Abstract
Since the evidence regarding statin therapy in PAH has not been conclusive, we assessed the impact of statin therapy in PAH through a systematic review and meta-analysis of available studies. We searched selected databases up to August 1, 2015 to identify the studies investigating the effect of statin administration on PAH. Meta-analysis was performed using either a fixed-effects or random-effect model according to I2 statistic. Meta-analysis of 8 studies with 665 patients did not suggest any significant improvement in 6-min walking distance (6MWD) by statin therapy (weighed mean difference [WMD]: −6.08 m, 95% confidence interval [CI]: −25.66, 13.50, p = 0.543; Q = 8.41, I2 = 28.64%). Likewise, none of the other indices including pulmonary arterial pressure (WMD: −0.97 mmHg, 95%CI: −4.39, 2.44, p = 0.577; Q = 14.64, I2 = 79.51%), right atrial pressure (WMD: 1.01 mmHg, 95%CI: −0.93, 2.96, p = 0.307; Q = 44.88, I2 = 95.54%), cardiac index (WMD: 0.05 L/min/m2, 95%CI: −0.05, 0.15, p = 0.323; Q = 3.82, I2 = 21.42%), and pulmonary vascular resistance (WMD: −1.42 dyn*s/cm5, 95%CI: −72.11, 69.27, p = 0.969; Q = 0.69, I2 = 0%) was significantly altered by statin therapy. In conclusion, the results of the meta-analysis did not show a statistically significant effect of statin therapy in the improvement of 6MWD, pulmonary arterial pressure, right atrial pressure, cardiac index and pulmonary vascular resistance.
Similar content being viewed by others
Introduction
Pulmonary hypertension (PAH) is defined according to the new 2015 Guidelines of the European Society of Cardiology (ESC) and European Respiratory Society (ERS) as an increase of the mean pulmonary arterial pressure (PAPm) above 25 mmHg at rest, assessed by right heart catheterization (RHC) and pulmonary vascular resistance (PVR) above 3 Wood units1. Compared with the 2008 PAH guidelines, the new guidelines eliminated the exercise criterion, added hemodynamic parameters, and defined post-capillary PAH subgroups1. The new guidelines categorized various PAH conditions into five groups: pulmonary arterial hypertension, pulmonary hypertension due to left heart disease, pulmonary hypertension due to lung diseases and/or hypoxia, chronic thromboembolic pulmonary hypertension and other pulmonary artery obstructions, and pulmonary hypertension with unclear and/or multifactorial mechanisms1. Several prognostic factors such as right ventricular failure, higher World Health Organization functional classification (WHO FC), shorter 6-min walk distance (6MWD) and hemodynamic factors such as right atrial pressure, and brain natriuretic peptide (BNP) levels are useful for PAH prognosis2,3. Furthermore, the American College of Chest Physicians’ (CHEST) Guideline suggests that the diagnosis of PAH should be made in a systematic and consistent manner with the use of a combination of WHO FC, exercise capacity, echocardiographic, laboratory and hemodynamic variables, and care should be provided by experts in the management of PAH. (Grade CB)4. However, the prognosis for patients with PAH remains poor, especially without appropriate treatment5.
Current therapy for PAH is based on endothelin receptor antagonists, phosphodiesterase-5 (PDE5) inhibitors, prostacyclin analogues (prostanoids), soluble guanylate cyclase stimulators, and prostacyclin receptor (IP receptor) agonist6,7. Despite the appearance of novel targets for PAH therapy tested in preclinical and clinical trials such as rituximab, endothelial progenitor cells, specific rho-kinase (ROCK) inhibitors (AT-877ER), FK506 (tacrolimus), fluoxetine, sertraline, paroxetine, bardoxolone methyl, aviptadil or tyrosine kinase inhibitors (nilotinib, sorafenib and imatinib), the diagnosis and treatment of PAH is still difficult7.
