Abstract
Single-molecule localization microscopy (SMLM) image quality and resolution strongly depend on the photoswitching properties of fluorophores used for sample labeling. Development of fluorophores with optimized photoswitching will considerably improve SMLM spatial and spectral resolution. Currently, evaluating fluorophore photoswitching requires protein-conjugation before assessment mandating specific fluorophore functionality, which is a major hurdle for systematic characterization. Herein, we validated polyvinyl alcohol (PVA) as a single-molecule environment to efficiently quantify the photoswitching properties of fluorophores and identified photoswitching properties predictive of quality SMLM images. We demonstrated that the same fluorophore photoswitching properties measured in PVA films and using antibody adsorption, a protein-conjugation environment analogous to labeled cells, were significantly correlated to microtubule width and continuity, surrogate measures of SMLM image quality. Defining PVA as a fluorophore photoswitching screening platform will facilitate SMLM fluorophore development and optimal image buffer assessment through facile and accurate photoswitching property characterization, which translates to SMLM fluorophore imaging performance.
Similar content being viewed by others
Introduction
Superresolution microscopy (SRM) has enabled fluorescence imaging at unprecedented spatial resolution1,2,3. Although a number of SRM techniques exist, single-molecule localization microscopy (SMLM) has gained in popularity due to its relative ease of instrumentation and compatibility with current labeling approaches for biological samples4,5. Two common SMLM techniques are stochastic optical reconstruction microscopy (STORM)2,6,7 and photoactivated localization microscopy (PALM)1,8, which enable visualization with ~10–20 nm resolution; however, resolution depends strongly upon the performance of the fluorophore used for sample labeling9,10,11,12.
SMLM requires dense labeling of features of interest with photoswitchable fluorophores that have the ability to stochastically switch between the fluorescent “on” state where photons are emitted and the nonfluorescent “off” or dark state13,14. Although the exact photoswitching mechanism is unknown for all fluorophore scaffolds, switching between the fluorescent on and off states is largely attributed to manipulation of the reductive and oxidative status of the imaging environment13,15,16. Subdiffractive localization of individual fluorophores throughout a series of images via activation of a stochastic, small population of fluorophores in the on state facilitates reconstruction of a superresolution image17. Thus, the photoswitching ability of the fluorescent labels is crucial to the quality of SMLM.
Currently utilized fluorophores largely consist of commercially available probes that have been demonstrated to photoswitch or be photoactivatable under certain imaging conditions10,18,19. However such probes are not designed to provide the optimal photoswitching as it is a disadvantage for conventional fluorescence microscopy, presenting an opportunity for photoswitching improvement. The most widely used fluorophores for SMLM include Alexa Fluor 647 and Cy5, which can achieve ~20 nm resolution, yet the majority of commercial fluorophores identified as the top SMLM candidates reach resolutions of only 30–40 nm at best6,10,18. Furthermore, as SMLM progresses to imaging multi-protein cellular complexes with multicolor SMLM, photoswitchable fluorophores with relatively narrow excitation and emission spectra will be required to minimize crosstalk10,18. Advancing the spatial and spectral resolution of SMLM will be realized through the synthesis of fluorophores specifically designed to photoswitch or photoactivate with ideal spectral properties for multicolor imaging. The chemical space to be investigated for development of ideal photoswitchable fluorophores in addition to the numerous imaging buffer formulations that significantly impact photoswitching behavior present a pressing need for a robust and efficient method to characterize fluorophore utility for SMLM.
A common approach to assess fluorophore photoswitching is through in vitro labeling of a known cellular structure in cells with descriptive image evaluation of the rendered SMLM image as this closely reflects most SMLM fluorophore applications10,18,20. However, the quality of the resulting image is often influenced by more than just fluorophore photoswitching properties. SMLM image quality can also reflect labeling issues arising from nonspecific binding or insufficient labeling density rather than the photoswitching ability of the fluorophore. Descriptive image evaluation of SMLM image quality is subjective and thus not readily comparable between fluorophores. Additionally, in vitro evaluation requires the fluorophore of interest to contain a readily conjugatable group, such as an N-hydroxysuccinimide (NHS) ester, maleimide or azide, for conjugation to a targeting molecule where the type of chemical attachment to the labeling protein may affect fluorophore photoswitching. Thus, in vitro evaluation of photoswitching requires additional fluorophore synthetic steps for conjugation, convolves the fluorophore photoswitching properties with labeling density and nonspecific background, and may be affected by the selected protein attachment strategy, making direct assessment of fluorophore photoswitching time consuming and largely descriptive instead of quantitative.
An alternative approach is studying fluorophore photophysical properties sans cells by spatially isolating and immobilizing single molecules. Such single-molecule systems include fluorophore adsorption to coverglass through protein-conjugation18,21,22,23,24 and fluorophores fixed in various polymer films25,26,27,28,29,30,31. Protein adsorption may more closely represent the environment utilized for biological SMLM imaging than polymer films while minimizing sample preparation time in comparison to evaluation of photoswitching properties in cells. However, protein adsorption still requires addition of a readily conjugatable group on the fluorophore of interest, where the chosen chemical conjugation strategy may affect photoswitching properties. By contrast, polymer films enable isolation of fluorophores devoid of conjugatable groups, which has significant utility for rapid screening of novel fluorophores as a photoswitch as well as isolation of the photoswitching properties from conjugation strategy. While fluorophore photoswitching performance evaluated via protein adsorption has been shown to qualitatively correlate to cell imaging applications18, it has not been established how the more advantageous polymer film isolation method compares, nor how photoswitching properties quantitatively compare to SMLM image quality.
In this study, we compared the polymer film and the protein adsorption single-molecule systems for their ability to predict in vitro SMLM image quality within cells through measurement of photoswitching properties. SMLM images ranging in quality were acquired of microtubules labeled in cells with eight commercial fluorophores. Photoswitching properties of the eight fluorophores were then obtained using dilute fluorophores dried into a polyvinyl alcohol (PVA) film and fluorophore-conjugated antibodies adsorbed to coverglass as the two single-molecule isolation platforms. Through statistical analysis, we identified the photoswitching properties measured using each isolation platform that correlate to, and ultimately predict, SMLM image quality. We demonstrated PVA films were efficacious and robust in evaluating fluorophores for SMLM imaging applications and hence, provide the necessary screening system for SMLM fluorophore development.
