Abstract
Variations within genes associated with dyslexia result in a language network vulnerability, and in patients with Frontotemporal Dementia (FTD), language disturbances represent a disease core feature. Here we explored whether variations within three related-dyslexia genes, namely KIAA0319, DCDC2, and CNTNAP, might affect cortical thickness measures in FTD patients. 112 FTD patients underwent clinical and neuropsychological examination, genetic analyses and brain Magnetic Resonance Imaging (MRI). KIAA0319 rs17243157 G/A, DCDC2 rs793842 A/G and CNTNAP2 rs17236239 A/G genetic variations were assessed. Cortical thickness was analysed by Freesurfer. Patients carrying KIAA0319 A*(AG or AA) carriers showed greater cortical thickness atrophy in the left fusiform and inferior temporal gyri, compared to KIAA0319 GG (p ≤ 0.001). Patients carrying CNTNAP2 G*(GA or GG) showed reduced cortical thickness in the left insula thenCNTNAP2 AA carriers (p≤0.001). When patients with both at-risk polymorphisms were considered (KIAA0319 A* and CNTNAP2 G*), greater and addictive cortical thickness atrophy of the left insula and the inferior temporal gyrus was demonstrated (p ≤ 0.001). No significant effect of DCDC2 was found. In FTD, variations of KIAA0319 and CNTNAP2 genes were related to cortical thickness abnormalities in those brain areas involved in language abilities. These findings shed light on genetic predisposition in defining phenotypic variability in FTD.
Similar content being viewed by others
Introduction
Frontotemporal dementia (FTD) is a heterogeneous group of neurodegenerative disorders characterized by progressive deterioration of social behavior and personality, deficits of executive functions and language impairment. The different phenotypes within the FTLD spectrum include two major syndromes, namely the behavioral variant of frontotemporal dementia (bvFTD) mainly characterized by cognitive, personality and social comportment impairment; and the primary progressive aphasia (PPA), characterized by a fastest and predominant disorder of language1,2,3.
These phenotypes are characterized by frontal and temporal atrophy, thus language disorders manifestations are considered a core future of PPAs but present even in bvFTD4,5. They include abnormalities in speech production, word retrieval, object naming, word and sentence comprehension, as well as difficulty in the mastery of reading and/or spelling skills (dyslexia)4,5,6,7,8.
A high frequency of neurodevelopmental learning disability, including dyslexia, has been reported in FTD patients and their first-degree relatives9. Considering the strong genetic background of language acquisition as well as its impairment, it might be argue for the involvement of specific genes potentially causative of a susceptibility of language-associated brain regions in FTD10. Additionally, dyslexic individuals showed structural and functional changes of the left temporal regions, those regions selectively damaged in FTD patients11,12,13. Language-related genes might drive the anatomical distribution of regional atrophy in FTD and clinical presentation.
In this scenario, imaging-derived grey matter measurements could help to demonstrate whether those genes that predispose to language impairment modulate FTD presentation. In that regards, by voxel-based morphometry (VBM) methods, previous studies highlighted that FOXP2 gene, linked to the acquisition of spoken language, is associated to greater hypoperfusion of the left frontal regions14,15. Similarly, we recently demonstrated that KIAA0319 gene, associated to reading ability, is related to greater grey matter volume atrophy in the left temporal regions in FTD patients16. In the same study, no effect of other two dyslexia genes, namely DCDC2 and CNTNAP2, was found by using VBM. However, previous findings underline the importance of selecting the appropriate neuroimaging measurement to successfully identify genes that influence brain structure or function17. More specifically, inimaging genetic studies it has been claimed that VBM may be limited by not discriminating genetically independent measurement as cortical thickness. Total grey matter volumes sum both cortical surface area and cortical thickness variables, these two measurements being demonstrated to depend on different genetic substrates18; thus, considering only one of these two variables, i.e. cortical thickness, might allow to obtain specific and reliable relationship with chosen genetic factors. Moreover, studies performed in a voxel-per-voxel basis generally require that the images are aligned to a common space, which has the potential for introducing additional biases, such as misalignment and misclassification17.
In the present study, we used a genetically independent and fully automated brain measure as cortical thickness to investigate the effect of three dyslexia genes, KIAA0319, DCDC2, CNTNAP2 on language networks in a cohort of FTD patients, with the attempt to confirm and extend our previous VBM findings.