The pleiotropic effects of statins through induction of endothelial nitric oxide (NO) expression as well as anti-inflammatory and antiproliferative mechanisms possibly confer various clinical advantages beyond the reduction of serum cholesterol levels8,9. Since statins have been suggested as potential drugs for PAH treatment10, we assessed the impact of statin therapy on multiple parameters in PAH in this systematic review and meta-analysis.
Methods
This study was designed according to the 2009 preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines11. Due to the study design (meta-analysis) no Institutional Review Board (IRB) approval, as well as no patients’ informed consents were obtained.
Search Strategy
PubMed-Medline, SCOPUS, Web of Science and Google Scholar databases were searched using the following search terms in titles and abstracts (also in combination with MESH terms): (atorvastatin OR simvastatin OR rosuvastatin OR fluvastatin OR pravastatin OR pitavastatin OR lovastatin OR cerivastatin OR “statin therapy” OR statins OR statin) AND (“pulmonary arterial hypertension” OR “pulmonary hypertension” OR “pulmonary artery hypertension” OR “pulmonary vascular disease” OR “pulmonary heart disease” OR “pulmonary cardiac disease” OR PAH). The wild-card term “*” was used to increase the sensitivity of the search strategy. The literature search was limited to articles published in English. The search was limited to studies in humans. The literature was searched from inception to August 1, 2015. Two reviewers (MRG and AGB) evaluated each article independently. Disagreements were resolved by discussion with a third party (MB).
Study Selection
Original studies were included if they met the following criteria: (i) being a clinical study with either observational or interventional design, (ii) recruiting patients with a clinical diagnosis of PAH according to echocardiography regardless of the etiology (congenital heart disease, connective tissue disease, chronic thrombembolism or iatrogenic), and, (iii) investigating the impact of statin therapy on a valid disease activity index including 6MWD, pulmonary arterial pressure, right atrial pressure, cardiac index and pulmonary vascular resistance.
Exclusion criteria were: (i) non-clinical studies, (ii) lack of a statin-free control group in the study design, and, (iii) lack of sufficient information on baseline or follow-up indices of PAH.
Data extraction
Eligible studies were reviewed and the following data were abstracted: 1) first author’s name 2) year of publication 3) study location 4) study design 5) number of participants in the statin and groups 5) age, gender and body mass index (BMI) of study participants 6) type and duration of statin therapy and 7) baseline and follow-up values of PAH-related indices.
Data extraction was performed independently by 2 reviewers; disagreements were resolved by a third reviewer.
Quality assessment
Methodological quality of the included studies was assessed using the Newcastle-Ottawa Scale (NOS)12. In this context, topic items of each eligible study are assessed: that is, the selection of the studied patients, the comparability of the studied populations and the ascertainment of the exposure. A study can be awarded a maximum of one point for each item. Risk-of-bias assessment was performed independently by 2 reviewers; disagreements were resolved by a third reviewer.
Quantitative Data Synthesis
Meta-analysis was conducted using Comprehensive Meta-Analysis (CMA) V2 software (Biostat, NJ)13. Net changes in measurements (change scores) were calculated as follows: measure at end of follow-up − measure at baseline. For single-arm cross-over trials, net change in each efficacy measure was calculated by subtracting the value after control intervention from that reported after treatment. Standard deviations (SDs) of the mean difference were calculated using the following formula: SD = square root [(SDpre-treatment)2 + (SDpost-treatment)2 − (2R × SDpre-treatment × SDpost-treatment), assuming a correlation coefficient (R) = 0.5. If the outcome measures were reported in median and range or 95% CI, mean and standard SD values were estimated using the method described by Wan et al.14. Where standard error of the mean (SEM) was only reported, SD was estimated using the following formula: SD = SEM × sqrt (n), where n is the number of subjects.
A random-effects model (using DerSimonian-Laird method) and the generic inverse variance method were used to compensate for the heterogeneity of studies in terms of demographic characteristics of populations being studied and also differences in study design and type of statin being studied15. Heterogeneity was quantitatively assessed using I2 index. Effect sizes were expressed as weighted mean difference (WMD) and 95% confidence interval (CI). In order to evaluate the influence of each study on the overall effect size, sensitivity analysis was conducted using leave-one-out method, i.e. removing one study each time and repeating the analysis.