Results
SMLM Image Quality Varies with Photoswitchable Fluorophore Label
We established in vitro SMLM performance of selected fluorophores through quantitative assessment of the structure of microtubules labeled with indirect immunofluorescence in fixed cells. Eight commercially available fluorophores were used: three blue absorbing fluorophores, Fluorescein, ATTO 488 (ATTO488), and BODIPY™ FL (BODIPY FL); two green absorbing fluorophores, Cy™3B (Cy3B), and Alexa Fluor™ 568 (AlexaFluor568); and three red absorbing fluorophores, Cy™5 (Cy5), Alexa Fluor™ 647 (AlexaFluor647), and ATTO 680 (ATTO680). These eight fluorophores covered the ultraviolet to red region of the light spectrum and represented four classic fluorophore scaffolds including xanthene, BODIPY, cyanine, and oxazine. Microtubule labeling was completed with equal concentrations of primary antibody and fluorophore concentration conjugated to secondary antibody to maintain equal labeling density across the different fluorophores. Images of all fluorophores were collected under constant imaging buffer, image capture settings, and fluence rate within an excitation laser line.
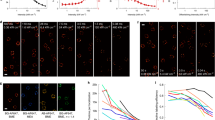
Microtubule structures were visible in all rendered images with image quality varying in fluorophore homogeneity (Fig. 1), measured width (Fig. 2a), and measured continuity (Fig. 2b) of the labeled structure. AlexaFluor647, Cy5, and ATTO488 provided the most homogeneous rendered microtubule structures, followed by AlexaFluor568, Fluorescein, BODIPY FL, and ATTO680. Cy3B had the worst fluorophore homogeneity across the microtubule structure and the lack of Cy3B fluorophores rendered required the quadrupling of image contrast to aid in visualization of the displayed images (Fig. 1a,b). The range in SMLM image quality was expected as the chosen imaging conditions were not optimized for each fluorophore, but rather kept constant to enable differences in resulting image quality to be attributed to the photoswitching properties of the selected fluorophore. SMLM image quality using the selected fluorophores could be improved for future studies by tailoring the imaging buffer and acquisition settings for each fluorophore12,32.
(a) Rendered SMLM images of microtubules (scale bar = 2 μm) with (b) magnified boxes to demonstrate reconstructed microtubule detail (scale bar = 0.5 μm). Panels labeled with “4x” are displayed with four times the brightness in comparison to other panels. Rendered images were organized by increasing observed quality from left to right: Cy3B, ATTO680, BODIPY FL, Fluorescein, AlexaFluor568, ATTO488, Cy5, and AlexaFluor647.
Calculated (a) average microtubule width and (b) average continuity of microtubules for each fluorophore, organized by observed quality. Average width and continuity measurements are graphed as the mean ± standard deviation of n = 15 measurements per image of the corresponding fluorophore. Images were collected at the following fluence rates: 0.28 kWcm−2 for the 488 nm laser line (blue points), 0.49 kWcm−2 for the 561 nm laser line (green points) and 1.11 kWcm−2 for the 647 nm laser line (red points).
SMLM image quality of the rendered microtubules resulting from each fluorophore was quantified by measuring the average width (Fig. 2a) and continuity (Fig. 2b). The width was measured as the full width at half maximum (FWHM) of the microtubule structure and the continuity was defined as the photons detected per nm2 of the measured microtubule. As expected, the average microtubule width measurements closely aligned with the observed quality of the rendered images (Figs 1a,b and 2a). The fluorophores with the narrowest average microtubule widths, AlexaFluor647 (51.3 ± 12.6 nm), Cy5 (60.2 ± 15.3 nm), and ATTO488 (62.9 ± 10.6 nm) had the highest quality microtubule images. These widths closely aligned with the expected 55 nm FWHM width, which are wider than the reported 25 nm width of microtubules as measured by electron crystallography due to the primary and secondary antibodies used for indirect immunofluorescence labeling9,18,33,34. Microtubule images of lower quality had larger widths including AlexaFluor568 (67.4 ± 20.7 nm), Fluorescein (76.1 ± 14.0 nm) and BODIPY FL (80.9 ± 23.1 nm). The poorest quality SRM images demonstrated the widest microtubules, which were found to be ATTO680 (83.1 ± 17.6 nm) and Cy3B (83.7 ± 37.3 nm) herein. The large microtubule width standard deviations reflect the inconsistent detection of fluorophores across even the most continuous microtubules within the SMLM image, which was likely attributed to their poor photoswitching properties and relatively low signal to background ratio (SBR). The average continuity measurements (Fig. 2b) also varied between fluorophores and showed a similar trend to observed image quality. Continuity was greatest for ATTO488 (1.48 ± 0.26 photons/nm2), AlexaFluor568 (1.35 ± 0.42 photons/nm2), Cy5 (1.20 ± 0.31 photons/nm2), and AlexaFluor647 (1.16 ± 0.29 photons/nm2). Fluorescein (1.11 ± 0.22 photons/nm2), ATTO680 (1.07 ± 0.23 photons/nm2), and BODIPY FL (0.97 ± 0.28 photons/nm2) demonstrated mid-level continuity, while Cy3B (0.52 ± 0.24 photons/nm2) showed the lowest average signal intensity and thus the least microtubule continuity. SMLM microtubule width and continuity measurements were used to identify correlation of image quality to photoswitching properties measured using each single-molecule isolation system.
Correlation of SMLM Image Quality to Fluorophore Photoswitching Properties
The photoswitching properties of the eight fluorophores were measured using both the antibody adsorption and PVA film single-molecule isolation systems (Table 1 and Fig. 3a–f). Previous studies of a variety of commercially available photoswitchable fluorophores35,36,37,38,39,40 have shown that SMLM image quality is influenced by photoswitchable fluorophore photon output and the amount of time each fluorophore spends in the fluorescent on and off states18,41,42. Herein, we quantified six photoswitching properties for the spatially isolated fluorophores where four properties characterized photon output and two properties characterized time in the fluorescent on and off states. Measurements were collected in triplicate to assess photoswitching property reproducibility over three fluence rates within each excitation laser line. Measurements across the three fluence rates were used to assess the relationship between photoswitching behavior in each single-molecule isolation system and fluence rate. Measurements collected at the highest fluence rate were used for photoswitching property correlation analysis, as this was the same fluence rate used to acquire the SMLM microtubule images.
