Abstract
An essential factor for the production of nitric oxide by nitric oxide synthase 1 (NOS1), major modulator of cardiac function, is the cofactor tetrahydrobiopterin (BH4). BH4 is regulated by GTP cyclohydrolase 1, the rate-limiting enzyme in BH4 biosynthesis which catalyses the formation of dihydroneopterin 3′triphosfate from GTP, producing BH4 after two further steps catalyzed by 6-pyruvoyltetrahydropterin synthase and sepiapterin reductase. However, there are other essential factors involved in the regulation of NOS1 activity, such as protein inhibitor of NOS1 (PIN), calmodulin, heat shock protein 90 and NOS interacting protein. All these molecules have never been analysed in human non-ischemic dilated hearts (DCM). In this study we demonstrated that the upregulation of cardiac NOS1 is not accompanied by increased NOS1 activity in DCM, partly due to the elevated PIN levels and not because of alterations in biopterin biosynthesis. Notably, the PIN concentration was significantly associated with impaired ventricular function, highlighting the importance of this NOS1 activity inhibitor in Ca2+ homeostasis. These results take a central role in the current list of targets for future studies focused on the complex cardiac dysfunction processes through more efficient harnessing of NOS1 signalling.
Similar content being viewed by others
Introduction
Heart failure (HF) is an increasingly prevalent clinical problem with high rate of morbidity and mortality in industrialised countries and no curative treatment is currently available. Dilated cardiomyopathy (DCM) is one of the most frequent causes of HF. This severe pathology of unknown aetiology is characterised by dilation and systolic contractile dysfunction, with an increase in ventricular mass and volume and wall thickness1,2. The role of the free radical signalling molecule, nitric oxide (NO), in modulating cardiac function is well established3. This molecule is constitutively released in cardiomyocytes by both neuronal and endothelial isoforms of nitric oxide synthase (NOS1 and NOS3, respectively). NOS1, the major modulator of cardiac function and intracellular Ca2+ fluxes, has been extensively studied in experimental models4,5,6. An essential factor for the production of NO by NOS1 is the presence of the cofactor tetrahydrobiopterin (BH4). BH4 is regulated by GTP cyclohydrolase 1 (GCH1), the rate-limiting enzyme in BH4 biosynthesis which catalyses the formation of dihydroneopterin 3’triphosfate from GTP, producing BH4 after two further steps catalyzed by 6-pyruvoyltetrahydropterin synthase and sepiapterin reductase (SPR)5,7,8. However, there are other essential factors involved in the regulation of NOS1 activity, such as protein inhibitor of NOS1 (PIN: DYNLL1 gene), calmodulin, heat shock protein 90 and NOS interacting protein9,10,11. Although we have studied this system in ischemic human hearts12, all these molecules has never been analysed in DCM.
Here, we focused on the assessment of NOS1 activity and cardiac tetrahydrobiopterins synthesis in left ventricular tissue samples obtained from DCM patients and compared the results with non-diseased controls (CNTs). We also identify differentially expressed genes closely involved in this process using the sensitive and powerful technique of RNA sequencing (RNA-seq).
Results
Clinical characteristics of patients
We analysed a total of 20 DCM human hearts obtained from patients undergoing cardiac transplantation, who had age of 48 ± 13 years and an NYHA functional classification of III–IV. Patients had previously been diagnosed with significant comorbidities, including hypertension (21%) and diabetes mellitus (18%). Table 1 summarises the clinical characteristics of the DCM patients. CNT samples were acquired from 10 non-diseased donor hearts. The CNT group mainly consisted of males (80%), with a mean age of 47 ± 16 years.
Gene expression analysis
Differences in transcriptome levels between DCM and CNT samples were investigated through a large-scale screening of 23 heart samples (13 from DCM patients, 10 from CNTs) using RNA-seq technology. On comparing the two groups, we found 1628 differentially expressed genes, of which 596 were upregulated (≥1.5-fold increase; P < 0.05 for all) and 1032 were downregulated (≥1.5-fold decrease; P < 0.05 for all).