Methods
Setting and participants
Patients fulfilling criteria for FTD were consecutively recruited from the Centre for Neurodegenerative Disorders, University of Brescia, Italy, from December 2001 to July 2014. All patients underwent somatic and neurologic evaluation, routine laboratory examination, and a complete mental status evaluation. Only patients with brain MRI and blood sampling for genetic analyses were considered in the present study.
Each patient was screened for monogenic inherited disease, such as GRN, MAPT, or C9orf72 mutations. Stringent exclusion criteria were applied as follows: (1) cerebrovascular disorders, previous stroke, hydrocephalus, and intracranial mass documented by MRI; (2) history of traumatic brain injury or another neurologic disease; (3) relevant medical problems (e.g., poorly controlled diabetes or hypertension; cancer within the past 5 years; clinically important hepatic, renal, cardiac, or pulmonary disorders); (4) history of major depressive disorder, bipolar disorder, schizophrenia, substance abuse disorder, or mental retardation according to DSM-IV criteria; (5) cerebrospinal fluid beta-amyloid/tau profile suggestive for Alzheimer disease (available in almost 60% of patients); and (6) logopenic variant of PPA (lvPPA), according to current clinical criteria2 as mainly associated with Alzheimer disease neuropathology19.
A comprehensive neuropsychological and behavioural assessment, including Basic Activities of Daily Living and Instrumental Activities of Daily Living, was carried out20,21. The neuropsychological testing was performed by a standardized neuropsychological battery including Mini-Mental State Examination, Frontotemporal Dementia-modified Clinical Dementia Rating scale (FTD-modified CDR), Raven Colored Progressive Matrices, Controlled Oral Word Association Test and Category Fluency, Clock Drawing Test, Rey Complex Figure Copy and Recall, Story Recall Test, Digit Span, Trail Making Test A and B, Token Test, and De Renzi Imitation Test22,23,24,25,26,27,28,29,30,31,32.
In addition, a language evaluation was further perform to patients with PPA. Behavioral disturbances were evaluated by using the Frontal Behavioral Inventory and Neuropsychiatry Inventory33,34.
Informed consent was obtained for blood collection and genetic analyses and for MRI scanning from each patient. The work was performed in accordance to the approved guidelines on human subjects research of Helsinki Declaration and it was approved by the local Ethics Committee of Brescia Hospital, Italy.
Genetic analyses
Total genomic DNA was isolated from peripheral blood according to standard procedures. Three single nucleotide polymorphisms located within KIAA0319/TTRAP/THEM2 locus (rs17243157 G/A), DCDC2 (rs793842 A/G), and CNTNAP2 (rs17236239 A/G) genes were evaluated. Primers for each polymorphism were reported in Supplementary Table 1.
The amplification protocols were designed as follows: 5 minutes at 95 °C for the first cycle, denaturation at 95 °C for 30 seconds, annealing ranging from 59 °C to 66 °C for 30 seconds (depending on the analyzed polymorphism), extension at 72 °C for 30 seconds for the subsequent 35 cycles, and a final extension at 72 °C for 5 minutes. The PCR products were analyzed on 2% agarose with 0.005% of ethidium bromide to reveal the reaction and to verify their size.
To assess rs17243157 and rs793842, denaturing high performance liquid chromatography (DHPLC) analysis on the WAVE nucleic acid fragment analysis system was performed (Transgenomic, Santa Clara, CA). Samples with an altered DHPLC profile were purified with Microcon Centrifugal filter devices (Amicon Bioseparations; Millipore Corp, Billerica, MA) and sequenced. Nucleotide direct sequencing was performed on genomic DNA for both strands by the ABI 3500xl DNA analyzer(Applied Biosystems, Foster City, CA) and analyzed using Chromas (Technelysium Pty Ltd, South Brisbane, Australia).
To assess rs17236239, direct sequencing was performed for both strands from purified PCR on the ABI 3500 DNA genetic analyzer (Applied Biosystems), according to the manufacturer’s instructions, and analyzed using Chromas (Technelysium Pty Ltd).
Genotype analyses were performed blinded to clinical diagnoses.