Average photoswitching properties including (a) photons per switching cycle, (b) number of switching cycles, (c) total photons, (d) localization precision, (e) photoswitching time, and (f) duty cycle are demonstrated. Average photoswitching properties represented the mean ± standard deviation of n = 3 SMLM imaging series for each fluorophore. Photoswitching property measurements were collected at fluence rates equivalent to those utilized for imaging: 0.28 kWcm−2 for the 488 nm laser line (blue bars), 0.49 kWcm−2 for the 561 nm laser line (green bars) and 1.11 kWcm−2 for the 647 nm laser line (red bars).
Of the six measured photoswitching properties, four characterized single-molecule photon output including: (1) photons per switching cycle, which represented the intensity of each molecule per fluorescent on cycle (Supplementary Fig. S1a), (2) number of switching cycles, which were the number of transitions from the dark off state to the fluorescent on state and were counted when photons were emitted above a set threshold (Supplementary Fig. S1b), (3) total photons, which were calculated as the photons per switching cycle multiplied by the number of switching cycles, and (4) localization precision, which reflected the deviation in the x and y position of the location of the maximum intensity of each molecule’s switching cycle43. The remaining two photoswitching properties characterized time in the fluorescent on and off states including: (5) photoswitching time, which reflected the length of time a molecule photoswitched before photobleaching occurred (Supplementary Fig. S1b), and (6) duty cycle, which described the fraction of time the molecule was on during the entire collected video.
Trends were seen when comparing the image quality of the eight selected fluorophores (Fig. 1) to their photoswitching property data (Fig. 3 and Table 1). Fluorophores with higher quality images tended to have higher photons per switching cycle, a greater number of switching cycles, higher total photons, and higher duty cycles. Statistically significant correlations were identified using Spearman two-tail correlation tests44 with significance reported as p < 0.05, which validated our observations. Both the antibody adsorption and PVA film systems produced total photon output results that correlated to SMLM image quality (Table 2) represented by the width (Antibody method: p = 0.037 and PVA method: p = 0.046). These findings agreed with previous studies that showed that SMLM image quality was improved by higher photon output as well as theoretical localization-precision-based calculations16,18,19. Additionally, both the antibody adsorption and PVA film systems produced duty cycle results that correlated to continuity measurements (Antibody method: p = 0.028 and PVA method: p = 0.037) (Table 2). Other photoswitching properties statistically correlated with continuity but the exact property varied based on the fluorophore isolation method used. Using the antibody adsorption method the localization precision correlated to continuity (p = 0.046). Using the PVA film isolation method, the switching cycles (p = 0.022) and total photons (p = 0.028) correlated to continuity (Table 2).
Equivalent Fluorophore Photoswitching Property Measurements Using Antibody Adsorption and PVA Film Fixation Methods
The antibody adsorption fluorophore isolation method recapitulated established photoswitching property measurements collected using similar fixation techniques10,18. Comparing photons per switching cycle and number of switching cycles with the previously published literature demonstrated similar trends with our antibody isolated fluorophore photoswitching data. Both studies demonstrated similar photon output for the blue fluorophores ATTO488 and Fluorescein. When comparing photon output of the green fluorophores, AlexaFluor568 had higher photon output than Cy3B, similar to previously published results. Both studies also demonstrated a similar trend for the red fluorophores where AlexaFluor647 and Cy5 showed higher photon output than ATTO68018. Switching cycles were also comparable between the two studies, where ATTO488 had more switching cycles than Fluorescein for the blue fluorophores and Cy3B showed a similar number of switching cycles to AlexaFluor568 for the green fluorophores. The only anomaly between our photoswitching property data and the previously published results was in the switching cycle number for the red fluorophores. While both studies reported AlexaFluor647 had more switching cycles than Cy5, Dempsey et al. reported ATTO680 had the fewest switching cycles, while we measured the greatest number of switching cycles from ATTO680 across the red fluorophores18. Of note, the exact numerical values for photons per switching cycle and number of switching cycles between our study and previously published work did differ as would be expected from collecting photoswitching property data using different instrument configurations. The difference in switching cycle number across the red fluorophores was most likely due to differences in illumination fluence between the two studies, which strongly affect number of switching cycles. Even with some slight discrepancies, our antibody fluorophore isolation data demonstrated very similar photoswitching properties to previously published literature, validating our assay for photoswitching property evaluation. Of note, we did not directly compare duty cycle to the previously published study as our calculation represented an average over the entire collected dataset rather than a subset of frames that excluded the initial photobleach step. The inclusion of data collected during the initial photobleaching step in our duty cycle calculation resulted in higher absolute numerical values than previously reported18. Localization precision was also not directly compared to previous studies, as it has not previously been calculated for a suite of fluorophores.
The validated antibody adsorption photoswitching properties were compared to the PVA film isolated photoswitching properties to determine the similarity of the fluorophore photoswitching performance in these different environments. All six measured photoswitching properties showed similar overall trends across the eight fluorophores between the antibody adsorption and PVA film isolation methods at the high fluence rate (Table 1), which was used to collect the SMLM images utilized to assess image quality. Localization precision had the strongest trend with antibody adsorption values closely matching PVA film values for all eight fluorophores tested (Fig. 3d). Photons per switching cycle (Fig. 3a), number of switching cycles (Fig. 3b), and total photons (Fig. 3c) also showed strong trends between the antibody adsorption and PVA film photoswitching property data. Duty cycle (Fig. 3f) and photoswitching time (Fig. 3e) trended together less strongly than the other measured photoswitching properties between the two different fluorophore isolation methods. Overall, the antibody and PVA fluorophore isolation methods demonstrated strongly correlated photoswitching properties, which was further demonstrated by the fact that the total photons measured using each method statistically correlated to microtubule width and that the duty cycle measured for each method statistically correlated to microtubule continuity (Table 2).