Publication bias
Potential publication bias was explored using visual inspection of Begg’s funnel plot asymmetry, Begg’s rank correlation, and Egger’s weighted regression. Duval and Tweedie “trim and fill” was used to adjust the analysis for the effects of publication bias16.
Results
Flow and characteristics of included studies
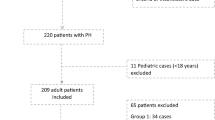
On the basis of a database search 859 published studies were found, 310 of which were non-clinical or review articles and thus they were not included in further analysis. We screened 549 articles but 533 of them were excluded because they did not meet inclusion criteria. Out of 16 eligible papers, 6 were excluded since they were not conducted in subjects with PAH, one of them did not assess any of the pre-specified efficacy measures, and one was not a clinical study. Finally, we included 8 articles in this meta-analysis17,18,19,20,21,22,23,24 (Fig. 1).
Most of the analyzed studies (7/8) were randomized, double- or triple-blind and placebo-controlled. This meta-analysis comprised 665 patients with PAH receiving either statins (rosuvastatin, simvastatin, pravastatin or atorvastatin) (311 patients) or placebo (354 patients) for 6 months. In analyzed patients, PAH was idiopathic, heritable, associated with congenital heart disease, atrial-septal defects or connective tissue disease, and many of them suffered from chronic obstructive pulmonary disease. The PAH-related indices 6MWD, pulmonary arterial pressure, right atrial pressure, cardiac index and pulmonary vascular resistance were measured in 6, 6, 2, 4 and 2 analyzed studies, respectively (Table 1).
Risk of bias assessment
The quality of the included studies assessed by the Newcastle-Ottawa Scale (NOS)12 is shown in Table 2.
Effect of statin therapy on PAH-related indices
Meta-analysis of included studies did not suggest any significant improvement in 6MWD by statin therapy (WMD: −6.08 m, 95% CI: −25.66, 13.50, p = 0.543; Q = 8.41, I2 = 28.64%) (Fig. 2A). This effect was robust in sensitivity analysis and the statistical significance was not influenced by any single study included in the meta-analysis (Fig. 2B). Likewise, none of the other indices including pulmonary arterial pressure (WMD: −0.97 mmHg, 95% CI: −4.39, 2.44, p = 0.577; Q = 14.64, I2 = 79.51%) (Fig. 3A), right atrial pressure (WMD: 1.01 mmHg, 95% CI: −0.93, 2.96, p = 0.307; Q = 44.88, I2 = 95.54%) (Fig. 3B), cardiac index (WMD: 0.05 L/min/m2, 95% CI: −0.05, 0.15, p = 0.323; Q = 3.82, I2 = 21.42%) (Fig. 3C), and pulmonary vascular resistance (WMD: −1.42 1.42 dyn*s/cm5, 95% CI: −72.11, 69.27, p = 0.969; Q = 0.69, I2 = 0%) (Fig. 3D) was significantly altered by statin therapy.
Lower plot (Fig. 2B) shows leave-one-out sensitivity analysis.
Publication bias
The funnel plot of the study standard error by effect size (mean difference) for the meta-analysis of statin effects on 6MWD was asymmetric suggesting a potential publication bias (Fig. 4). Using “trim and fill” correction, two potentially missing studies were imputed on the left side of funnel plot, yielding an effect size of −11.72 m (95% CI: −31.27, 7.82). The results of Begg’s rank correlation (Kendall’s Tau with continuity correction = 0.38, z = 1.20, two-tailed p-value = 0.230) and Egger’s linear regression (intercept = 1.97, standard error = 1.25; 95% CI = −1.24, 5.18, t = 1.58, df = 5, two-tailed p = 0.176) tests excluded the possibility of publication bias in the meta-analyses.
Discussion
To our knowledge, the current systematic review and meta-analysis is the first to evaluate the effects of statins on PAH. Contrary to the findings from some studies18,25, this meta-analysis of 8 studies did not suggest any significant improvement in 6MWD, pulmonary arterial pressure, right atrial pressure, cardiac index, and pulmonary vascular resistance by statin therapy. The lack of benefit was robust in sensitivity analysis and the statistical significance was not influenced by any single study included in the meta-analysis.