MRI data acquisition and analyses
In the present study, brain images were collected using 2 different MRI scanners: (1) 1.5T MR scanner (Siemens Symphony, Erlangen, Germany), equipped with a circularly polarized transmit–receive coil to acquire 3D magnetization-prepared rapid gradient echo (MPRAGE) T1-weighted scan (repetition time [TR] 2.010 milliseconds, echo time [TE] 3.93 milliseconds, matrix 1 × 1 × 1, in-plane field of view [FOV] 250 3 250 mm2, slice thickness 1 mm, flip angle 15°); and (2) 1.5T MR scanner (Siemens Avanto) to acquire 3D MPRAGE T1-weighted scan (TR 2.050 milliseconds, TE 2.56 milliseconds, matrix 1 × 1 × 1, in-plane FOV 256 mm2, slice thickness 1 mm, flip angle 15°).
T1-weighted MRI were analyzed with FreeSurfer version 5.0 35. The surface-based pipeline consists of several stages previously described in detail35,36,37. Briefly, for each subject the T1-weighted, anatomical 3-dimensional MRI data sets were corrected for the intensity variations and a normalized intensity image was created. The volume was registered with the Talairach atlas through an affine registration. Next, the skull was stripped and extracerebral voxels were removed. Through a segmentation procedure, voxels were classified as white or grey matter, and cutting planes were chosen to separate the hemispheres. Cortical thickness measurements were than obtained by calculating the boundary between white matter and cortical grey matter in the left and right hemisphere separately at each of approximately 160,000 points per hemisphere across the cortical mantle.
To compare anatomic features across subjects, the surface of each subject was inflated to determine the large-scale folding patterns of the cortex and subsequently transformed into a sphere to minimize metric distortion. The folding patterns of each individual were then aligned with an average folding pattern using a high-resolution surface-based averaging. Thickness measures were mapped to the inflated surface of each participant’s brain reconstruction allowing visualization of data across the entire cortical surface. Finally, cortical thickness was smoothed with a 10-mm full width at half height Gaussian kernel to reduce local variations in the measurement.
Statistical analysis
The data were analyzed by SPSS 21.0 software (http://www.spss.com). Subjects were analyzed according to genotype status for each evaluated polymorphism. Genotype distribution and allele frequencies were computed by Chi-Square test. Socio-demographic and clinical data were assessed by Mann-Whitney test and Chi-Square test, as appropriate.
Cortical thickness differences between groups were assessed using a vertex-by-vertex analysis and a two-sample t test in FreeSurfer, and the effect of each polymorphism was carried out by analysing patients carrying at-risk genotype vs. non carriers[i.e., KIAA0319 A*(GA or AA) vs KIAA0319 GG; DCDC2 G*(GA or GG) vs. DCDC2 AA; CNTNAP2 G*(GA or GG) vs. CNTNAP2 AA]. Moreover, the cumulative effect of at-risk polymorphisms was considered by analysing patients carrying all significant at-risk polymorphism vs. non-carriers.
Age, FTD-modified CDR, GRN autosomal dominant disorder (as the high number of patients bearing GRN mutation) and scanner type were introduced as covariates; the significance was set to P < 0.001 uncorrected for multiple comparisons, and cluster size was set to >300 vertices.
Finally, structural covariance analysis was further performed by means of FreeSurfer, to demonstrate that damaged areas were disconnected with intra- and inter- hemispheric brain regions. As previously demonstrated38, this approach relies on the assumption that functionally correlated brain regions show greater concordance in GM and WM volumes as a result of mutually trophic influences or common experience-related plasticity39,40. To this, a significant cluster of cortical thickness obtained in the previous analysis was selected and used as “seed” regions, exploring the pattern of covariance between the cortical thickness of this “seed” and the cortical thickness throughout the whole brain. More specifically the correlation analysis on a vertex-by-vertex basis was performed using the design matrix available in the Freesurfer - Qdec interface: a correlation model was estimated introducing as covariate of interest the thickness values of the selected seed region for each subjects. First the analysis was carried out in the three groups separately (no, one or two at-risk polymorphism carriers) to explore the pattern of covariance in FTD patients according to KIAA0319 and CNTNAP2 genotypes (p < 0.05 corrected for False Discovery Rate (FDR)). Then a statistical vertex-by-vertex direct comparisons of the connectivity pattern between groups was carried out (p ≤ 0.001 uncorrected).