Correlations seen between the photoswitching properties measured with both the antibody adsorption and PVA film isolation methods at the highest fluence rate translated to trends seen across all three fluence rates (Fig. 4). Localization precision (Fig. 4d,j) and switching events (Fig. 4b,h) demonstrated the strongest correlation at the highest fluence rate. Photons (Fig. 4a,g) and total photons (Fig. 4c,i) trended similarly for both the antibody adsorption and PVA film isolation methods. Photoswitching time (Fig. 4e,k) and duty cycle (Fig. 4f,l) showed little relationship to fluence rate.
Correlation of photoswitching properties and fluence rate for antibody adsorption (a–f) and PVA (g–l) isolated fluorophores. Linear regression analysis of each average photoswitching property versus fluence rate was completed and R2 values were calculated (see key for values) for (a,g) photons per switching cycle, (b,h) switching cycles, (c,i) total photons, (d,j) localization precision, (e,k) photoswitching time, and (f,l) duty cycle. Mean photoswitching properties for n = 3 SMLM imaging series for each fluorophore are displayed.
Effect of Excitation Fluence Rate on Photoswitching Properties
Correlative relationships were observed between select photoswitching properties and fluence rate using both the antibody adsorption and PVA film single-molecule systems (Fig. 4). Linear regressions were calculated using the average value (n = 3 SMLM image series/fluorophore) for each photoswitching property at each fluence rate to quantify the degree of correlation using R2 values. Photons per switching cycle (Antibody R2 = 0.81–1.0, PVA R2 = 0.89–1.0) and number of switching cycles (Antibody R2 = 0.88–1.0, PVA R2 = 0.72–0.99) were strongly correlated with fluence rate, where increased fluence rate resulted in greater photon output and fewer switching cycles. Total photons (Antibody R2 = 0.23–0.99, PVA R2 = 0.01–0.99) and localization precision (Antibody R2 = 0.35–0.99, PVA R2 = 0.73–0.99) showed general correlation with fluence rate, however neither property was as strongly correlated as photons per switching cycle or number of switching cycles. Photoswitching time and duty cycle were largely unaffected by fluence rate (Fig. 4e,f,k,l).
Intersample Stability of Photoswitching Property Measurements
The reported photoswitching properties represented mean and standard deviation calculated from triplicate imaging series collected for each fluorophore at three fluence rates (Table 1 and Supplementary Figs S2 and S3). The coefficient of variation (CV) was less than 1 for all fluorophores across the computed photoswitching properties, demonstrating that the photoswitching property measurements were reproducible using both the antibody adsorption and PVA film fluorophore isolation methods. Some properties had lower standard deviation than others exhibiting better reproducibility. For both the antibody adsorption and PVA film fixation methods the least variable photoswitching property was localization precision (CV < 0.1 for all fluorophores), followed by photons with CVs ranging 0.03–0.4. The most variable photoswitching properties was photoswitching time with CVs ranging up to 0.7. However, overall both fluorophore isolation methods provided robust, reproducible measurements of the defined photoswitching properties.
Discussion
In summary, we developed a methodology to correlate fluorophore photoswitching properties to SMLM image quality using two single molecule isolation systems. We examined PVA film as a method for facile characterization of fluorophores developed for SMLM or optimization of imaging buffer systems, where the photoswitching properties of fluorophores measured using both PVA film and the previously utilized antibody adsorption method were correlated with SMLM image quality (Figs 1 and 2). While prior studies have characterized photoswitching10,14,18, our study is the first to demonstrate a statistically significant correlation between SMLM image quality and photoswitching properties including total photons and duty cycle (Table 2). We found microtubule width was statistically correlated with total photon output using both the antibody absorption and PVA film fluorophore isolation methods, while duty cycle was significantly correlated to microtubule continuity using both fluorophore isolation methods (Table 2 and Fig. 3). Interestingly, previous studies have demonstrated strong correlation between photons per switching cycle and SRM image quality. In the current study, total photons was significantly correlated to image quality rather than photons per switching cycle, which is likely explained by the different photophysical environments provided by the antibody adsorption and PVA film isolation methods. Although the imaging buffer utilized in both fluorophore isolation systems contained oxygen scavengers, the PVA film may have further reduced oxygen permeation into the system, thus affecting photoswitching physics6,22,45, potentially accounting for the differences seen in photon output per switching cycle and the number of switching cycles compared with the antibody adsorption method. However, the calculation of total photon output as the product of these two photoswitching properties recapitulated the relationship seen in the antibody adsorption fluorophore isolation method, where significant correlation was demonstrated between image quality and overall photon output (Table 2).
Interestingly, we found that fluorophores resulting in higher quality images had higher duty cycle than those of lower quality SRM images, which is in contradiction to previous studies where shorter duty cycle was thought to improve SRM image quality18. We hypothesize that higher duty cycle was significantly correlated with image quality in our study because of the difference in duty cycle calculation. In our study, duty cycle was calculated over the entire collected photoswitching analysis video, where images were collected as soon as the laser was turned on and thus, no pre-photobleaching step was included. Therefore, our higher duty cycle measurements represent individual fluorophore molecules that were less susceptible to photobleaching. Thus, higher duty cycles reflected fluorophore molecules spending more time in the fluorescent on state. While higher duty cycles could enhance imaging by improving the ability to detect individual fluorophore molecules, it could potentially be detrimental for fluorophores to have high cycles as it increases the chances of detected fluorophore overlap, which degrades SMLM image quality46. The low fluorophore density used for photoswitching property analysis in this study were well below the threshold where fluorophore overlap would affect accurate quantification of photoswitching property measurements, explaining the strong imaging quality correlation to the higher duty cycle photoswitching property in both the antibody absorption and PVA fluorophore isolation methods.
We also found that the photon output related photoswitching properties measured using both fluorophore isolation methods correlated to fluence rate, while the fluorescent on and off time parameters showed little correlation with fluence rate. While previous studies observed that the fluence rate used to excite photoswitchable fluorophores affects SMLM image quality18,47, the clear impact of fluence rate on photoswitching properties and image quality was demonstrated here. We found a strong correlation between photons per switching cycle and number of switching cycles as well as general correlation between fluence rate, total photons and localization precision. Higher quality SMLM images were achieved with fluorophores having higher total photons and duty cycle (Figs 1 and 3). Therefore, the highest fluence rate tested for each laser line was found to be the best for SMLM imaging, and hence most effective for future screening of developed fluorophores for SMLM. Importantly, similarities in photoswitching property rankings and response to fluence rate will enable either fluorophore isolation method to be used for accurate fluorophore evaluation and comparison. Lastly, we also quantified the repeatability of photoswitching measurements in both the antibody adsorption and PVA film based systems, demonstrating a robust platform for screening developed fluorophores for SMLM using single-molecule isolation (Fig. 3 and Table 1).
