Abstract
The cross talk among hydrogen peroxide (H2O2), nitric oxide (NO) and UV RESISTANCE LOCUS8 (UVR8) in UV-B-induced anthocyanin accumulation in the hypocotyls of radish sprouts was investigated. The results showed that UV-B irradiation significantly increased the anthocyanin accumulation and the expression of UVR8, and a similar trend appeared in radish sprouts subjected to cadmium, chilling and salt stresses regardless of light source. However, these responses disappeared under dark exposure. These results suggest that abiotic stress-induced anthocyanin accumulation and UVR8 expression were light-dependent. Moreover, abiotic stresses all enhanced the production of H2O2 and exogenous H2O2 addition significantly increased the anthocyanin concentration and UVR8 transcription, while these increases were severely inhibited by addition of dimethylthiourea (DMTU, a chemical trap for H2O2). It seems to suggest that H2O2 played an important role in the anthocyanin biosynthesis. Furthermore, addition of 0.5 mM sodium nitroprusside (SNP, a NO-releasing compound) substantially induced the anthocyanin accumulation, and H2O2-induced anthocyanin accumulation and UVR8 expression were significantly suppressed by co-treatment with 2-phenyl-4,4,5,5-tetramethylimidazoline-3-oxide-1-oxyl (PTIO, a NO scavenger), which was parallel with the expression of anthocyanin biosynthesis-related transcription factors and structural genes. All these results demonstrate that both H2O2 and NO are involved in UV-B-induced anthocyanin accumulation, and there is a crosstalk between them as well as a classical UVR8 pathway.
Similar content being viewed by others
Introduction
Anthocyanins are plant secondary metabolites synthesized through the flavonoid pathway, generating the characteristic reddish, bluish, and purple hues, which contribute to flower pigmentation, attraction of pollinators and seed dispersers1. In addition, they are also important as antioxidant molecules to protect plant cells against damage by reactive oxygen species (ROS)2, allelopathy, or UV irradiation3, and the production of anthocyanins in autumn leaves reduces the risk of photo-oxidative damage and delays leaf senescence4.On the other hand, possessing valuable nutritional antioxidant activities, anthocyanins are recognized as compounds with anti-inflammatory and anticancer effect, and potential health-benefits, such as prevention of cardiovascular diseases and obesity5,6.
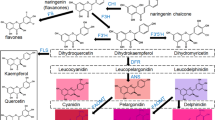
The biosynthesis of anthocyanins is through phenylpropanoid pathway, which is one of the best studied examples of secondary metabolism in higher plants, and most genes responsible for anthocyanin biosynthesis have been cloned and analyzed7,8,9,10. The anthocyanins are synthesized from phenylalanine which is subsequently catalyzed by PAL, CHS, CHI, F3H, DFR, ANS, LDOX and UFGT11, and the accumulation of anthocyanins is induced by the expression of these genes which are regulated by a ternary transcriptional complex (MBW complex) containing an R2R3-MYB-type transcription factor, a bHLH transcription factor, and a WD40 repeat (WDR) protein12,13,14. The MBW complex is highly organized, and each subunit plays a specific function such as binding to DNA, activation of expression of a target gene, or stabilization of the transcription factor complex15. The physical interactions of WDR and bHLH proteins with more specific MYB proteins determine the regulation of anthocyanin accumulation13,14. The MYB superfamily consists of more than 100 members in higher plants11, among which both anthocyanin pigment 1 (PAP1) and anthocyanin pigment 2 (PAP2) are redundant R2R3-MYB transcription factors and positive regulate the expression of anthocyanin biosynthesis-related genes16.
The induction of anthocyanin accumulation in vegetative tissues is often considered to be a response of plants to biotic or abiotic stress conditions, such as nutrient (nitrogen and phosphorus) deficiency, wounding, pathogen infection, water stress, and ultraviolet (UV) light17,18. Among these various environmental stimuli, UV-B is reported to be a main factor in anthocyanin accumulation, which is concomitant with up-regulation of MYB and biosynthetic genes19,20,21,22,23. UV RESISTANCE LOCUS8 (UVR8) is a photoreceptor that specifically mediates photomorphogenic responses to UV-B in plants24. UVR8 is required for 1) a UV-B-stimulated compensatory increase in epidermal leaf cell size, 2) normal progression of endoreduplication in response to UV-B, 3) stomatal differentiation and functions in plant acclimation, and 4) survival under solar UV25,26. It is also found that the expression of UVR8 is up-regulated in plants grown under salt or osmotic stress27. Furthermore, the ectopic expression of UVR8 causes pleiotropic effects on plant growth, such as reduced plant organ size and root growth, and increased accumulation of flavonoids28.
UVR8 is a homodimer in its ground state, and UV-B exposure results in its instantaneous monomerization (the active form) followed by interaction with CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1), a major factor in UV-B signaling29,30,31. Recently, some studies32,33,34 found that constitutively active UVR8 variants elevated levels of anthocyanins but UVR8 deficient mutant had lower levels of anthocyanins, suggesting that UVR8 may be involved in regulation of the biosynthesis of anthocyanins. However, details in the primary mechanisms of the involvement of UVR8 in anthocyanin biosynthesis are still scant.