These findings are of clinical interest, since the use of statins in PAH treatment is thought to be beneficial due to their antiproliferative, anti-inflammatory, and pro-apoptotic pleiotropic effects as well as the ability to restore endothelial vasoactive mediator production26. Various studies linked statin effects with the pathogenesis of pulmonary hypertension and various biomarkers27, through analyzing the relation between statins, endothelin-1 and PAH28,29, statins, asymmetric dimethylarginine and PAH30,31,32, statins, D-dimers and PAH33,34, statins, von Willebrand factor antigen and PAH35,36 or fibrinogen and PAH37,38. Until now, the results obtained using statins on PAH parameters in different animal models have been contradictory. Some experimental studies suggested that statins might attenuate the development or even regress established experimental PAH by decreasing proliferation and increasing apoptosis of pathological smooth muscle cells in the medial walls and neointima of pulmonary arteries and reducing right ventricular hypertrophy39,40. Other experimental studies on murine models reported an inhibition of progression of emphysema and pulmonary hypertension related to tobacco exposure after statin therapy41,42. One experimental study showed that low doses of fluvastatin have beneficial effects on adventitial fibroblasts from chronic hypoxic animals through actions on the Rac1-p38 MAP kinase-signaling pathway43. Another experimental study showed that rosuvastatin improved ischemia-reperfusion injury by decreasing macrophage infiltration and up-regulating endothelial nitric oxide synthase44. In contrast, atorvastatin neither improved survival nor reduced PAH, vascular remodeling and right ventricular hypertrophy in an experimental study on a rat monocrotaline PAH model45. However, some experimental studies reported a decrease of right ventricular (RV) hypertrophy associated with statin therapy in PAH models46, but no study showed a reduction in pulmonary arterial pressure47,48. An experimental study on guinea pigs exposed to cigarette smoking during 6 months showed no effects of simvastatin on small airway remodeling49.
The data on humans from short-term randomized trials are few and contradictory. In an open-label observational trial, simvastatin was successfully used as adjunctive therapy in patients with PAH associated with various pathologies25. In another study, atorvastatin reduced pulmonary artery pressure and raised the migration and adhesion of endothelial progenitor cells (EPCs) in patients with chronic pulmonary heart disease20. Other studies described a decreased frequency of exacerbations and intubations50, a reduced decline of pulmonary function51 and decreased pulmonary pressures associated with improved exercise capacity after statin treatment19 in patients with chronic obstructive pulmonary diseases. A multicenter propensity score study on 2,363 patients showed for the first time that statins improve survival in PAH patients by decreasing one-year all-cause mortality52.
Similar to our results obtained in this meta-analysis, and in contrast to previous arguments, another study did not find any effect of statins on 6MWD23, but the short duration of this trial and background therapy of PAH patients may be potential causes for the lack of effects. Another trial on 220 PAH patients showed no benefit of atorvastatin on cardio-pulmonary hemodynamics, 6 MWD and survival at 6 months of treatment24, suggesting that atorvastatin should not be prescribed as specific treatment in PAH.
Limitations
This meta-analysis has several limitations. First, the studies were relatively small and heterogeneous concerning study design, patients characteristics, major outcomes, PAH etiology and severity. Second, the patients included were classified in various functional classification groups. Third, the positive effects of statins in one study might have been overlapped by lack of effects of statins in the rest of studies since the effects of four statins (rosuvastatin, simvastatin, pravastatin and atorvastatin) were analyzed. Fourth, the patients received supportive drugs such as diuretics, warfarin, and digoxin, which could have influenced the results. The follow-up of the study was relatively short (6 months), so the long-term effects cannot be concluded and it is possible that longer therapy with statin might have been effective. Taking into account the given small sample size and short duration of the included studies, the meta-analysis could not also look at CVD outcomes that would be of the greatest interest. Finally, in the most of included studies PAH was diagnosed mainly based on echocardiography, which is also an important limitation.
