Abstract
This study aimed to investigate the optimal degree of weight gain across the gestational spectrum in 1971 children enrolled at birth and followed up to age 7 years. Weight gain in infancy was categorized into four groups based on weight gain z-scores: slow (<−0.67), on track (−0.67 to 0.67), rapid (0.67 to 1.28), and extremely rapid (>1.28). Underweight and overweight or obesity (OWO) were defined as a body mass index ≤5th and ≥85th percentile, respectively, for age and gender. In our population, OWO was far more common than underweight (39.7% vs. 3.6%). Weight gain tracked strongly from age 4 to 24 months, and was positively associated with OWO and an unfavorable pattern of metabolic biomarkers, although the degree of weight gain for the risk was different across gestational categories. Extremely rapid weight gain led to a particularly high risk of OWO among children born early term and late preterm: odds ratio: 3.3 (95% confidence interval: 1.9 to 5.5) and 3.7 (1.8 to 7.5), respectively, as compared to those with on track weight gain. Our findings suggest that monitoring and ensuring optimal weight gain across the entire gestational spectrum beginning from birth represents a first step towards primary prevention of childhood obesity.
Similar content being viewed by others
Introduction
The persistently high prevalence of obesity is a major clinical and public health challenge in the U.S. and globally. Of concern, 8.1% of U.S. infants and toddlers were overweight, and 16.9% of 2- to 19-year-olds were obese in 2011–20121. More importantly, obesity in young children leads not only to short-term morbidity but also to later obesity and its adverse consequences across the lifespan and generations2,3,4,5. Growing evidence indicates that obesity may originate in early life. A recent study has lent even further evidence, showing that incident obesity between age 5 and 14 years was more likely to originate at younger ages6. However, questions remain regarding what modifiable early life factors can increase the risk of childhood obesity.
Growth in early infancy is more rapid than at any other time during postnatal life. In particular, infants born preterm or early term usually compensate by engaging in rapid “catch-up” growth in the first year of life. Although studies of term births suggest that rapid weight gain in early life (ranging from 6 to 24 months) is associated with an increased risk of childhood obesity7,8, there is a lack of prospective birth cohort studies to investigate whether the associations persist across the entire gestational spectrum. Pediatricians monitor infant weight gain closely for “failure to thrive” and make recommendations to increase calories in babies born preterm. However, most current recommendations regarding appropriate growth velocity in infancy and early childhood largely do not consider the long term risk of obesity or metabolic disorders, nor do these recommendations address an infants’ specific gestational age category9. Moreover, current recommendations regarding the age to begin screening for obesity in children do not cover infancy: the U.S. Preventive Services Task Force recommends screening by age 6 years10, whereas the Expert Committee recommends screening by age 2 years11.
Using a prospective birth cohort enriched by preterm births, we aimed to investigate weight gain patterns and their associations with the risk of both underweight and OWO as well as metabolic biomarkers during early childhood (median [Interquartile range] age: 6[4–7] years) among children born across the gestational spectrum (full term, early term, late preterm and early preterm). This line of investigation is needed to provide evidence to establish optimal growth targets to balance the need for normal or catch-up growth and reduce the risk of OWO in children. Such information is critical to help identify children at high-risk of developing OWO during early infancy when interventions could be highly cost-effective and have lifelong impact.
Results
Characteristics of the study participants
Of the 1971 children (980 boys, 991 girls), 1442 were born term (926 full term, 516 early term) and 529 were born preterm (300 late preterm, 229 early preterm). Prenatal and postnatal anthropometric and growth parameters are presented in Table 1. Compared to those born full term, infants born preterm had lower birthweights and their mothers had higher rates of smoking, diabetes and hypertensive disorders during pregnancy. In total, 39.7% of the children (39.2% of boys and 40.2% of girls) were OWO, whereas only 72 (3.6%) children were underweight at age 2–7 years.
Persistence of weight gain during infancy
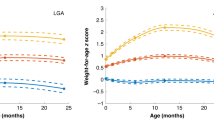
Infants born preterm had a higher percentage of rapid or extremely rapid weight gain in the first four months of life relative to babies born at term (76.4% vs. 29.5%); the highest rate was among early preterm births (87.8%). Data from all three time points (first 4 mo, 1y, and 2y) were available for 1643 study participants. Weight gain was highly correlated for the first four months and the first year (r = 0.81, p < 0.001), as well as for the first four months and at age 2 years (r = 0.74, p < 0.001). Rapid and extremely rapid weight gain tracked well across all of infancy. Rapid or extremely rapid weight gain persisted from the first four months to age 2 years (Fig. 1). In total, 94.5% and 91.5% of children who experienced rapid or extremely rapid growth in the first four months remained on the same track at years 1 and 2, respectively.