UV-B irradiation may induce the production of ROS35, among which H2O2 is a major species generated in plants36. Because H2O2 is relatively stable and diffusible through membrane, it is generally thought to serve as a signal molecule under various forms of abiotic stress37. It has been reported that H2O2 could regulate the biosynthesis of anthocyanins38,39, and mediate the regulatory effect of UVR8 in stomatal closure40. In addition, nitric oxide (NO) is regarded as a downstream gaseous molecule of H2O2 to mediate UV-B-induced stomatal closure40,41. Based on all these reports, we hypothesize that NO, H2O2 and UVR8 may interact to regulate UV-B-induced anthocyanin biosynthesis.
In the present study, cherry radish sprouts were chosen as the materials, which contain substantial amounts of antioxidants, vitamin C and health-promoting compounds such as glucosinolates and phenolic compounds42,43,44. Consumption of radish sprouts has been confirmed to reduce the risk of cancer and oxidative stress both in vivo and in vitro45,46,47. Furthermore, with red hypocotyls resulting from anthocyanin accumulation, cherry radish sprouts could provide visual evidence for the biosynthesis of anthocyanins, and consequently, the high concentration of anthocyanin accumulated in cherry radish sprouts could underpin their high antioxidant activity. Using radish sprouts, in the present paper we confirmed the involvement of the UVR8 pathway in UV-B-induced anthocynin accumulation. Moreover, our results demonstrated that H2O2-dependent production of NO interacted with UVR8 to regulate the biosynthesis of anthocyanins.
Results
UVR8 was involved in the biosynthesis of anthocyanins
To determine the involvement of UVR8 in the biosynthesis of anthocyanins, the concentration of anthocyanin and the transcript level of UVR8 in the hypocotyls of radish sprouts grown under dark, white light and UV-B were analyzed. As expected, compared with dark environment, white light significantly increased the concentration of anthocyanins, while the highest anthocyanin concentration (2 times greater than that under white light) was observed after 24 h UV-B irradiation (Fig. 1A). Similarly, the expression of UVR8 was significantly induced by UV-B, which was 2-fold that under white light (Fig. 1B).
The concentration of anthocyanins (A) and the expression of UVR8 (B) in the hypocotyls of radish sprouts under dark (D), white light (W) and UV-B. After 36 h dark incubation, the radish sprouts were then exposed to dark, white light or UV-B for another 36 h when the samples were collected and detected. The data are means ± SD of three independent experiments. Significance between experimental values was assessed by Duncan’s test (P < 0.05).
Previous studies show that UVR8 is involved in salt or osmotic stress conditions17. To investigate whether there is a relationship between UVR8 and abiotic stresses in the biosynthesis of anthocyanins, we compared the anthocyanin accumulation and UVR8 expression under control condition and abiotic stress. The results showed that regardless of white light or UV-B irradiation, cadmium, chilling and NaCl treatments all induced significant increases in anthocyanin accumulation and the transcript abundance of UVR8, compared with those under control (Fig. 2).
The concentration of anthocyanins (A,C) and the expression of UVR8 (B,D) in the hypocotyls of radish sprouts under different treatments. After 24 h dark culture, sprouts were subjected to cadmium (Cd), chilling, NaCl or nothing (Con) for 12 h still under dark, then these sprouts were transferred to white light or UV-B for 24 h. Values are the means ± standard error of three independent experiments with at least three replicates for each. Measurements in the same column followed by different letters are significantly different at P < 0.05 level by Duncan’s test.
However, it remains unknown whether abiotic stress-induced anthocyanin accumulation and UVR8 expression are light-dependent. To test this, we repeated the above experiment under a dark environment. In contrast to exposure to light, abiotic stresses under dark had no effects on the anthocyanin accumulation and the expression of UVR8 (Fig. 3), suggesting that light was a necessary factor in stress-induced anthocyanin accumulation and UVR8 expression.
The concentration of anthocyanins (A) and the expression of UVR8 (B) in the hypocotyls of radish sprouts under different treatments. After 24 h dark culture, sprouts were subjected to nothing (Con), cadmium (Cd), chilling or NaCl for 12 h still under dark, then these sprouts were transferred to white light, UV-B or still dark for 24 h. The data represent means ± SD of three independent experiments. Significance between experimental values was assessed by Duncan’s test (P < 0.05).
H2O2 was involved in UV-B-induced biosynthesis of anthocyanins
To identify the role of H2O2 under different stresses and the relationship between H2O2 and the anthocyanin accumulation, the H2O2 concentrations under stresses and the effects of H2O2 on anthocyanin accumulation and UVR8 expression were tested. Similarly, cadmium, chilling and salt treatments as well as UV-B irradiation all significantly increased the H2O2 concentration, with the maximum observed under UV-B, which is about 20 times as great as that of control (Fig. 4A). The accumulation of anthocyanins was positively correlated with the H2O2 concentration, though no significant difference was noted between control and the treatment with 0.2 mM H2O2 (Fig. 4B). The expression levels of UVR8 were also up-regulated by H2O2 ranging from 0.5 to 10 mM, while no positive correlation between them was observed (Fig. 4C). Therefore, 0.5 mM H2O2 was selected as the appropriate treatment concentration in the following experiments.