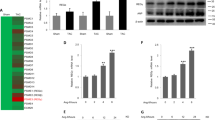
We focused on 28 NOS1-related genes involved in the regulation of myocardial Ca2+ fluxes and found significant differences in seven NOS1-related genes between the DCM samples and the CNTs. A heat map and hierarchical clustering were performed using MeV (v. 4.9.0) program to compare the altered genes in DCM samples with the corresponding genes in CNTs. Notably, this analysis identified two divergent gene expression profiles, showing a clear demarcation between the DCM and the CNT groups (Fig. 1A).
mRNA expression levels of altered NOS1-related genes involved in regulating the physiological function of myocytes in human dilated hearts.
Heat map and hierarchical clustering based on the fold change (FC) values. The heat map and hierarchical clustering analyses show the separation of the DCM and CNT groups. Columns: genes; rows: samples. Colours depict the relative expression level of each gene; blue: lowest, yellow: highest (A). The graph shows the values obtained by RNA-sequencing. The values from the controls were set to 1. FC units represent the fold induction over the CNT mRNA relative expression levels. Bars indicate FC ± SEM (standard error of the mean) (B). *P < 0.05 and **P < 0.01 versus the CNT group.
As shown in Table 2 and Fig. 1B, NOS1 was overexpressed in the DCM samples compared to the CNTs, while we did not find similar changes in NOS2 and NOS3. Important NOS1-related genes such as GCH1 and ATP2A2 had significantly decreased mRNA levels in the DCM samples, while mRNA levels of SPR, DYNLL1, ATP2A3, and RyR3 were upregulated. RT-qPCR analysis validated these results. We found that the expression of NOS1 (3.781 fold, P < 0.05), DYNLL1 (2.854 fold, P < 0.05) and SPR (3.018 fold, P < 0.05) were higher in DCM group than in CNT and GCH1 mRNA levels were decreased (−1.320 fold, P < 0.05).
NOS1 protein levels, activity and protein inhibitor of NOS1 (PIN) in human dilated hearts
Using western blotting, with total heart samples increased to 30, NOS1 protein levels were significantly increased in the DCM samples, as shown in Fig. 2A (140 ± 24 au versus 100 ± 34 au, P < 0.01). However, total NOS and NOS1 activity did not reach statistical significance when we compared pathological and CNT hearts (Fig. 2B). In addition, we found an increase in PIN levels in DCM hearts (126 ± 25 au versus 100 ± 6 au, P < 0.01) (Fig. 2C). Interestingly, we observed a significant negative correlation between PIN protein levels and LV ejection fraction (r = −0.682, P < 0.01, Fig. 2D). The complete parameters of LV function were available for 18 of 20 samples. We found protein-protein interactions of NOS1 with PIN (Fig. 2E) and a positive correlation between the levels of these proteins (r = 0.729, P < 0.01).
NOS1 protein levels, activity and protein inhibitor of NOS1 (PIN) in human dilated hearts.
NOS1 protein levels (A). Total NOS and NOS1 activity (B). PIN protein levels (C). Relationship between PIN protein levels and left ventricular ejection fraction (D). Association between NOS1 and PIN. Immunoprecipitates (IP) were immunoblotted for NOS1 and PIN, respectively. For the protein analysis (A,C), the values from the CNT group were set to 100. The data are expressed as optical density, arbitrary units (au). The values were normalised to GAPDH and finally to the CNTs. All data are expressed as means ± SEM (standard error of the mean). **P < 0.01 versus the CNT group.
Changes in GCH1 and SPR protein levels in human dilated hearts and tetrahydrobiopterin biosynthesis
The protein levels of GCH1 and SPR also differed between the DCM and CNT groups. The DCM group had lower levels of GCH1 (71 ± 21 au versus 100 ± 22 au, P < 0.01; Fig. 3A) and higher levels of SPR (126 ± 23 au versus 100 ± 34 au, P < 0.05; Fig. 3B) than the CNT group.
Differential GCH1 and SPR protein levels in human dilated hearts and tetrahydrobiopterin biosynthesis.