Weight gain z-score was defined as the change in weight for age z–score between birth and age 4 months and was categorized into four groups: slow (weight gain z-score less than −0.67); on track (weight gain z-score between −0.67 and 0.67), rapid (weight gain z-score between 0.67 and 1.28), and extremely rapid (weight gain z-score greater than 1.28).
Weight gain, Body Mass Index (BMI) z-score and the risk of overweight or obesity
Weight gain z-score in the first four months of life was positively associated with BMI z-score and OWO at age 2–7 years. The overall risk of OWO increased by 50% for every increase of one standard deviation (SD) in weight gain in the first four months (multivariate odds ratio [OR], 1.5; 95% confidence interval [CI], 1.4 to 1.7). The corresponding ORs (95%CI) were 1.9 (1.6 to 2.3) and 1.8 (1.4 to 2.3) for children who were born early term (37–38 weeks) and late preterm birth (34–36 weeks), respectively. When weight gain in the first four months of life was further categorized into four subgroups (on track, slow, rapid, and extremely rapid), extremely rapid weight gain was associated with a 2.3 (95%CI, 1.7 to 3.0) times greater risk of OWO than the on track group after adjusting for prenatal and postnatal risk factors (maternal race/ethnicity, educational attainment, smoking, parity, prepregnancy BMI category, diabetes status, hypertensive disorder, fetal growth status, gestational age categories, and breastfeeding) (Table 2). For full term infants, extremely rapid weight gain increased the risk of OWO by 60%, OR = 1.6, 95%CI, 1.0 to 2.4. An increased risk of OWO associated with extremely rapid weight gain appeared to be especially prominent among participants who were born early term and late preterm. The corresponding ORs (95%CI) were 3.3 (1.9 to 5.5) and 3.7 (1.8 to 7.5) for children born early term and late preterm, respectively (Table 2). Notably, the association pattern showed a difference across the gestational spectrum. In those born full term, children with both rapid weight gain and extremely rapid growth were at a similarly high risk of OWO. In those born early term, extremely rapid growth further increased the risk of OWO beyond those with rapid growth. However, in those born late preterm, rapid growth was not associated with OWO, but extremely rapid growth significantly increased the risk of OWO. In contrast, in those born early preterm, even extremely rapid weight gain was not associated with the risk of OWO (Fig. 2). Of note, after additional adjustment for duration of breastfeeding and timing of solid food introduction, the results did not substantially change. At the same time, excessive weight gain showed no clear benefit for reducing underweight, even among preterm or early term infants who need catch-up growth (Fig. 2).
Sensitivity analysis
When we repeated our primary analyses stratified by child’s age group (2–4 years and 5–7 years) the findings were substantially unchanged (Table 3). Likewise, when we examined the associations between weight gain in the first and second year relative to the risk of OWO, the associations were consistent. Point estimates were slightly higher when 1-year or 2-year windows of weight gain were used in the analyses rather than the shorter 4-month window (Table 4).
Weight gain and metabolic biomarkers: plasma leptin, adiponectin and adiponectin/leptin ratio
In sum, 1136 participants were available for the analyses of blood leptin concentrations, 1139 for adiponectin, and 1092 for the adiponectin/leptin ratio. The median (Interquartile range) age of biomarker measurement was 1.7 (0.9–3.5) years. Weight gain z-score in the first four months was positively associated with plasma leptin, whereas it was negatively associated with the adiponectin/leptin ratio. Every one unit increase in the weight gain z-score was associated with a 0.18 unit (log-transformed) increase in plasma leptin, and a 0.17 unit decrease in the adiponectin/leptin ratio (log-transformed). The associations were similar across the entire gestational spectrum. A corresponding 0.19 and 0.23 unit increase in leptin and a 0.22 and 0.27 unit decrease in the adiponectin/leptin ratio were observed among early term and late preterm children, respectively (Table 5).