Herein, we demonstrate that PVA is a facile fluorophore isolation method that can be used to screen fluorophores or imaging buffer conditions to predict SMLM image quality based on the quantification of total photon output and duty cycle. The PVA film method eliminates the functionality hurdle in screening novel fluorophores, facilitating a potentially high throughput approach for large studies. The utilization of PVA film fluorophore isolation system provides the necessary means to evaluate and guide future fluorophore development, as well as screen for optimal image buffer conditions, to improve the spatial and spectral resolution of SRM through quantification of photoswitching properties that predict SRM image quality.
Methods
Fluorophores
The eight fluorophores utilized in this study were obtained commercially in their succinimidyl ester form including Fluorescein, BODIPY FL, AlexaFluor568, AlexaFluor647 (Thermo Fisher Scientific), ATTO488, ATTO680 (ATTO-TEC), Cy3B and Cy5 (GE Healthcare Life Sciences).
Fluorophore-labeled Antibodies
Each fluorophore was conjugated to donkey anti-mouse antibody (Jackson ImmunoResearch) for SMLM microtubule imaging and single-molecule photoswitching property measurements. The antibody was buffer exchanged into 1x phosphate buffered saline (PBS) and pH adjusted to 8.0 with 35 mM disodium phosphate. The conjugation reactions were set up with fluorophore and antibody mixed at a 5:1 molar ratio for SMLM microtubule imaging and 1:1 molar ratio for single-molecule photoswitching property measurements. The fluorophore-antibody conjugation reactions were rocked gently at room temperature protected from light for 3 hr and were concentrated in 10 kDa MWCO spin filters followed by purification using a 7 kDa desalting column. The fluorophore to antibody-labeling conjugation ratios ranged from 1.1:1 to 2.3:1 for SMLM microtubule imaging and ranged from 0.4:1 to 0.5:1 for single-molecule photoswitching property measurements, which were determined using an absorbance spectrometer (SpectraMax M5 Microplate Reader).
Single-Molecule Localization Super Resolution Microscope Configuration
Imaging was completed on a Nikon ECLIPSE Ti-U inverted microscope equipped with a 60x oil immersion objective (NA = 1.49) using total internal reflection fluorescence configuration of the light path. Excitation laser lines included 488-nm (Coherent), 561-nm (Opto Engine LLC), and 647-nm (Coherent), with images collected through a 525/45, 605/64, or 708/75 nm single-bandpass filter (Semrock Inc.), respectively. An EMCCD camera (Andor Technology) recorded images in a 512 × 512 pixel area at 107 nm/pixel, with a 100 ms exposure time and a EM gain setting of 300 via Micro-Manager48,49. Images of single molecules were processed with modified custom-written Matlab scripts (Mathworks)50. Microtubule images were processed with the ThunderSTORM51 plugin for ImageJ where the approximate localization of individual fluorophores was identified as signal intensities above a set threshold. Image processing conditions were kept constant across all 8 selected fluorophores.
Imaging Buffer
Tris-buffered saline (TN buffer, 50 mM Tris pH 8.0 and 10 mM NaCl) with oxygen scavenger components including 0.5 mgml−1 glucose oxidase, 40 μgml−1 catalase (Sigma Aldrich), and 10% w/v glucose, as well as the reducing component 10 mM β-mercaptoethylamine, were utilized for all imaging and photoswitching property measurements18,52.
SMLM Microtubule Images
Cell Culture
U2OS cells were cultured in Dulbecco’s Modified Eagle media without phenol red supplemented with 10% FBS and 1% Penicillin-Streptomycin-Glutamine at 37 °C and 5% CO2. LabTek 8-well coverglass chambers were washed with SparKLEEN (5 min), milli-Q water (3 × 5 min), 1 M NaOH (90 min), and milli-Q water (3 × 5 min) prior to plating cells at ~80,000 cells per well. Cells were incubated for 2 days to reach ~75% confluency.
Immunofluorescence Labeling
Cells were preextracted with 0.5% Triton X-100 in PEM (100 mM PIPES buffer pH 7.0, 1 mM EGTA and 1 mM MgCl2) for 20 s, fixed with 0.4% glutaraldehyde (Electron Microscopy Science) and 0.25% Triton X-100 in PEM for 90 s, washed with PBS before fixing with 3% glutaraldehyde in PEM 15 min. Cells were washed with PBS (3 × 5 min), then reduced with 10 mM sodium borohydride for 10 min, washed again with PBS (3 × 5 min), and blocked with 5% bovine serum albumin (BSA) in PBS. Cells were incubated with bovine alpha-tubulin mouse primary antibody (Thermo Fisher Scientific) at 2 μgml−1 in BSA for 4 h at room temperature and washed with PBS (3 × 5 min). Lastly, cells were incubated with fluorophore-labeled donkey anti-mouse secondary antibody at 0.2 μM fluorophore concentration across the eight fluorophores in 5% BSA for 30 min at room temperature protected from light and washed with PBS (3 × 5 mins)20.
Imaging
A mixture of 200 μl imaging buffer and 2 μl gold nanoparticles (BBI International) fiducial markers was added to the labeled cells prior to imaging. Images were collected at a frame rate of 10 Hz for 10,000 frames. Samples labeled with Fluorescein, ATTO488, or BODIPY FL were excited using 488-nm laser line (0.28 kWcm−2). Samples labeled with Cy3B or AlexaFluor568 were excited by the 561-nm laser line (0.49 kWcm−2). Samples labeled with Cy5, AlexaFluor647, or ATTO680 were excited by the 647-nm laser line (1.11 kWcm−2). Imaging began with exposure to the excitation laser and a rapid decrease in the density of “on” fluorophores was observed within the first few hundred frames of the 10,000 frame movies, after which the observed “on” fluorophores in each frame were spatially separated.