Results
Subjects
One-hundred and eighteen subjects fulfilled inclusion/exclusion criteria. From this sample, 6 subjects were excluded for crashing of freesurfer pipeline and 112 entered in the present study, namely 79 patients with bvFTD, and 33 with PPA.
Demographic and clinical characteristics were reported in Table 1. No significant differences between bvFTD and PPA were found, with exception of gender.
No significant difference of genotype distribution and allele frequency of KIAA0319 (rs17243157 G/A), DCDC2 (rs793842 A/G) and CNTNAP2 (rs17236239 A/G) polymorphisms between bvFTD and PPA was reported (see Supplementary Table 2).
MRI analyses
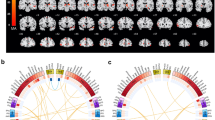
As reported in Fig. 1A and Table 2, patients carrying KIAA0319 A*(GA or AA) showed reduced cortical thickness in the left fusiform and inferior temporal gyri as compared to KIAA0319 GG (p ≤ 0.0001).
Maps of cortical thickness reduction in FTD patients carrying only one at-risk genotype: KIAA0319 A* vs. KIAA0319 GG (A* < GG) (Panel A), CNTNAP2 G * vs. CNTNAP2 AA (G* < AA) (Panel B). Panel (C): maps of cortical thickness reduction in FTD patients carrying both at-risk genotypes (KIAA0319 A* and CNTNAP G*) vs. patients carrying only one at-risk polymorphism (KIAA0319 A* or CNTNAP G*) and non-carriers (KIAA0319 GG and CNTNAP AA). P ≤ 0.001 uncorrected, clusters ≥ 300 voxels. L = left; R = right.
As shown in Fig. 1B and Table 2, patients carrying CNTNAP2 G*(GA or GG) showed reduced cortical thickness in the left insula as compared to CNTNAP2 AA (p ≤ 0.001).
No significant differences in cortical thickness were found when DCDC2 (rs793842 A/G) was considered.
We then evaluated the cumulative effect of the two at-risk polymorphisms taken together (KIAA0319 A* and CNTNAP G*). Patients carrying both genetic variations showed greater cortical thickness atrophy of the left insula and inferior temporal gyrus again, in addition to the left middle temporal gyrus, as compared to patients with only one at-risk polymorphisms (KIAA0319 A* or CNTNAP G*) or none (KIAA0319 GG and CNTNAP AA) (p ≤ 0.001, see Fig. 1C and Table 2).
The inverse comparisons for each above analysis did not show any significant clusters above the pre-established threshold.
To assess inter- and intra-hemispheric connectivity according to KIAA0319 and CNTNAP2 genotypes, structural correlation analysis was carried out. As shown in Fig. 2, a progressive decreased structural correlation between the left middle temporal gyrus (peak MNI coordinates x, y, z: −55,−36,−10) and other intra-hemispheric brain regions was highlighted (p ≤ 0.05, FDR corrected). Patients with no at-risk polymorphism (KIAA0319 GG and CNTNAP AA) showed a widespread structural correlation of left temporal gyrus with the left hemisphere (Panel A); this correlation progressively worse in those patients carrying one at-risk polymorphism (KIAA0319 A* or CNTNAP G*) (Panel B), to patients carrying both at-risk polymorphisms (KIAA0319 A* and CNTNAP G*) (Panel C) (see also Supplementary Table 3a). Plots of the data in Panel D show the linear correlation of decreased structural association between brain regions in FTD patients carrying only one or both at-risk polymorphisms than patients carrying no at-risk genotype (see also Supplementary Table 3b).
Pattern of structural correlation of the left middle temporal gyrus with other regions of the brain in: (Panel A) FTD patients carrying no at-risk polymorphism (KIAA0319 GG and CNTNAP AA), (Panel B) FTD patients carrying only one at-risk polymorphism (KIAA0319 A* or CNTNAP G*), (Panel C) FTD patients carrying both at-risk genotypes (KIAA0319 A* and CNTNAP G*). P ≤ 0.05 FDR corrected. L = left; R = right. Panel (D) Linear correlation of decreased structural association between brain regions in FTD patients carrying only one at-risk polymorphism (KIAA0319 A* or CNTNAP G*) or both at-risk genotypes (KIAA0319 A* and CNTNAP G*) (black lines) than patients carrying no at-risk polymorphism (red lines). P < 0.001 uncorrected.