Discussion
In the U.S., preterm birth affects 1 in 9 of all live births and 1 in 5 Black infants12. To date, the majority of studies that have associated rapid weight gain in early life with later obesity have been performed in children and adults born at term7,13,14. Although those born preterm display a faster rate of infant growth, data regarding the linkage between rapid weight gain in early life and the risk of childhood and adult obesity among preterm births are limited. Recently, the American Congress of Obstetricians and Gynecologists (ACOG) recommended defining children who are born between at 37–38 weeks as early term births; this group of births has received increased attention due to their increased risk of morbidity in the early and later postnatal period15.
To our knowledge, this is the first large-scale birth cohort study to investigate the effect of rapid weight gain in infancy on the risk of childhood OWO, as well as on metabolic biomarkers in children born preterm and early term, in a U.S., predominantly urban minority population. Our analyses showed a high prevalence of childhood OWO in this racial/ethnic minority population, which was 1.7 times higher than the rates for U.S. children aged 2–5 years1. The higher prevalence of OWO in our sample is consistent with the well documented socioeconomic and racial/ethnic disparities for childhood obesity16. The prevalence of underweight was much lower in our study population, especially among term births. Our data also showed a remarkable tracking of rapid or extremely rapid growth in infancy across the first two years of life, suggesting that monitoring and ensuring optimal weight gain should begin at birth.
We found that rapid weight gain during infancy was associated with an increased risk of OWO at age 2–7 years in a dose-response fashion among all children except for early preterm births, even after adjusting for pertinent prenatal and postnatal covariables. Most remarkably, we found that the effect of excessive weight gain on childhood OWO appeared to be more pronounced in children born early term or late preterm. However, excessive weight gain showed no clear benefit for reducing underweight, even among preterm or early term infants who need catch-up growth. In light of this finding, we should be sure not to lose sight of the potential adverse effects of inadequate weight gain, particularly on neurodevelopmental outcomes17. Given that a low degree of rapid weight gain did not increase the risk of OWO in those born late preterm, optimal catch-up growth for those infants is recommended. More importantly, since rapid weight gain may benefit neurodevelopment and did not increase the risk of OWO in early preterm births, catch-up growth is seen to be critical for early preterm infants. Our findings underscore the importance of identifying an “optimal” weight gain to help establish a balance between the need to prevent childhood obesity and metabolic consequences versus the need to ensure healthy neurodevelopment.
Adipose tissue has been recognized as an important endocrine organ that secretes adipokines. Two major adipokines, leptin and adiponectin, are thought to play important roles in the regulation of insulin sensitivity and metabolic homeostasis. Previous studies have shown that plasma leptin concentrations increase with obesity, whereas plasma adiponectin concentrations are decreased in obese individuals18,19. The leptin/adiponectin ratio is strongly correlated with the glucose infusion rate as measured by the euglycemic hyperinsulinemic clamp method20. In children, a high leptin/adiponectin ratio is associated with cardiovascular risk and systematic inflammation21. More importantly, studies have also demonstrated that the leptin/adiponectin ratio serves as a marker of insulin resistance that is superior to leptin or adiponectin alone20,22,23. Conversely, the adiponectin/leptin ratio serves as a marker of insulin sensitivity24. It is well understood that BMI is not an exact measure of adiposity, especially in young children25,26. Although it is conventionally accepted, the accuracy of the diagnosis of obesity based on BMI categories is limited. Therefore, we determined obesity-related biomarkers to further verify our findings. We measured plasma leptin concentrations (as a surrogate of body fat) and adiponectin concentrations (as a marker of insulin sensitivity). We also calculated the adiponectin/leptin ratio as an alternative marker of insulin sensitivity. Our data showed that weight gain in the first four months was significantly associated with increased plasma leptin levels and a decreased adiponectin/leptin ratio, but the associations with plasma adiponectin concentrations alone were weak. These findings suggest that the adiponectin/leptin ratio, which indicates the relative abundance of adiponectin over leptin, may present the best way to capture their combined contribution to the association with child weight gain.
Our findings have important clinical and public health implications. Adequate weight gain in preterm infants appears to benefit neurodevelopment27. However, despite the potential benefit of “catch-up” growth, our study revealed that extremely rapid weight gain in the first four months of life is a risk factor for OWO in all childhood except those born early preterm, and particularly for those born early term and late preterm. Our findings underscore the premise that optimal weight gain targets in early infancy may benefit from being tailored to a specific gestational age and to first 4-month weight gain trajectories. These efforts may help to reduce childhood obesity and minimize adverse metabolic consequences in later life.