Microtubule Quantitation
The image quality of the rendered SMLM images was quantitated based on the microtubule width and continuity, which were measured at 15 representative points per image where labeling was most complete and the structure was well defined. The width was calculated by taking the FWHM from line profiles summed over 13 nm lengths of the microtubule structure. The continuity was reported as the photon density of microtubules per square nanometer. Continuity was calculated as the summed photons detected along a 670 nm length sections of microtubules using the calculated width to determine the measured microtubule area in nm2.
Single-molecule Fixation
Single-molecule samples were fixed in 96-well glass bottom plates with #1.5 coverglass bottom (In Vitro Scientific) and washed as detailed for the LabTek plates above. Single-molecule isolation was performed using two fixation methods: antibody adsorption and PVA film formation. For the antibody adsorption method, 100 μl of fluorophore-labeled antibody diluted to a final concentration of 1 × 10−10 M fluorophore in 1x PBS was used per well. Antibody solutions were incubated for 16 h at room temperature sealed and protected from light, then washed and stored in PBS at 4 °C. For the PVA film method, fluorophores were diluted to a final concentration of 5 × 10−9 M in 1 wt% PVA (72,000 MW) and 50 μl was used per well before drying in a fume hood overnight. Sample preparation resulted in 150–300 observed particles dispersed throughout the 512 × 512 pixel field of view. At 107 nm/pixel, this equated to a density of 0.05–0.1 fluorophores/μm2.
Single-molecule Photoswitching Measurements
All measurements were carried out in the imaging buffer described above. For adsorbed antibody samples, storage PBS was aspirated prior to the addition of imaging buffer. For PVA film samples, the dried PVA films were quickly flushed with PBS 3x to remove any molecules not isolated by the dried film prior to addition of imaging buffer. PVA film measurements were collected between 10 and 60 minutes after adding the imaging buffer to allow for adequate penetration of the buffer into the solid film, but prior to any compromise of the film by rehydration that could allow for molecule drift out of the PVA matrix.
Each sample was imaged three times in three different regions of the 96-well plate at each of the selected fluence rates termed low, mid and high for each laser line. Fluorescein, ATTO488 and BODIPY FL were excited with a 488-nm laser line at fluence rates of 0.08, 0.14, and 0.28 kWcm−2. Cy3B and AlexaFluor568 were excited with a 561-nm laser line at fluence rates of 0.12, 0.26, and 0.49 kWcm−2. Cy5, AlexaFluor647, and ATTO680 were excited with a 647-nm laser line at fluence rates of 0.30, 0.58, and 1.11 kWcm−2. Imaging videos were collected for 5,000 frames at 10 Hz.
Single-molecule Data Analysis
Single molecules were identified as fluorescent signals detected above a threshold 6 times root mean square (RMS) of the average detected signal. Molecules were tracked positionally throughout the 5,000-frame image series while recording the x and y coordinates of their point-spread function. A minimum of 100 molecules were identified in each 5,000-frame image series. Photoswitching properties were quantitated as follows.
Photons per Switching Cycle
The number of photons emitted per switching cycle was calculated algebraically by summing the fluorescence emission signal collected over consecutive frames from a single molecule that was above 6 times RMS, minus the baseline intensity. This was considered a single switching cycle. The summed signal (analog digital units, ADU) was converted to photons using the camera’s reported analog-to-digital conversion factor (DCF, electrons/ADU), quantum efficiency (QE), and the acquisition gain setting (gain) as follows: photons = ADU x DCF x 1/QE x 1/gain.
Switching Cycles
The number of switching cycles was determined by counting the instances a tracked particle emitted photons above 6 times RMS, reflecting how often the molecule transitioned from the off to the on state.
Total Photons
The total photon values were calculated by multiplying the photons per switching cycle by the number of switching cycle, resulting in the total photons a single molecule emitted throughout the image series.
Localization Precision
The lateral localization precision, σxy, was determined using the standard deviation of the x (σx) and y (σy) coordinates recorded for each molecule throughout the 5,000-frame image series calculated as σxy = (σx2 + σy2)1/2.
Photoswitching Time
The photoswitching time was calculated as the amount of time in seconds a single molecule photoswitched during the entire 5,000-frame image series. This was calculated as the time difference between the first switching cycle and the last switching cycle. Results were calculated only for molecules that had their initial cycle during the first 100 s to prevent truncating photoswitching time calculations for molecules that started blinking at a later time point in the image series.
Duty Cycle
The duty cycle represents the fraction of time fluorophores emit photons during the 500 s acquisition time. Duty cycle was calculated as the number of frames the fluorophore was considered on (above the set threshold of RMS 6) divided by the total number of frames.
Statistical Analysis of Photoswitching Properties
Statistical analysis was completed using GraphPad Prism (GraphPad Software). To determine the correlation of each photoswitching property to image quality, a spearman two-tail correlation test44 was completed comparing the set of average widths (n = 15/fluorophore, 8 fluorophores) and the set of average continuity (n = 15/fluorophore, 8 fluorophores) to the average of each photoswitching property (n = 3/fluorophore, 8 fluorophores) (Table 2). This test was conducted on data from both the antibody adsorption and PVA film fixation methods. Correlation was reported as significant for p < 0.05.
To study the relationship between fluence rate and photoswitching properties, linear regressions were completed using the average value (n = 3 SMLM image series/fluorophore) for a particular photoswitching property at each fluence rate. Computed R2 values, defined as the coefficient of determination, were reported to characterize the correlation between photoswitching property and fluence rate. R2 values closer to 1 indicate a more linear relationship while R2 values closer to 0 indicate a less linear relationship53.
To study the intersample stability of photoswitching property measurements, the coefficient of variance (CV) was calculated by dividing the standard deviation of the triplicate measurements by the mean of the triplicate measurements.
Additional Information
How to cite this article: Bittel, A. M. et al. Methodology for Quantitative Characterization of Fluorophore Photoswitching to Predict Superresolution Microscopy Image Quality. Sci. Rep. 6, 29687; doi: 10.1038/srep29687 (2016).
References
Betzig, E. et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645, doi: 10.1126/science.1127344 (2006).
Rust, M. J., Bates, M. & Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nature methods 3, 793–795, doi: 10.1038/nmeth929 (2006).
Vaughan, J. C., Jia, S. & Zhuang, X. Ultrabright photoactivatable fluorophores created by reductive caging. Nature methods 9, 1181–1184, doi: 10.1038/nmeth.2214 (2012).
