, L. et al. Prospective comparison of transient elastography, FibroTest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 128, 343–350 (2005).
Mueller, S. & Sandrin, L. Liver stiffness: a novel parameter for the diagnosis of liver disease. Hepat Med. 2, 49–67 (2010).
Shen, Q. L. et al. Assessment of liver fibrosis by Fibroscan as compared to liver biopsy in biliary atresia. World J Gastroenterol. 21, 6931–6936 (2015).
Gerber, L. et al. Assessment of liver fibrosis with 2-D shearwave elastography and acoustic radiation force impulse imaging in patients with chronic liver disease. Ultrasound in Med. & Biol. 41, 2350–2359 (2015).
Cassinotto, C. et al. Non-invasive assessment of liver fibrosis with impulse elastography: comparison of Supersonic Shear Imaging with ARFI and FibroScan. J Hepatol. 61, 550–557 (2014).
Millonig, G. et al. Extrahepatic Cholestasis Increases Liver Stiffness (FibroScan) Irrespective of Fibrosis. Hepatology 48, 1718–1723 (2008).
Leung, D. H. et al. Aspartate aminotransferase to platelet ratio and fibrosis-4 as biomarkers in biopsy-validated pediatric cystic fibrosis liver disease. Hepatology 62, 1576–83 (2015).
Kim, S. Y., Seok, J. Y., Han, S. J. & Koh, H. Assessment of liver fibrosis and cirrhosis by aspartate aminotransferase-to-platelet ratio index in children with biliary atresia. J Pediatr Gastroenterol Nutr. 51, 198–202 (2010).
Hahn, S. M., Kim, S., Park, K. I., Han, S. J. & Koh, H. Clinical benefit of liver stiffness measurement at 3 months after Kasai hepatoportoenterostomy to predict the liver related events in biliary atresia. PLoS One 8, e80652 (2013).
Acknowledgements
This study was supported by National Natural Science Foundation of China (NO: 81501480) and Natural Science Foundation of Guangdong province (NO: 2015A030313060). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
L.Z. and S.C. designed and conceived the study; S.C., B.L., Z.Z., Y.Z., B.L. and Q.S. collected data, performed the statistical analysis and interpreted the data; S.C., B.L. and L.Z. drafted the manuscript; L.Z., X.X. and S.C. revised and recheck the articles; all the authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chen, S., Liao, B., Zhong, Z. et al. Supersonic shearwave elastography in the assessment of liver fibrosis for postoperative patients with biliary atresia. Sci Rep 6, 31057 (2016). https://doi.org/10.1038/srep31057
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep31057
This article is cited by
-
Performance of two-dimensional shear wave elastography for detecting advanced liver fibrosis and cirrhosis in patients with biliary atresia: a systematic review and meta-analysis
Pediatric Radiology (2023)
-
Development and validation of non-invasive models in predicting advanced fibrosis of choledochal cyst
Pediatric Surgery International (2023)
-
Diagnosis of liver cirrhosis with two-dimensional shear wave elastography in biliary atresia before Kasai portoenterostomy
Pediatric Surgery International (2022)
-
Comparative study between liver biopsy and non-invasive biomarkers in assessment of hepatic fibrosis in children with chronic liver diseases
Egyptian Pediatric Association Gazette (2021)
-
The usefulness of noninvasive liver stiffness assessment using shear-wave elastography for predicting liver fibrosis in children
BMC Medical Imaging (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.