The latest American Academy of Pediatrics (AAP) report emphasizes the critical role of pediatricians in preventing childhood obesity28. Between infancy and a baby’s first birthday, there are a total of seven recommended well-child visits. However, the recommendations regarding the age to begin screening for obesity in children do not currently cover infancy: the U.S. Preventive Services Task Force recommends screening by age 6 years10 whereas the Expert Committee recommends screening much earlier—by age 2 years11. However, numerous studies including our own have recognized the prenatal and early childhood stages as being sensitive periods in the development of obesity because of the rapid cellular proliferation and differentiation and metabolic programming that occurs during this time29,30. In addition, several studies have documented that the proliferation and differentiation of adipocytes occurs primarily in utero31, and that the total number of adipocytes, a major determinant of adult fat mass, is established in early childhood32,33,34. Previous studies also have shown an increased risk of obesity in older children following rapid weight gain as early as the first 4–6 months of life in term births7,13,14, and incident obesity between the ages of 5 and 14 years subsequent to being overweight in kindergarten6. Our study findings raise the prospect that the first steps toward primary prevention of OWO might need to occur very early in infancy, and that conducting rigorous studies of early life risk factors and intervention targets would not only be an important step in reducing OWO but could yield commensurate long-term health benefits.
Some particular strengths of this study were that it was based on a large prospective birth cohort. The fact that the study sample was enriched with preterm births enabled us to examine weight gain patterns in infancy among infants born early term and preterm. This study was further strengthened by the measurements of metabolic biomarkers, leptin and adiponectin. Our study also had some limitations. The year of participant enrollment varied due to rolling recruitment in the Boston Birth Cohort. However, we examined growth parameters based on gender- and age-specific z-scores, which allowed us to account for variation in age at follow-up. Because we did not quantify the intake of caloric or specific macro- or micronutrients during infancy, we could not examine the effects of these on weight gain patterns and the risk of childhood obesity.
Conclusions
We demonstrated a high prevalence of OWO across the gestational age spectrum within this urban, predominantly low-income minority sample. The association patterns of weight gain in infancy with childhood OWO were different according to gestational age categories. Extremely rapid weight gain in the first four months of life was a strong predictor of OWO at age 2–7 years for all children except early preterm births. Because early infancy is a critical period in which to establish an optimal growth trajectory and prevent childhood obesity, as we observed in this study, an optimal growth pattern should be established according to gestational age. Pediatricians can play an important role in monitoring and educating families to achieve optimal weight gain in infants beginning at birth.
Methods
Study population
From 2004–2014, we followed 2937 children who were born at the Boston Medical Center, MA, between 1998–2012 and recruited into the Boston Birth Cohort, a large, prospective, predominantly urban minority birth cohort35. We excluded 740 participants due to missing a visit in the first four months and 226 participants due to missing a visit during years 2–7. The final study analyses included 1971 (67% of the total follow-up children) participants who had a visit within the first four months of life and at least one follow-up visit from age 2–7 years. Children who were included relative to the total follow-up sample in the study were similar with regard to prenatal variables as well as birth outcomes. The evaluation of enrollment and follow-up included a medical questionnaire and interview and anthropometric measurements. The study protocol was approved by the Institutional Review Boards of the Boston University Medical Center, the Ann & Robert H. Lurie Children’s Hospital of Chicago (formerly Children’s Memorial Hospital), and the Johns Hopkins University Bloomberg School of Public Health. The methods were carried out in accordance with the approved guidelines. Written informed consent was obtained from mothers.
Measurement of major variables and outcomes
Major Prenatal and Postnatal Variables
Maternal race/ethnicity, educational attainment, smoking, parity and prepregnancy weight and height were collected via structured interview 24 to 72 hours after delivery. Maternal prepregnancy BMI was calculated as weight in kilograms divided by height in meters squared. Maternal smoking during pregnancy was coded as “never a smoker”, “quit for the pregnancy”, or “continuous smoker” based on whether the mother smoked cigarettes throughout the pregnancy, only smoked in the three months before pregnancy, or only smoked during the first trimester35. Race/ethnicity was based on maternal response to fixed categories in the questionnaire and classified as Black, Hispanic, or other. Parity was grouped into nulliparous and multiparous. Maternal diabetes status was categorized as either “pre-gestational/gestational diabetes” or “no diabetes” based on the medical record36. Hypertensive disorders were coded as being present if the mother had experienced one or more of the following: pre-eclampsia, eclampsia, chronic hypertension or hemolysis, elevated liver enzymes, or low platelet (HELLP) syndrome37. Information on infant feeding was obtained using a standardized interview performed at follow-up study visits over the first few years of life, and subjects were classified into three groups: (1) formula-fed exclusively, (2) breast-fed exclusively, or (3) both38.