Klein, T., Proppert, S. & Sauer, M. Eight years of single-molecule localization microscopy. Histochemistry and cell biology 141, 561–575, doi: 10.1007/s00418-014-1184-3 (2014).
Huang, B. Super-resolution optical microscopy: multiple choices. Current opinion in chemical biology 14, 10–14, doi: 10.1016/j.cbpa.2009.10.013 (2010).
Heilemann, M. et al. Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes. Angew Chem Int Ed Engl 47, 6172–6176, doi: 10.1002/anie.200802376 (2008).
van de Linde, S., Sauer, M. & Heilemann, M. Subdiffraction-resolution fluorescence imaging of proteins in the mitochondrial inner membrane with photoswitchable fluorophores. Journal of structural biology 164, 250–254, doi: 10.1016/j.jsb.2008.08.002 (2008).
Hess, S. T., Girirajan, T. P. & Mason, M. D. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophysical journal 91, 4258–4272, doi: 10.1529/biophysj.106.091116 (2006).
Adam, V. et al. Rational design of photoconvertible and biphotochromic fluorescent proteins for advanced microscopy applications. Chemistry & biology 18, 1241–1251, doi: 10.1016/j.chembiol.2011.08.007 (2011).
Lehmann, M., Lichtner, G., Klenz, H. & Schmoranzer, J. Novel organic dyes for multicolor localization-based super-resolution microscopy. Journal of biophotonics, doi: 10.1002/jbio.201500119 (2015).
van de Linde, S. et al. Investigating cellular structures at the nanoscale with organic fluorophores. Chemistry & biology 20, 8–18, doi: 10.1016/j.chembiol.2012.11.004 (2013).
van de Linde, S. et al. Direct stochastic optical reconstruction microscopy with standard fluorescent probes. Nature protocols 6, 991–1009, doi: 10.1038/nprot.2011.336 (2011).
Ha, T. & Tinnefeld, P. Photophysics of fluorescent probes for single-molecule biophysics and super-resolution imaging. Annual review of physical chemistry 63, 595–617, doi: 10.1146/annurev-physchem-032210-103340 (2012).
van de Linde, S., Wolter, S., Heilemann, M. & Sauer, M. The effect of photoswitching kinetics and labeling densities on super-resolution fluorescence imaging. J Biotechnol 149, 260–266, doi: 10.1016/j.jbiotec.2010.02.010 (2010).
van de Linde, S. et al. Photoinduced formation of reversible dye radicals and their impact on super-resolution imaging. Photochemical & photobiological sciences: Official journal of the European Photochemistry Association and the European Society for Photobiology 10, 499–506, doi: 10.1039/c0pp00317d (2011).
Dempsey, G. T. et al. Photoswitching mechanism of cyanine dyes. Journal of the American Chemical Society 131, 18192–18193, doi: 10.1021/ja904588g (2009).
Nienhaus, K. & Nienhaus, G. U. Fluorescent proteins for live-cell imaging with super-resolution. Chemical Society reviews 43, 1088–1106, doi: 10.1039/c3cs60171d (2014).
Dempsey, G. T., Vaughan, J. C., Chen, K. H., Bates, M. & Zhuang, X. Evaluation of fluorophores for optimal performance in localization-based super-resolution imaging. Nature methods 8, 1027–1036, doi: 10.1038/nmeth.1768 (2011).
Endesfelder, U. et al. Chemically induced photoswitching of fluorescent probes-a general concept for super-resolution microscopy. Molecules 16, 3106–3118, doi: 10.3390/molecules16043106 (2011).
Whelan, D. R. & Bell, T. D. Image artifacts in single molecule localization microscopy: why optimization of sample preparation protocols matters. Scientific reports 5, 7924, doi: 10.1038/srep07924 (2015).
Funatsu, T., Harada, Y., Tokunaga, M., Saito, K. & Yanagida, T. Imaging of single fluorescent molecules and individual ATP turnovers by single myosin molecules in aqueous solution. Nature 374, 555–559, doi: 10.1038/374555a0 (1995).
Dulin, D. et al. Reduced photobleaching of BODIPY-FL. Physics Procedia 3, 1563–1567, doi: 10.1016/j.phpro.2010.01.222 (2010).
Altman, R. B. et al. Cyanine fluorophore derivatives with enhanced photostability. Nature methods 9, 68–71, doi: 10.1038/nmeth.1774 (2012).
Gust, A. et al. A starting point for fluorescence-based single-molecule measurements in biomolecular research. Molecules 19, 15824–15865, doi: 10.3390/molecules191015824 (2014).
Dickson, R. M., Norris, D. J., Tzeng, Y. L. & Moerner, W. E. Three-dimensional imaging of single molecules solvated in pores of poly(acrylamide) gels. Science 274, 966–969 (1996).
Dickson, R. M., Cubitt, A. B., Tsien, R. Y. & Moerner, W. E. On/off blinking and switching behaviour of single molecules of green fluorescent protein. Nature 388, 355–358, doi: 10.1038/41048 (1997).
Betzig, E. & Chichester, R. J. Single molecules observed by near-field scanning optical microscopy. Science 262, 1422–1425, doi: 10.1126/science.262.5138.1422 (1993).
Belov, V. N., Bossi, M. L., Folling, J., Boyarskiy, V. P. & Hell, S. W. Rhodamine spiroamides for multicolor single-molecule switching fluorescent nanoscopy. Chemistry 15, 10762–10776, doi: 10.1002/chem.200901333 (2009).
Bock, H. et al. Two-color far-field fluorescence nanoscopy based on photoswitchable emitters. Applied Physics B 88, 161–165, doi: 10.1007/s00340-007-2729-0 (2007).
Zondervan, R., Kulzer, F., Kol’chenko, M. A. & Orrit, M. Photobleaching of Rhodamine 6G in Polyvinyl alcohol at the Ensemble and Single-Molecule Levels. J Phys Chem A 108, 1657–1665, doi: 10.1021/jp037222e (2004).
Folling, J. et al. Fluorescence nanoscopy by ground-state depletion and single-molecule return. Nature methods 5, 943–945, doi: 10.1038/nmeth.1257 (2008).