In conclusion, the results of this meta-analysis of available studies did not show a statistically significant effect of statin therapy in the improvement of 6 MWD, pulmonary arterial pressure, right atrial pressure, cardiac index and pulmonary vascular resistance. Additional large, long term and well-designed trials are necessary to investigate the impact of statins for the treatment of PAH including their use as pulmonary vascular antiproliferative agents.
Additional Information
How to cite this article: Rysz-Górzynska, M. et al. Efficacy of Statin Therapy in Pulmonary Arterial Hypertension: A Systematic Review and Meta-Analysis. Sci. Rep. 6, 30060; doi: 10.1038/srep30060 (2016).
References
Galie, N. et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37, 67–119 (2016).
Austin, E. D. & Loyd, J. E. Toward Precision Medicine in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 192, 1272–1274 (2015).
Delcroix, M. & Howard, L. Pulmonary arterial hypertension: the burden of disease and impact on quality of life. European Respiratory Review: an Official Journal of the European Respiratory Society 24, 621–629 (2015).
Taichman, D. B. et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest Journal 146, 449–475 (2014).
Sinha, N. et al. Can echocardiographically estimated pulmonary arterial elastance be a non-invasive predictor of pulmonary vascular resistance? Arch Med Sci 10, 692–700 (2014).
Sharma, M., Pinnamaneni, S., Aronow, W. S., Jozwik, B. & Frishman, W. H. Existing drugs and agents under investigation for pulmonary arterial hypertension. Cardiology in Review 22, 297–305 (2014).
Galiè, N. et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37, 67–119 (2016).
Krishna, R. K. et al. Pleiotropic effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors in pulmonary diseases: A comprehensive review. Pulmonary Pharmacology & Therapeutics 30, 134–140 (2015).
Banach, M. et al. Impact of statin therapy on coronary plaque composition: a systematic review and meta-analysis of virtual histology intravascular ultrasound studies. BMC Med 13, 229 (2015).
Faul, J. et al. In Pulmonary Hypertension Contemporary Cardiology™ (eds NicholasS Hill & HarrisonW Farber ) Ch. 15, 321–336 (Humana Press, 2008).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group, P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535 (2009).
Wells, G. et al. Shea B, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www,ohri.ca/programs/clinical_epidemiology.oxford.htm (2000).
Borenstein, M., Hedges, L., Higgins, J. & Rothstein, H. Comprehensive Meta-Analysis Version 2. Englewood, NJ: Biostat 104 (2005).
Wan, X., Wang, W., Liu, J. & Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 14, 135 (2014).
Sutton, A. J., Abrams, K. R. & Jones, D. R. Methods for meta-analysis in medical research.
Duval, S. & Tweedie, R. Trim and fill: a simple funnel‐plot–based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 56, 455–463 (2000).
Barreto, A., Maeda, N., Soares, R., Cícero, C. & Lopes, A. Rosuvastatin and vascular dysfunction markers in pulmonary arterial hypertension: a placebo-controlled study. Brazilian Journal of Medical and Biological Research 41, 657–663 (2008).
Kawut, S. M. et al. Randomized clinical trial of aspirin and simvastatin for pulmonary arterial hypertension: ASA-STAT. Circulation 123, 2985–2993 (2011).
Lee, T. M., Chen, C. C., Shen, H. N. & Chang, N. C. Effects of pravastatin on functional capacity in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Clinical science (London, England: 1979) 116, 497–505 (2009).
Liu, H.-f., Qi, X.-w., Long-le Ma, D.-K. Y. & Wang, L. Atorvastatin improves endothelial progenitor cell function and reduces pulmonary hypertension in patients with chronic pulmonary heart disease. Experimental & Clinical Cardiology 18, e40 (2013).
Moosavi, S. A. J., Raji, H., Faghankhani, M., Yazdani, R. & Esmaeili, M. Evaluation of the effects of atorvastatin on the treatment of secondary pulmonary hypertension due to chronic obstructive pulmonary diseases: a randomized controlled trial. Iranian Red Crescent medical journal 15, 649 (2013).