GCH1 (A) and SPR (B) protein levels. Values from the CNT group were set to 100. The data are expressed in optical density, arbitrary units (au). The values were normalised to GAPDH and finally to the CNT group. Biopterin content: BH2 (C), BH4 (D), total biopterins (E), BH4:BH2 + B ratio (F). All data are expressed as means ± SEM (standard error of the mean). *P < 0.05 and **P < 0.01 versus the CNT group.
To evaluate whether downregulated myocardial GCH1 leads to a decrease in biopterin content in pathological hearts, we compared the biopterin concentrations of DCM and CNT samples. As shown in Fig. 3C, we detected a small increase in BH2 in CNT hearts (3.47 ± 0.88 pmol/mg protein versus 4.96 ± 1.33 pmol/mg, P < 0.05). By contrast, we did not find differences in the BH4 level, total biopterins level and BH4:BH2 + B ratio (7.73 ± 2.77 pmol/mg protein versus 7.28 ± 1.70 pmol/mg protein, 12.18 ± 2.48 pmol/mg protein versus 13.87 ± 3.02 pmol/mg protein and 1.90 ± 0.98 versus 1.19 ± 0.47; respectively, Fig. 3D–F).
Discussion
In the present study we reveal that the upregulation of cardiac NOS1 in human dilated hearts is not accompanied by an increase in NOS activity or by alterations in BH4 levels. BH4 is an important regulator of cardiovascular homeostasis5,7,13. Several studies indicates that maintaining adequate BH4 levels in the endothelium is likely to be critical in regulating the balance between NO and O2− synthesis5,8. To date, studies have largely focused on the role of BH4 in inflammation and vascular pathologies14,15,16,17, but less is known about its role in the heart. Thus, the cardiomyocyte-specific implications of BH4 in cardiac pathologies remain unclear. Previous works have demonstrated that pressure overload induced by transverse aortic constriction in mice reduces cardiac BH4 levels, leading to myocyte hypertrophy, cardiac dilation, interstitial fibrosis and ventricular dysfunction8. In addition, the effect of NOS uncoupling in a mouse model of diastolic dysfunction was shown to result in increased cardiac oxidative stress, principally from uncoupled NOS1 and an reduced BH4:oxidised biopterins ratio18. A key factor for NO production by NOS1 is the presence of BH4, regulated by GCH1, the rate-limiting enzyme in BH4 biosynthesis, which catalyses the formation of dihydroneopterin 3’triphosfate from GTP, producing BH4 in two further steps through the activities of 6-pyruvoyltetrahydropterin synthase and SPR5,7,8. In the present work, we found an increase in cardiac NOS1 levels in pathological hearts, but in no change in its activity. Thus, we aimed to identify the possible factors, which are responsible for the NOS1 activity to be similar in dilated and control hearts in spite of the elevated levels of NOS1 found in the pathological samples. We measured the biopterin concentrations in the DCM and CNT samples and did not find changes in the BH4 level, total biopterin level, or BH4:BH2 + B ratio, despite finding significantly lower levels of GCH1. This decrease in GCH1 could be compensated for the high levels of SPR found in these dilated hearts, highlighting the role of SPR as a compensatory factor to maintain the normal levels of biopterins in conditions of low GCH1 concentrations and supporting previous results from an experimental model14.
Other essential molecules involved in the regulation of NOS1 activity, such as calmodulin, heat shock protein 90 and NOS interacting protein, did not differ between the DCM and CNT groups. However, we found a substantial increase in expression and protein levels of PIN (DYNLL1 gene) in DCM hearts and a good relationship with NOS1 levels. Although is it not possible to determine if NOS1 activity show dose-dependency in our samples under the assay conditions, these results could help explain the lack of change in NOS1 activity despite the high levels found in the pathological samples, as PIN physically interacts and inhibits the activity of NOS111. Fan et al. purified large quantities of PIN overexpressed in bacterial cells and showed that the PIN-binding region of NOS1 specifically mapped to a 17-residue peptide fragment from Met-228 to His-244 of NOS1. They also reported that PIN binds to NOS1 with a 1:2 stoichiometry19. We found a significant relationship between PIN protein levels and LV ejection fraction, indicating that increased PIN levels are associated with functional deterioration and supporting the direct involvement of this inhibitor in myocardial Ca2+ homeostasis. Therefore, our findings indicate new interesting directions for research on dilated function pathogenesis and may provide targets in this complex process that deserve further investigation.