Gestational Age Category
Gestational age was assessed based on the last menstrual period and verified by early ultrasound (<20 weeks of gestation)35. Term births were defined as gestational age ≥37 weeks, and were further categorized into two groups: full term (gestational age ≥39 weeks) and early term (37–38 weeks). Preterm births were defined as gestational age <37 weeks, and were further grouped into late preterm (34–36 weeks) and early preterm (<34 weeks).
Measures of Infancy Weight Gain and Overweight or Obesity
Child height and weight were obtained at well-child visits as part of pediatric primary care at the Boston Medical Center. Weight-for-age z-scores in the first two years were calculated using WHO reference values39. Weight gain z-scores in infancy were defined as the change in weight-for-age z-scores from birth to the target time points, and were categorized into four groups: slow (weight gain z-score <−0.67), on track (−0.67 to 0.67), rapid (>0.67 to 1.28), and extremely rapid (>1.28). These groups are equivalent to “crossing downward one or more”, “no crossing”, “crossing upwards one” and “crossing upwards two or more” of the major weight percentile lines (2rd, 10th, 25th, 50th, 75th, 90th, and 98th) on the WHO standard growth chart, respectively.
BMI z-scores and percentiles were calculated using U.S. national reference data39. Although there are different growth charts for term and preterm infants, we chose one national standard for all study participants because our objective was to assess the association between weight gain in early infancy and childhood obesity across the gestational age spectrum. Underweight and OWO were defined as BMI <5th and BMI ≥85th percentile, respectively, for age and gender based on the U.S. Centers for Disease Control and Prevention (CDC) definition40.
Leptin and Adiponectin Measures
Leptin concentrations were determined using sandwich immunoassays based on flow metric xMAP technology on Luminex 200 machines (Luminex Corp., Austin, TX) with an inter-assay coefficient of variation (CV) of 4.5%. Adiponectin was measured by ELISA with an inter-assay CV of <5.8%. The immunoassay kit was obtained commercially from Millipore Corp. Each sample was run in duplicate, and the intra-assay CVs for leptin and adiponectin were 4.3% and 2.9%, respectively.
Statistical analysis
We examined participant characteristics and outcomes by gestational age category. To calculate unadjusted trend P values across gestational age category, we used the Mantel-Haenszel χ2 test for categorical characteristics and linear regression for continuous characteristics. Weight gain z-score was evaluated both as a continuous variable and as a categorical variable by weight gain category (slow, on track, rapid and extremely rapid weight gain). Pearson correlation coefficients were computed to examine the association between weight gain z-score from age 4 to 24 months. In order to examine the independent effects of weight gain on BMI z-scores, multiple linear regressions were applied to the total samples and four subgroups (full term, early term, late preterm, and early preterm) and adjusted for prenatal and postnatal risk factors including maternal race/ethnicity, educational attainment, smoking status, parity, pre-pregnancy BMI, pre-gestational or gestational diabetes, hypertensive disorders, fetal growth status, and infant breastfeeding status. For total sample models, we additionally adjusted for gestational age categories. Similarly, multiple logistic regressions were used to examine the effects of weight gain on the risk of OWO, adjusted for the aforementioned covariables. As the weight gain z-scores, BMI z-scores and BMI percentiles already controlled for child age and sex, analyses were not further adjusted for these parameters.
We performed several sensitivity analyses to test for the influence of child age on the above associations by stratifying the age group (2–4 years, 5–7 years). In order to examine the impact of the time window of infancy weight gain, we expanded the time window for calculating the average weight gain levels (to 1 or 2 years rather than 4 months). Those associations with P values (2-sided tests) less than 0.05 were regarded as statistically significant. All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
Additional Information
How to cite this article: Wang, G. et al. Weight Gain in Infancy and Overweight or Obesity in Childhood across the Gestational Spectrum: a Prospective Birth Cohort Study. Sci. Rep. 6, 29867; doi: 10.1038/srep29867 (2016).