Discussion
FTD is a complex disorderthat encompassesheterogeneous phenotypes despite the same underlying neurodegenerative process41. The cause of this variability is not yet known. However, the well known genetic substrate of FTD might suggest that heritable factors interact with the neurodegenerative process and drive the anatomical distribution of damage.
Language impairment is a key feature of FTD2, and learning disabilities, including dyslexia, have been reported in FTD patients and their first degree relatives19. Consequently, it has been hypothesized that an antecedent genetic vulnerability leads to the involvement of brain language networks10. Recent genetic studies have corroborated this issue, and genetic variants linked to spoken language and reading abilities, i.e. FOXP2, KIAA0319, DYX1C1, DCDC2, have been demonstrated to modify grey/white matter volumes and activation, in healthy controls and in patients with dyslexia42,43,44,45,46.
In the same view, FOXP2 and KIAA0319 genetic variations have been associated with greater brain atrophy in the language areas in FTD patients14,15,16.
In the present study, we firstly assessed the effect of dyslexia-related genes by evaluating variations of cortical thickness on a wide cohort of FTD patients. Indeed, cortical thickness is considered a preferred measure, then gray matter volumes, to carefully identify how genetic background may influence brain structure17.
By using this approach, we showed that KIAA0319 and CNTNAP2 genetic variations affect cortical thickness in the left temporal regions. Moreover, bearing both genetic polymorphisms had an addictive effect on cortical thickness damage within language network areas. This was further suggested by the presence of an intra-hemispheric breakdown between damaged regions and the rest of the brain in FTD patients with both at-risk polymorphisms.
The genetic association with KIAA0319 genotype confirmed and extended our previous voxel-based morphometry study16, and herein, by cortical thickness approach, we additionally provided evidence of CNTNAP2 influence on language. Both KIAA0319 and CNTNAP2 are considered two of the most consistently replicated genes associated with dyslexia47. There are several evidence that variants in KIAA0319 may contribute to reading abilities48,49,50,51, and previous genome-wide association studies have linked CNTNAP2 to specific language impairment and dyslexia52,53,54. These genes are involved in neuronal migration and maturation during early development, and the genetic polymorphisms evaluated in the present study have been demonstrated to affect brain volume, brain connectivity and brain activation in language-related areas, and to drive hemispheric asymmetry42,45,55,56.
Indeed, temporal regions and perisylvian areas, including insula, represent an important neural correlate of dyslexia57,58,59,60.
In conclusion, we demonstrated that selective cortical thickness reduction in language-related areas is driven by dyslexia susceptibility genes in FTD patients. These results argue for the relevant role of language-related genes in modulating the clinical phenotype.
We acknowledge that this study entails some limits. Statistical analysis were not corrected for multiple comparisons and neuropathological confirmation was missing, and further studies considering only Primary Progressive Aphasia subtypes and Alzheimer dementia, are warranted. Furthermore, we have no reliable information on learning disabilities in these patients and in their siblings.
New development in molecular genetics have a great potential for characterizing speech and language impairments and for understanding their etiology and developmental course in the neurodegenerative conditions.
Additional Information
How to cite this article: Paternicó, D. et al. Frontotemporal dementia and language networks: cortical thickness reduction is driven by dyslexia susceptibility genes. Sci. Rep. 6, 30848; doi: 10.1038/srep30848 (2016).
References
Neary, D. et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51, 1546–1554 (1998).
Gorno-Tempini, M. L. et al. Classification of primary progressive aphasia and its variants. Neurology 76, 1006–1014, doi: 10.1212/WNL.0b013e31821103e6 (2011).
Rascovsky, K. et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477, doi: 10.1093/brain/awr179 (2011).
Macoir, J., Laforce, R. Jr., Monetta, L. & Wilson, M. Language deficits in major forms of dementia and primary progressive aphasias: an update according to new diagnostic criteria. Geriatr Psychol Neuropsychiatr Vieil 12, 199–208, doi: 10.1684/pnv.2014.0466 (2014).
Rogalski, E. J. et al. Association between the prevalence of learning disabilities and primary progressive aphasia. JAMA Neurol 71, 1576–1577, doi: 10.1001/jamaneurol.2014.2805 (2014).