Le Gall, A. et al. Improved photon yield from a green dye with a reducing and oxidizing system. Chemphyschem: a European journal of chemical physics and physical chemistry 12, 1657–1660, doi: 10.1002/cphc.201100085 (2011).
Nogales, E., Whittaker, M., Milligan, R. A. & Downing, K. H. High-resolution model of the microtubule. Cell 96, 79–88 (1999).
Olivier, N., Keller, D., Go, P. & Manley, S. Resolution Doubling in 3D-STORM Imaging through Improved Buffers. PloS one 8, doi: 10.1371/journal.pone.0069004 (2013).
Chozinski, T. J., Gagnon, L. A. & Vaughan, J. C. Twinkle, twinkle little star: photoswitchable fluorophores for super-resolution imaging. FEBS letters 588, 3603–3612, doi: 10.1016/j.febslet.2014.06.043 (2014).
Flors, C., Ravarani, C. N. & Dryden, D. T. Super-resolution imaging of DNA labelled with intercalating dyes. Chemphyschem: a European journal of chemical physics and physical chemistry 10, 2201–2204, doi: 10.1002/cphc.200900384 (2009).
Lukinavicius, G. et al. A near-infrared fluorophore for live-cell super-resolution microscopy of cellular proteins. Nature chemistry 5, 132–139, doi: 10.1038/nchem.1546 (2013).
Shim, S. H. et al. Super-resolution fluorescence imaging of organelles in live cells with photoswitchable membrane probes. Proceedings of the National Academy of Sciences of the United States of America 109, 13978–13983, doi: 10.1073/pnas.1201882109 (2012).
Stein, I. H. et al. Linking single-molecule blinking to chromophore structure and redox potentials. Chemphyschem: a European journal of chemical physics and physical chemistry 13, 931–937, doi: 10.1002/cphc.201100820 (2012).
Vogelsang, J., Cordes, T., Forthmann, C., Steinhauer, C. & Tinnefeld, P. Controlling the fluorescence of ordinary oxazine dyes for single-molecule switching and superresolution microscopy. Proceedings of the National Academy of Sciences of the United States of America 106, 8107–8112, doi: 10.1073/pnas.0811875106 (2009).
Thompson, R. E., Larson, D. R. & Webb, W. W. Precise nanometer localization analysis for individual fluorescent probes. Biophysical journal 82, 2775–2783, doi: 10.1016/S0006-3495(02)75618-X (2002).
Yildiz, A. et al. Myosin V walks hand-over-hand: single fluorophore imaging with 1.5-nm localization. Science 300, 2061–2065, doi: 10.1126/science.1084398 (2003).
Deschout, H. et al. Precisely and accurately localizing single emitters in fluorescence microscopy. Nature methods 11, 253–266, doi: 10.1038/nmeth.2843 (2014).
Glantz, S. A. In Primer of Biostatistics 7th edn (eds Diedrich, C. et al.) Ch. 8, 169–173 (McGraw-Hill, 2012).
Zondervan, R., Kulzer, F., Koh’chenko, M. A. & Orrit, M. Photobleaching of Rhodamine 6G in Polyvinyl alcohol at the Ensemble and Single-Molecule Levels. J Phys Chem A 108, 1657–1665 (2004).
Shroff, H., Galbraith, C. G., Galbraith, J. A. & Betzig, E. Live-cell photoactivated localization microscopy of nanoscale adhesion dynamics. Nature methods 5, 417–423, doi: 10.1038/nmeth.1202 (2008).
Burgert, A., Letschert, S., Doose, S. & Sauer, M. Artifacts in single-molecule localization microscopy. Histochemistry and cell biology 144, 123–131, doi: 10.1007/s00418-015-1340-4 (2015).
Edelstein, A. D. et al. Advanced methods of microscope control using muManager software. Journal of biological methods 1, doi: 10.14440/jbm.2014.36 (2014).
Edelstein, A., Amodaj, N., Hoover, K., Vale, R. & Stuurman, N. Computer control of microscopes using microManager. Current protocols in molecular biology /edited by Frederick, M. Ausubel … [et al.] Chapter 14, Unit14 20, doi: 10.1002/0471142727.mb1420s92 (2010).
Nan, X. et al. Single-molecule superresolution imaging allows quantitative analysis of RAF multimer formation and signaling. Proceedings of the National Academy of Sciences of the United States of America 110, 18519–18524, doi: 10.1073/pnas.1318188110 (2013).
Ovesný, M., Křížek, P., Borkovec, J., Svindrych, Z. & Hagen, G. M. ThunderSTORM: a comprehensive ImageJ plug-in for PALM and STORM data analysis and super-resolution imaging. Bioimage informatics 30, 2389–2390, doi: 10.1093/bioinformatics/btu202 (2014).
van de Linde, S. & Sauer, M. How to switch a fluorophore: from undesired blinking to controlled photoswitching. Chemical Society reviews 43, 1076–1087, doi: 10.1039/c3cs60195a (2014).
Glantz, S. A. In Primer of Biostatistics 7th edn (eds Diedrich, C. et al.) Ch. 8, 165–167 (McGraw-Hill, 2012).
Acknowledgements
We would like to thank Drs. Tao Huang and Li-Jung Lin for experimental assistance. This study was supported in part by a collaborative research agreement between Thermo Fisher Scientific and Oregon Health and Science University (SLG).
Author information
Authors and Affiliations
Contributions
A.M.B., N.J.D., X.N. and S.L.G. designed the experiments. A.M.B., A.N. and I.S.S. performed experiments. A.M.B., A.N., N.J.D., X.N. and S.L.G. performed data analysis and interpretation. A.M.B. and S.L.G. wrote the manuscript. S.L.G. supervised the project.
Corresponding author
Ethics declarations
Competing interests
N.J.D is an employee of Thermo Fisher Scientific.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Bittel, A., Nickerson, A., Saldivar, I. et al. Methodology for Quantitative Characterization of Fluorophore Photoswitching to Predict Superresolution Microscopy Image Quality. Sci Rep 6, 29687 (2016). https://doi.org/10.1038/srep29687
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep29687
This article is cited by
-
Blinking characteristics of organic fluorophores for blink-based multiplexing
Communications Chemistry (2024)
-
Photoregulated fluxional fluorophores for live-cell super-resolution microscopy with no apparent photobleaching
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.