Reed, R. M. et al. Statin therapy is associated with decreased pulmonary vascular pressures in severe COPD. COPD: Journal of Chronic Obstructive Pulmonary Disease 8, 96–102 (2011).
Wilkins, M. R. et al. Simvastatin as a treatment for pulmonary hypertension trial. American Journal of Respiratory and Critical Care Medicine 181, 1106–1113 (2010).
Zeng, W.-J. et al. Atorvastatin in pulmonary arterial hypertension (APATH) study. European Respiratory Journal 40, 67–74 (2012).
Kao, P. N. Simvastatin treatment of pulmonary hypertension: an observational case series. Chest 127, 1446–1452 (2005).
Faul, J. L. et al. Statins for Treatment of Pulmonary Hypertension, In: Pulmonary Hypertension 321–336 (Springer, 2008).
Al-Naamani, N. et al. Prognostic Significance of Biomarkers in Pulmonary Arterial Hypertension. Annals of the American Thoracic Society 13, 25–30 (2016).
Giannakoulas, G., Mouratoglou, S.-A., Gatzoulis, M. A. & Karvounis, H. Blood biomarkers and their potential role in pulmonary arterial hypertension associated with congenital heart disease. a systematic review. International Journal of Cardiology 174, 618–623 (2014).
Sahebkar, A. et al. Statin therapy reduces plasma endothelin-1 concentrations: A meta-analysis of 15 randomized controlled trials. Atherosclerosis 241, 433–442 (2015).
Serban, C. et al. A systematic review and meta-analysis of the effect of statins on plasma asymmetric dimethylarginine concentrations. Scientific Reports 5, 9902 (2015).
Parikh, R. V. et al. Increased levels of asymmetric dimethylarginine are associated with pulmonary arterial hypertension in HIV infection. AIDS (London, England) 28, 511 (2014).
Andersen, C. U. et al. Diagnostic and prognostic role of biomarkers for pulmonary hypertension in interstitial lung disease. Respiratory medicine 106, 1749–1755 (2012).
Barnes, T. et al. Baseline vWF factor predicts the development of elevated pulmonary artery pressure in systemic sclerosis. Rheumatology 51, 1606–1609 (2012).
Sahebkar, A. et al. Association between statin use and plasma D-dimer levels. Thrombosis and Haemostasis 114, 546–557 (2015).
Sahebkar, A. et al. The impact of statin therapy on plasma levels of von Willebrand factor antigen. Systematic review and meta-analysis of randomised placebo-controlled trials. Thromb Haemost 115, 520–32 (2016).
Lopes, A. A. et al. Plasma von Willebrand factor as a predictor of survival in pulmonary arterial hypertension associated with congenital heart disease. Brazilian Journal of Medical and Biological Research 44, 1269–1275 (2011).
Kato, F. et al. Association of plasma fibrinogen and plasminogen with prognosis of inoperable chronic thromboembolic pulmonary hypertension. Circulation Journal: Official Journal of the Japanese Circulation Society 78, 1754–1761 (2014).
Sahebkar, A. et al. Head-to-head comparison of statins versus fibrates in reducing plasma fibrinogen concentrations: A systematic review and meta-analysis. Pharmacol Res 103, 236–252, (2016).
Lee, J.-H. et al. Simvastatin inhibits cigarette smoking–induced emphysema and pulmonary hypertension in rat lungs. American Journal of Respiratory and Critical Care Medicine 172, 987–993 (2005).
Nishimura, T. et al. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation 108, 1640–1645 (2003).
Girgis, R. E. et al. Attenuation of chronic hypoxic pulmonary hypertension by simvastatin. American Journal of Physiology-Heart and Circulatory Physiology 285, H938–H945 (2003).
Girgis, R. E. et al. Regression of chronic hypoxic pulmonary hypertension by simvastatin. American Journal of Physiology-Lung Cellular and Molecular Physiology 292, L1105–L1110 (2007).