In addition, we detected the presence of important alterations in different NOS1-related counterparts also involved in the regulation of the physiological function of cardiomyocytes in the cardiac tissue of DCM patients. The central role of the cardiac sarcoplasmic reticulum and its Ca2+-handling proteins, including RyRs, SERCA2a and phospholamban (PLB), in excitation-contraction coupling is well known20,21,22,23. NOS1 is normally located in the sarcoplasmic reticulum, where it associates with RyR and it is involved in the activities of calcium-handling molecules. In HF, the level and activity of SERCA2a are decreased, contributing directly to impaired cardiac contraction and relaxation20,21. It has been reported that SERCA2a is impaired in the absence of NOS124. As expected, we found SERCA2a to be downregulated in our study. Furthermore, we observed alterations in the expression of RyR3 but not in that of RyR2; this result could open an unexplored area of research into the role of this isoform in HF.
A common limitation of studies that examine cardiac tissues from end-stage HF patients is the presence of extensive variability in both disease aetiology and treatment.
In conclusion, we demonstrate that the upregulation of cardiac NOS1 is not accompanied by an increase in NOS1 activity in dilated hearts, due in part to the elevated levels of PIN found and not to alterations in biopterins biosynthesis. Notably, the protein concentrations of PIN were significantly associated with impaired ventricular function, highlighting the importance of this inhibitor of NOS1 activity in Ca2+ homeostasis. These results take a central role in the current list of targets for future studies focused on the complex cardiac dysfunction process that occurs in DCM through more efficient harnessing of NOS signalling.
Methods
Collection of samples
Experiments were performed with LV samples from 30 explanted human hearts: 20 from patients with DCM undergoing cardiac transplantation and 10 from non-diseased CNTs. Clinical history, hemodynamic study, ECG and Doppler echocardiography data were available from these patients. Non-ischemic DCM was diagnosed when patients had LV systolic dysfunction (EF < 40%) with a dilated non-hypertrophic left ventricle (LVEDD > 55 mm) on echocardiography. Moreover, patients did not show existence of primary valvular disease and familial DCM. All patients were functionally classified according to the New York Heart Association (NYHA) criteria and they were receiving medical treatment following the guidelines of the European Society of Cardiology25. Clinical characteristics of patients are summarised in Table 1.
All CNTs had normal LV function (EF > 50%), as determined by Doppler echocardiography. None had any history of cardiac disease. CNT samples were obtained from non-diseased donor hearts that had been rejected for cardiac transplantation owing to size or blood type incompatibility, or due to non-availability of a suitable recipient within the requisite time period. All these donors died of either cerebrovascular or motor vehicle accidents.
Our hospital has been ranked as the national leader in heart transplantation for the third time with more than 700 transplants accomplished in the last 25 years. In accordance we acquire high-quality samples as revealed by high values (greater or equal to 9) of RNA integrity number (RIN). We had access to operating rooms during interventions and full explanted hearts in all cases. For each procedure, we chose tissue samples from the same area of the left ventricle to standardize our research methodology. In our study, all tissue samples were obtained from near the apex of the left ventricle, were maintained in 0.9% NaCl and were preserved at 4 °C for a maximum of 6 hours after the loss of coronary circulation. Samples were stored at −80 °C until used.
This study was approved by the Ethics Committee (Biomedical Investigation Ethics Committee of La Fe University Hospital of Valencia, Spain). Prior to tissue collection, signed informed consent was obtained from each patient. The study was conducted in accordance with the guidelines of the Declaration of Helsinki26.