Blair, M., Marczinski, C. A., Davis-Faroque, N. & Kertesz, A. A longitudinal study of language decline in Alzheimer’s disease and frontotemporal dementia. J Int Neuropsychol Soc 13, 237–245, doi: 10.1017/s1355617707070269 (2007).
Kertesz, A., Davidson, W., McCabe, P., Takagi, K. & Munoz, D. Primary progressive aphasia: diagnosis, varieties, evolution. J Int Neuropsychol Soc 9, 710–719, doi: 10.1017/s1355617703950041 (2003).
Kertesz, A., McMonagle, P., Blair, M., Davidson, W. & Munoz, D. G. The evolution and pathology of frontotemporal dementia. Brain 128, 1996–2005, doi: 10.1093/brain/awh598 (2005).
Rogalski, E., Johnson, N., Weintraub, S. & Mesulam, M. Increased frequency of learning disability in patients with primary progressive aphasia and their first-degree relatives. Arch Neurol 65, 244–248, doi: 10.1001/archneurol.2007.34 (2008).
Rogalski, E., Weintraub, S. & Mesulam, M. M. Are there susceptibility factors for primary progressive aphasia? Brain Lang 127, 135–138, doi: 10.1016/j.bandl.2013.02.004 (2013).
Brambati, S. M. et al. Regional reductions of gray matter volume in familial dyslexia. Neurology 63, 742–745 (2004).
Brambati, S. M., Ogar, J., Neuhaus, J., Miller, B. L. & Gorno-Tempini, M. L. Reading disorders in primary progressive aphasia: a behavioral and neuroimaging study. Neuropsychologia 47, 1893–1900, doi: 10.1016/j.neuropsychologia.2009.02.033 (2009).
Wilson, S. M. et al. The neural basis of surface dyslexia in semantic dementia. Brain 132, 71–86, doi: 10.1093/brain/awn300 (2009).
Padovani, A. et al. The speech and language FOXP2 gene modulates the phenotype of frontotemporal lobar degeneration. J Alzheimers Dis 22, 923–931, doi: 10.3233/jad-2010-101206 (2010).
Premi, E. et al. FOXP2, APOE, and PRNP: new modulators in primary progressive aphasia. J Alzheimers Dis 28, 941–950, doi: 10.3233/jad-2011-111541 (2012).
Paternico, D. et al. Dyslexia susceptibility genes influence brain atrophy in frontotemporal dementia. Neurol Genet 1, e24, doi: 10.1212/nxg.0000000000000024 (2015).
Winkler, A. M. et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage 53, 1135–1146, doi: 10.1016/j.neuroimage.2009.12.028 (2010).
Panizzon, M. S. et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex 19, 2728–2735, doi: 10.1093/cercor/bhp026 (2009).
Mesulam, M. M. et al. Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia. Brain 137, 1176–1192, doi: 10.1093/brain/awu024 (2014).
Katz, S., Ford, A. B., Moskowitz, R. W., Jackson, B. A. & Jaffe, M. W. Studies of illness in the aged. The index of adl: a standardized measure of biological and psychosocial function. Jama 185, 914–919 (1963).
Lawton, M. P. & Brody, E. M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9, 179–186 (1969).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198 (1975).
Knopman, D. S. et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain 131, 2957–2968, doi: 10.1093/brain/awn234 (2008).
Bingham, W. C., Burke, H. R. & Murray, S. Raven’s progressive matrices: construct validity. J Psychol 62, 205–209, doi: 10.1080/00223980.1966.10543785 (1966).
Isaacs, B. & Kennie, A. T. The Set test as an aid to the detection of dementia in old people. Br J Psychiatry 123, 467–470 (1973).
Sunderland, T. et al. Clock drawing in Alzheimer’s disease. A novel measure of dementia severity. J Am Geriatr Soc 37, 725–729 (1989).
Loring, D. W., Martin, R. C., Meador, K. J. & Lee, G. P. Psychometric construction of the Rey-Osterrieth Complex Figure: methodological considerations and interrater reliability. Arch Clin Neuropsychol 5, 1–14 (1990).
Babcock, H. & Levy, L. The measurement of mental efficiency of mental functioning (revised examination) (1940).