References
Ogden, C., Carroll, M., Kit, B. & Flegal, K. Prevalence of Childhood and Adult Obesity in the United States, 2011–2012. Jama 11, 806–814 (2014).
Freedman, D. S. et al. The relation of childhood BMI to adult adiposity: the Bogalusa Heart Study. Pediatrics 115, 22–27 (2005).
Guo, S. S., Wu, W., Chumlea, W. C. & Roche, A. F. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr 76, 653–658 (2002).
Freedman, D. S. et al. Classification of body fatness by body mass index-for-age categories among children. Arch Pediatr Adolesc Med 163, 805–811 (2009).
Freedman, D. S., Khan, L. K., Dietz, W. H., Srinivasan, S. R. & Berenson, G. S. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Pediatrics 108, 712–718 (2001).
Cunningham, S. A., Kramer, M. R. & Narayan, K. M. Incidence of childhood obesity in the United States. N Engl J Med 370, 403–411 (2014).
Taveras, E. M. et al. Crossing growth percentiles in infancy and risk of obesity in childhood. Arch Pediatr Adolesc Med 165, 993–998 (2011).
Ong, K. K., Ahmed, M. L., Emmett, P. M., Preece, M. A. & Dunger, D. B. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. Bmj 320, 967–971 (2000).
Torrazza, R. & Neu, J. Evidence-Based Guidelines for Optimization of Nutrition for the Very Low Birthweight Infant. NeoReviews 14, e340–e349 (2013).
Barton, M. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. Pediatrics 125, 361–367 (2010).
Barlow, S. E. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 120 Suppl 4, S164–192 (2007).
Centers for Disease Control and Prevention. Morbidity and mortality weekly report. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/su6001a16.htm. Accessed: 26 November 2013.
Taveras, E. M. et al. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics 123, 1177–1183 (2009).
Stettler, N., Zemel, B. S., Kumanyika, S. & Stallings, V. A. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics 109, 194–199 (2002).
ACOG committee opinion no. 560: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol 121, 908–910 (2013).
Gordon-Larsen, P., Adair, L. S. & Popkin, B. M. The relationship of ethnicity, socioeconomic factors, and overweight in US adolescents. Obes Res 11, 121–129 (2003).
Belfort, M. B. et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics 128, e899–906 (2011).
Considine, R. V. et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334, 292–295 (1996).
Arita, Y. et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257, 79–83 (1999).
Oda, N. et al. The ratio of leptin to adiponectin can be used as an index of insulin resistance. Metabolism 57, 268–273 (2008).
Stakos, D. A. et al. Plasma leptin and adiponectin concentrations correlate with cardiometabolic risk and systemic inflammation in healthy, non-obese children. J Pediatr Endocrinol Metab 27, 221–228 (2014).
Donoso, M. A. et al. Increased leptin/adiponectin ratio and free leptin index are markers of insulin resistance in obese girls during pubertal development. Horm Res Paediatr 80, 363–370 (2013).
Zhuo, Q. et al. Comparison of adiponectin, leptin and leptin to adiponectin ratio as diagnostic marker for metabolic syndrome in older adults of Chinese major cities. Diabetes Res Clin Pract 84, 27–33 (2009).
Vega, G. L. & Grundy, S. M. Metabolic risk susceptibility in men is partially related to adiponectin/leptin ratio. J Obes 2013, 409679 (2013).
Daniels, S. R., Khoury, P. R. & Morrison, J. A. The utility of body mass index as a measure of body fatness in children and adolescents: differences by race and gender. Pediatrics 99, 804–807 (1997).
Demerath, E. W. et al. Do changes in body mass index percentile reflect changes in body composition in children? Data from the Fels Longitudinal Study. Pediatrics 117, e487–495 (2006).
De Curtis, M. & Rigo, J. The nutrition of preterm infants. Early Hum Dev 88 Suppl 1, S5–7 (2012).
Daniels, S. R. & Hassink, S. G. The Role of the Pediatrician in Primary Prevention of Obesity. Pediatrics 136, e275–292 (2015).
Dietz, W. H. Overweight in childhood and adolescence. N Engl J Med 350, 855–857 (2004).
Wang, G., Chen, Z., Bartell, T. & Wang, X. Early Life Origins of Metabolic Syndrome: The Role of Environmental Toxicants. Curr Envir Health Rpt 1 (2014).