RNA sequencing, RT-qPCR validation and protein analysis
Methods used for RNA extraction, RNA-seq and computational analysis of the RNA-seq data were performed as previously described27. For this analysis, LV tissue samples were obtained from 13 DCM patients and 10 non-diseased CNTs. The data presented in this publication have been deposited in the NCBI Gene Expression Omnibus (GEO) and can be retrieved using the GEO Series accession number GSE55296 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE55296). Methods used for RT-qPCR analysis are included as Supplementary Information and to improve the numerical base with a higher number of samples in this validation we increased the DCM samples up to 20. We determined the protein levels of NOS1, PIN, GCH1 and SPR with total heart samples increased to 30. We also studied protein-protein interactions of NOS1 with PIN. Data are included as Supplementary Information.
Nitric oxide synthase 1 activity and tetrahydrobiopterins determination
Total NOS and NOS1 activity and myocardial biopterins were assessed by high performance liquid chromatography (HPLC), as described previously7,28. Details of these methods are included in the Supplementary Information.
Statistical methods
Data were expressed as mean ± standard deviation for continuous variables and as percentage for discrete variables. Significant mean differences between groups were analysed using the Mann-Whitney U test. Spearman’s correlation coefficients were calculated to determine the relationships between altered proteins and echocardiographic parameters. P <0.05 was considered statistically significant. All statistical analysis was performed using the SPSS software for Windows (version 20.0; IBM SPSS Inc; Chicago. IL, USA).
Additional Information
How to cite this article: Roselló-Lletí, E. et al. Protein Inhibitor of NOS1 Plays a Central Role in the Regulation of NOS1 Activity in Human Dilated Hearts. Sci. Rep. 6, 30902; doi: 10.1038/srep30902 (2016).
References
Richardson, P. et al. Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 93, 841–842 (1996).
Towbin, J. A. et al. Incidence, causes and outcomes of dilated cardiomyopathy in children. JAMA. 296, 1867–1876 (2006).
Massion, P. B., Feron, O., Dessy, C. & Balligand, J. L. Nitric oxide and cardiac function: ten years after and continuing. Circ Res. 93, 388–398 (2003).
Umar, S. & van der Laarse, A. Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic and failing heart. Mol Cell Biochem. 333, 191–201 (2010).
Carnicer, R., Crabtree, M. J., Sivakumaran, V., Casadei, B. & Kass, D. A. Nitric oxide synthases in heart failure. Antioxid Redox Signal. 18, 1078–1099 (2013).
Ashley, E. A., Sears, C. E., Bryant, S. M., Watkins, H. C. & Casadei, B. Cardiac nitric oxide synthase 1 regulates basal and beta-adrenergic contractility in murine ventricular myocytes. Circulation. 105, 3011–3016 (2002).
Carnicer, R. et al. Cardiomyocyte GTP cyclohydrolase 1 and tetrahydrobiopterin increase NOS1 activity and accelerate myocardial relaxation. Circ Res. 111, 718–727 (2012).
Bendall, J. K., Douglas, G., McNeill, E., Channon, K. M. & Crabtree, M. J. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid Redox Signal. 20, 3040–3077 (2014).
Bender, A. T. et al. Neuronal nitric-oxide synthase is regulated by the Hsp90-based chaperone system in vivo. J Biol Chem. 274, 1472–1478 (1999).
Nedvetsky, P. I., Sessa, W. C. & Schmidt, H. H. There’s NO binding like NOS binding: protein-protein interactions in NO/cGMP signaling. Proc Natl Acad Sci USA 99, 16510–16512 (2002).
Jaffrey, S. R. & Snyder, S. H. The protein inhibitor of neuronal nitric oxide synthase (PIN): characterization of its action on pure nitric oxide synthases. Science. 1, 274, 774–777 (1996).
Roselló-Lletí, E. et al. Human ischemic cardiomyopathy shows cardiac Nos1 translocation and its increased levels are related to left ventricular performance. Sci Rep. 6, 24060 (2016).