Blackburn, H. L. & Benton, A. L. Revised administration and scoring of the digit span test. J Consult Psychol 21, 139–143 (1957).
Reitan, R. M. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills 8, 271–276, doi: 10.2466/PMS.8.7.271-276 (1958).
De Renzi, E., Motti, F. & Nichelli, P. Imitating gestures. A quantitative approach to ideomotor apraxia. Arch Neurol 37, 6–10 (1980).
De Renzi, E. & Vignolo, L. A. The token test: A sensitive test to detect receptive disturbances in aphasics. Brain 85, 665–678 (1962).
Kertesz, A., Davidson, W. & Fox, H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci 24, 29–36 (1997).
Cummings, J. L. et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 44, 2308–2314 (1994).
Fischl, B. & Dale, A. M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97, 11050–11055, doi: 10.1073/pnas.200033797 (2000).
Fischl, B., Sereno, M. I. & Dale, A. M. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 9, 195–207, doi: 10.1006/nimg.1998.0396 (1999).
Gronenschild, E. H. et al. The effects of FreeSurfer version, workstation type, and Macintosh operating system version on anatomical volume and cortical thickness measurements. PLoS One 7, e38234, doi: 10.1371/journal.pone.0038234 (2012).
Seeley, W. W., Crawford, R. K., Zhou, J., Miller, B. L. & Greicius, M. D. Neurodegenerative diseases target large-scale human brain networks. Neuron 62, 42–52, doi: 10.1016/j.neuron.2009.03.024 (2009).
Fornito, A., Zalesky, A. & Breakspear, M. The connectomics of brain disorders. Nat Rev Neurosci 16, 159–172, doi: 10.1038/nrn3901 (2015).
Montembeault, M. et al. The impact of aging on gray matter structural covariance networks. Neuroimage 63, 754–759, doi: 10.1016/j.neuroimage.2012.06.052 (2012).
Seelaar, H., Rohrer, J. D., Pijnenburg, Y. A., Fox, N. C. & van Swieten, J. C. Clinical, genetic and pathological heterogeneity of frontotemporal dementia: a review. J Neurol Neurosurg Psychiatry 82, 476–486, doi: 10.1136/jnnp.2010.212225 (2011).
Pinel, P. et al. Genetic variants of FOXP2 and KIAA0319/TTRAP/THEM2 locus are associated with altered brain activation in distinct language-related regions. J Neurosci 32, 817–825, doi: 10.1523/jneurosci.5996-10.2012 (2012).
Liegeois, F. et al. Language fMRI abnormalities associated with FOXP2 gene mutation. Nat Neurosci 6, 1230–1237, doi: 10.1038/nn1138 (2003).
Liegeois, F., Morgan, A. T., Connelly, A. & Vargha-Khadem, F. Endophenotypes of FOXP2: dysfunction within the human articulatory network. Eur J Paediatr Neurol 15, 283–288, doi: 10.1016/j.ejpn.2011.04.006 (2011).
Darki, F., Peyrard-Janvid, M., Matsson, H., Kere, J. & Klingberg, T. Three dyslexia susceptibility genes, DYX1C1, DCDC2, and KIAA0319, affect temporo-parietal white matter structure. Biol Psychiatry 72, 671–676, doi: 10.1016/j.biopsych.2012.05.008 (2012).
Whalley, H. C. et al. Genetic variation in CNTNAP2 alters brain function during linguistic processing in healthy individuals. Am J Med Genet B Neuropsychiatr Genet 156b, 941–948, doi: 10.1002/ajmg.b.31241 (2011).
Newbury, D. F. et al. Investigation of dyslexia and SLI risk variants in reading- and language-impaired subjects. Behav Genet 41, 90–104, doi: 10.1007/s10519-010-9424-3 (2011).
Francks, C. et al. A 77-kilobase region of chromosome 6p22.2 is associated with dyslexia in families from the United Kingdom and from the United States. Am J Hum Genet 75, 1046–1058, doi: 10.1086/426404 (2004).
Cope, N. et al. Strong evidence that KIAA0319 on chromosome 6p is a susceptibility gene for developmental dyslexia. Am J Hum Genet 76, 581–591, doi: 10.1086/429131 (2005).