Strauss, R. S. Effects of the intrauterine environment on childhood growth. Br Med Bull 53, 81–95 (1997).
Knittle, J. L., Timmers, K., Ginsberg-Fellner, F., Brown, R. E. & Katz, D. P. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J Clin Invest 63, 239–246 (1979).
Muhlhausler, B. & Smith, S. R. Early-life origins of metabolic dysfunction: role of the adipocyte. Trends Endocrinol Metab 20, 51–57 (2009).
Rolland-Cachera, M. F., Deheeger, M., Maillot, M. & Bellisle, F. Early adiposity rebound: causes and consequences for obesity in children and adults. Int J Obes (Lond) 30 Suppl 4, S11–17 (2006).
Wang, X. et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. Jama 287, 195–202 (2002).
Kumar, R. et al. Gestational diabetes, atopic dermatitis, and allergen sensitization in early childhood. J Allergy Clin Immunol 124, 1031–1038 e1031-1034 (2009).
Yu, Y. et al. The combined association of psychosocial stress and chronic hypertension with preeclampsia. Am J Obstet Gynecol 209, 438 e431-438 e412 (2013).
Hong, X. et al. Gene polymorphisms, breast-feeding, and development of food sensitization in early childhood. J Allergy Clin Immunol 128, 374–381 e372 (2011).
Centers for Disease Control and Prevention. Growth charts. Available at: http://www.cdc.gov/growthcharts/clinical_charts.htm. Accessed: 26 November 2013.
Centers for Disease Control and Prevention. Division of Nutrition, Physical Actibity, and Obesity. Available at: http://www.cdc.gov/obesity/childhood/defining.html. Accessed: 8 October 2015.
Acknowledgements
We wish to thank all of the study participants and the Boston Medical Center Labor and Delivery Nursing Staff for their support and help with the study. We thank T.R. Bartell (Stanley Manne Children’s Research Institute, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, USA) for English editing. We are also grateful for the dedication and hard work of the entire field team at the Department of Pediatrics, Boston University School of Medicine. None of the above named received compensation for their role in this study. The Boston Birth Cohort (the parent study) is supported in part by the March of Dimes PERI grants (20-FY02-56, #21-FY07-605), and the National Institutes of Health (NIH) grants (R21 ES011666, R01 HD041702, R21HD066471, R21AI088609, U01AI090727, R01HD086013). We also wish to acknowledge generous philanthropic support from The Ludwig Family Foundation.
Author information
Authors and Affiliations
Contributions
G.W. and X.W. conceptualized and designed the study. G.W. and Y.G. analyzed and drafted the initial manuscript. X.W. supervised the analysis of data and performance of statistical analyses. Y.G., S.J., S.P., X.H., S.D., S.R., D.P., M.M., G.M., M.-C.W., B.Z. and T.L.C. contributed to the interpretation of results. X.H., S.D., S.R., D.P., M.M., G.M. and M.-C.W. reviewed and revised the manuscript. D.C., C.P., S.O.W., E.M. and Z.C. designed the data collection instruments, coordinated and supervised data collection, and critically reviewed the manuscript. S.J., S.P., B.Z. and T.L.C. had primary responsibility for the final content of the manuscript. All authors reviewed the manuscript for important intellectual content and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, G., Johnson, S., Gong, Y. et al. Weight Gain in Infancy and Overweight or Obesity in Childhood across the Gestational Spectrum: a Prospective Birth Cohort Study. Sci Rep 6, 29867 (2016). https://doi.org/10.1038/srep29867
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep29867
This article is cited by
-
Childhood growth outcomes 2 years after hypertensive versus normotensive pregnancy: a P4 study
Pediatric Research (2024)
-
Associations between KCNQ1 and ITIH4 gene polymorphisms and infant weight gain in early life
Pediatric Research (2022)
-
Childhood obesity and adverse cardiometabolic risk in large for gestational age infants and potential early preventive strategies: a narrative review
Pediatric Research (2022)
-
Protocol of the Snuggle Bug/Acurrucadito Study: a longitudinal study investigating the influences of sleep-wake patterns and gut microbiome development in infancy on rapid weight gain, an early risk factor for obesity
BMC Pediatrics (2021)
-
Underdiagnosis of obesity in pediatric clinical care settings among children born preterm: a retrospective cohort study
International Journal of Obesity (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.