Carlin, C. M. et al. Low-dose fluvastatin reverses the hypoxic pulmonary adventitial fibroblast phenotype in experimental pulmonary hypertension. Am J Respir Cell Mol Biol 47, 140–148 (2012).
Matsuo, S. et al. Single-dose rosuvastatin ameliorates lung ischemia-reperfusion injury via upregulation of endothelial nitric oxide synthase and inhibition of macrophage infiltration in rats with pulmonary hypertension. J Thorac Cardiovasc Surg 149, 902–909 (2015).
McMurtry, M. S. et al. Statin therapy, alone or with rapamycin, does not reverse monocrotaline pulmonary arterial hypertension: the rapamcyin-atorvastatin-simvastatin study. American Journal of Physiology-Lung Cellular and Molecular Physiology 293, L933–L940 (2007).
Murata, T. et al. Statin protects endothelial nitric oxide synthase activity in hypoxia-induced pulmonary hypertension. Arterioscler Thromb Vasc Biol 25, 2335–2342 (2005).
Sun, X. & Ku, D. D. Rosuvastatin provides pleiotropic protection against pulmonary hypertension, right ventricular hypertrophy, and coronary endothelial dysfunction in rats. American Journal of Physiology. Heart and Circulatory Physiology 294, H801–809 (2008).
Zhao, L. et al. Simvastatin and sildenafil combine to attenuate pulmonary hypertension. The European Respiratory Journal 34, 948–957 (2009).
Wright, J. L. et al. Statin reverses smoke-induced pulmonary hypertension and prevents emphysema but not airway remodeling. Am J Respir Crit Care Med 183, 50–58 (2011).
Blamoun, A. I. et al. Statins may reduce episodes of exacerbation and the requirement for intubation in patients with COPD: evidence from a retrospective cohort study. Int J Clin Pract 62, 1373–1378 (2008).
Alexeeff, S. E., Litonjua, A. A., Sparrow, D., Vokonas, P. S. & Schwartz, J. Statin use reduces decline in lung function: VA Normative Aging Study. Am J Respir Crit Care Med 176, 742–747 (2007).
Eshtehardi, P. et al. Statin Therapy Improves Survival in Patients With Severe Pulmonary Hypertension: A Propensity Score Matching Study. Circulation 130, A11717 (2014).
Acknowledgements
The meta-analysis has been prepared within Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC)Group (www.lbpmcgroup.umed.pl). Four authors (M.R-G., A.G-B., J.R., M.B.) are partially supported by theHealthy Ageing Research Centre project of Medical University of Lodz, Lodz, Poland (REGPOT-2012-2013-1, 7FP).
Author information
Authors and Affiliations
Contributions
M.B. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. M.R.-G. and A.G.-B. contributed to the data acquisition, data analysis and interpretation, and drafting of the manuscript; A.S. contributed to the statistical analysis and the writing and revising of the manuscript; M.C.-S. contributed to the data acquisition, drafting and critical revision of the manuscript; D.P.M., S.U., P.P.T., V.B., G.F.W., G.Y.H.L., J.R. and A.L.C. contributed to critical revision of the manuscript; and M.B. contributed to the study concept and design, drafting of the manuscript, and critical revision and final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Rysz-Górzynska, M., Gluba-Brzózka, A., Sahebkar, A. et al. Efficacy of Statin Therapy in Pulmonary Arterial Hypertension: A Systematic Review and Meta-Analysis. Sci Rep 6, 30060 (2016). https://doi.org/10.1038/srep30060
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep30060
This article is cited by
-
A Systematic Review of Novel Therapies of Pulmonary Arterial Hypertension
American Journal of Cardiovascular Drugs (2024)
-
High-fat diet attenuates the improvement of hypoxia-induced pulmonary hypertension in mice during reoxygenation
BMC Cardiovascular Disorders (2021)
-
Statin treatment prevents the development of pulmonary arterial hypertension in a nonhuman primate model of HIV-associated PAH
Scientific Reports (2019)
-
Low-density lipoprotein cholesterol and survival in pulmonary arterial hypertension
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.