Harold, D. et al. Further evidence that the KIAA0319 gene confers susceptibility to developmental dyslexia. Mol Psychiatry 11, 1085–1091, 1061, doi: 10.1038/sj.mp.4001904 (2006).
Paracchini, S. et al. Association of the KIAA0319 dyslexia susceptibility gene with reading skills in the general population. Am J Psychiatry 165, 1576–1584, doi: 10.1176/appi.ajp.2008.07121872 (2008).
Rodenas-Cuadrado, P., Ho, J. & Vernes, S. C. Shining a light on CNTNAP2: complex functions to complex disorders. Eur J Hum Genet 22, 171–178, doi: 10.1038/ejhg.2013.100 (2014).
Vernes, S. C. et al. A functional genetic link between distinct developmental language disorders. N Engl J Med 359, 2337–2345, doi: 10.1056/NEJMoa0802828 (2008).
Peter, B. et al. Replication of CNTNAP2 association with nonword repetition and support for FOXP2 association with timed reading and motor activities in a dyslexia family sample. J Neurodev Disord 3, 39–49, doi: 10.1007/s11689-010-9065-0 (2011).
Paracchini, S. et al. The chromosome 6p22 haplotype associated with dyslexia reduces the expression of KIAA0319, a novel gene involved in neuronal migration. Hum Mol Genet 15, 1659–1666, doi: 10.1093/hmg/ddl089 (2006).
Clemm von Hohenberg, C. et al. CNTNAP2 polymorphisms and structural brain connectivity: a diffusion-tensor imaging study. J Psychiatr Res 47, 1349–1356, doi: 10.1016/j.jpsychires.2013.07.002 (2013).
Binder, J. R., Desai, R. H., Graves, W. W. & Conant, L. L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex 19, 2767–2796, doi: 10.1093/cercor/bhp055 (2009).
Steinbrink, C., Ackermann, H., Lachmann, T. & Riecker, A. Contribution of the anterior insula to temporal auditory processing deficits in developmental dyslexia. Hum Brain Mapp 30, 2401–2411, doi: 10.1002/hbm.20674 (2009).
Ackermann, H. & Riecker, A. The contribution(s) of the insula to speech production: a review of the clinical and functional imaging literature. Brain Struct Funct 214, 419–433, doi: 10.1007/s00429-010-0257-x (2010).
Henry, M. L., Beeson, P. M., Stark, A. J. & Rapcsak, S. Z. The role of left perisylvian cortical regions in spelling. Brain Lang 100, 44–52, doi: 10.1016/j.bandl.2006.06.011 (2007).
Acknowledgements
This work was supported by NETALS project founding.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to conception and design, and/or acquisition of data, and/or analysis and interpretation of data. In detail: D.P.: first draft of the manuscript, imaging analysis and interpretation of data. M.M.: critical revision of the manuscript for content, imaging analysis and interpretation of data. E.P.: imaging analysis and interpretation of data. M.C.: patients’ cohort, critical revision of the manuscript for content. S.G.: imaging analysis and interpretation of data. A.A.: patients’ cohort, analysis and interpretation of data. S.A.: genetic analysis, critical revision of the manuscript for content. E.B.: genetic analysis, critical revision of the manuscript for content. M.S.C.: critical revision of the manuscript for content. M.C.: first draft of the manuscript and interpretation of data. M.T.: patients’ cohort, critical revision of the manuscript for content. A.M.: patients’ cohort, critical revision of the manuscript for content. G.R.: critical revision of the manuscript for content. A.P.: patient cohort, critical revision of the manuscript for content. B.B.: first draft of the manuscript, patient cohort, analysis and interpretation of data, study conceptualization and design.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Paternicó, D., Manes, M., Premi, E. et al. Frontotemporal dementia and language networks: cortical thickness reduction is driven by dyslexia susceptibility genes. Sci Rep 6, 30848 (2016). https://doi.org/10.1038/srep30848
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep30848
This article is cited by
-
Development of quantitative and continuous measure for severity degree of Alzheimer’s disease evaluated from MRI images of 761 human brains
BMC Bioinformatics (2022)
-
Differences in aphasia syndromes between progressive supranuclear palsy–Richardson’s syndrome, behavioral variant frontotemporal dementia and Alzheimer’s dementia
Journal of Neural Transmission (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.