Nishijima, Y. et al. Tetrahydrobiopterin depletion and NOS2 uncoupling contribute to heart failure-induced alterations in atrial electrophysiology. Cardiovasc Res. 91, 71–79 (2011).
Gao, L. et al. Sepiapterin reductase regulation of endothelial tetrahydrobiopterin and nitric oxide bioavailability. Am J Physiol Heart Circ Physiol. 297, H331–H339 (2009).
Zhang, P. et al. Inducible nitric oxide synthase deficiency protects the heart from systolic overload-induced ventricular hypertrophy and congestive heart failure. Circ Res. 100, 1089–1098 (2007).
Thöny, B., Auerbach, G. & Blau, N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 347, 1–16 (2000).
Widder, J. D. et al. Regulation of tetrahydrobiopterin biosynthesis by shear stress. Circ Res. 101, 830–838 (2007).
d’Uscio, L. V. & Katusic, Z. S. Increased vascular biosynthesis of tetrahydrobiopterin in apolipoprotein E-deficient mice. Am J Physiol Heart Circ Physiol. 290, H2466–H2471 (2006).
Fan, J. S. et al. Protein inhibitor of neuronal nitric-oxide synthase, PIN, binds to a 17-amino acid residue fragment of the enzyme. J Biol Chem. 273, 33472–33481 (1998).
Mercadier, J. J. et al. Altered sarcoplasmic reticulum Ca2(+)-ATPase gene expression in the human ventricle during end-stage heart failure. J Clin Invest. 85, 305–309 (1990).
Arai, M., Alpert, N. R., MacLennan, D. H., Barton, P. & Periasamy, M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res. 72, 463–469 (1993).
Stokke, M. K. et al. Reduced SERCA2 abundance decreases the propensity for Ca2+ wave development in ventricular myocytes. Cardiovasc Res. 86, 63–71 (2010).
Stokke, M. K. et al. Ca(2+) wave probability is determined by the balance between SERCA2-dependent Ca(2+) reuptake and threshold SR Ca(2+) content. Cardiovasc Res. 90, 503–512 (2011).
Zhang, Y. H. et al. Reduced phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice. Circ Res. 102, 242–249 (2008).
McMurray, J. J. et al. ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 33, 1787–1847 (2012).
Macrae, D. J. The Council for International Organizations and Medical Sciences (CIOMS) guidelines on ethics of clinical trials. Proc Am Thorac Soc. 4, 176–178, discussion 178–9 (2007).
Roselló-Lletí, E. et al. ATP synthase subunit alpha and LV mass in ischaemic human hearts. J Cell Mol Med. 19, 442–451 (2015).
Crabtree, M. J., Tatham, A. L., Hale, A. B., Alp, N. J. & Channon K. M. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways. J Biol Chem. 284, 28128–28136 (2009).
Acknowledgements
The authors thank the Transplant Coordination Unit (Hospital Universitario y Politécnico La Fe) for their help in obtaining the myocardium samples. National Institute of Health “Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III” [PI13/00100 and PI14/01506], RETICS, RD12/0042/0003 and co-financed by European Regional Development Fund (FEDER).
Author information
Authors and Affiliations
Contributions
Conception and design the research: E.R.-L. and E.T. Analysis and interpretation of data: E.R.-L., A.O., E.T., R.C., M.R., F.L. and C.G.-C. Drafting the manuscript: E.R.-L. Critical revision of the manuscript for important intellectual content: E.R.-L., E.T., M.R., M.P. and J.R.G.-J. Final approval of the manuscript: E.R.-L., R.C., E.T., A.O., C.G.-C., F.L., J.R.G.-J., M.P. and M.R.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Roselló-Lletí, E., Tarazón, E., Ortega, A. et al. Protein Inhibitor of NOS1 Plays a Central Role in the Regulation of NOS1 Activity in Human Dilated Hearts. Sci Rep 6, 30902 (2016). https://doi.org/10.1038/srep30902
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep30902
This article is cited by
-
Circulating Sphingosine-1-Phosphate as A Non-Invasive Biomarker of Heart Transplant Rejection
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.