The H2O2 concentration in the hypocotyls of radish sprouts subjected to Cd, chilling, NaCl or UV-B (A). After 24 h dark incubation, the radish sprouts were then subjected to different abiotic stresses for 12 h, and then they were exposed to white light for another 24 h. The anthocyanin accumulation (B) and the expression of UVR8 (C) in the hypocotyls of radish sprouts treated with increasing exogenous H2O2. After 24 h dark incubation, the radish sprouts were then subjected to H2O2 with different concentrations for 12 h, and then they were exposed to white light for another 24 h. The data represent means ± SD of three independent experiments. Significance between experimental values was assessed by Duncan’s test (P < 0.05).
Addition of 0.5 mM H2O2 significantly enhanced the pigmentation in the hypocotyls of radish sprouts, regardless of exposure to white light or UV-B, while addition of DMTU (dimethylthiourea, a chemical trap for H2O2) effectively inhibited UV-B-induced anthocyanin accumulation (Fig. 5A,B). The concentration of H2O2 and the level of UVR8 both showed similar trends with the accumulation of anthocyanins (Fig. 5C,D).
Effects of exogenous addition of H2O2 or DMTU on the pigmentation of hypocotyls (A), anthocyanin accumulation (B), H2O2 concentration (C) and UVR8 expression (D). After 36 h dark incubation, the radish sprouts were then subjected to H2O2 or DMTU under white light or UV-B for 24 h. The data represent means ± SD of three independent experiments. Significance between experimental values was assessed by Duncan’s test (P < 0.05).
NO was involved in UV-B-induced biosynthesis of anthocyanins
To verify the involvement of NO in activating anthocyanin biosynthesis, the endogenous NO levels and anthocyanin concentrations in response to various concentrations of exogenous SNP (sodium nitroprusside, a NO-releasing compound) were determined. As shown in Fig. S1, the endogenous levels of NO almost increased linearly with increasing SNP concentrations (Fig. S1A). The anthocyanin accumulation was significantly increased by addition of 0.5 mM SNP, and decreased afterward with further increasing of SNP (Fig. S1B).
The relationship underlying NO, H2O2 and UVR8 in anthocyanin accumulation
Similar to the results above, addition of H2O2, SNP or their combination all considerably enhanced the anthocyanin biosynthesis, while in comparison with SNP, co-treatment with DMTU slightly offset this increase, though it was not statistically significant. Moreover, H2O2-induced increase of anthocyanins was dramatically suppressed by co-treatment with PTIO (Fig. 6A). The NO level and UVR8 expression were both consistent with this trend, except that the combination of DMTU and SNP significantly inhibited SNP-induced increase of UVR8 expression (Fig. 6B,C).
The anthocyanin accumulation (A), endogenous NO level (B) and UVR8 expression (C) under different treatments. After 36 h dark incubation, the radish sprouts were then subjected to H2O2, DMTU, SNP, PTIO or their combination under white light for 24 h. The data represent means ± SD of three independent experiments. Significance between experimental values was assessed by Duncan’s test (P < 0.05).
Considering that H2O2 induces the generation of NO which is involved in many plant physiologic responses, we examined the effect of H2O2 on the enzymes and genes responsible for NO generation. As reported previously48,49, H2O2 significantly enhanced the activities of NOS and NR, and up-regulated the expression of NOA1 and NR by 5 and 1.2-fold, respectively (Fig. 7).
Finally, the expression levels of transcription factors and structural genes responsible for anthocyanin biosynthesis were further determined by qRT-PCR. The relative expressions of COP1, PAP1 and PAP2 displayed similar trends. Compared with the control, H2O2, SNP and their combination all significantly up-regulated the transcript levels, while DMTU and PTIO had no effects on the transcription of COP1, PAP1 and PAP2, with the exception of the considerable decrease of COP1 under DMTU treatment. Compared with H2O2, co-treatment with PTIO significantly down-regulated the transcripts of COP1, PAP1 and PAP2. Compared with SNP, combined treatment with DMTU and SNP decreased the transcription of COP1 and PAP1 (Fig. S3). The transcript levels of anthocyanin-biosynthesis-related structural genes (PAL, CHS, CHI, F3H, DFR, ANS, LDOX and UFGT) displayed the similar trends to the transcription factors (Fig. 8). In detail, H2O2, SNP and their combination significantly up-regulated the transcript levels of these genes, while H2O2 + PTIO and SNP + DMTU substantially suppressed their expressions.
The transcript levels of anthocyanin biosynthesis-related genes PAL (A), CHS (B), CHI (C), F3H (D), DFR (E), ANS (F), LDOX (G) and UFGT (H) under different treatments. After 36 h dark incubation, the radish sprouts were then subjected to H2O2, DMTU, SNP, PTIO or their combination under white light for 24 h. The data represent means ± SD of three independent experiments. Significance between experimental values was assessed by Duncan’s test (P < 0.05).
Discussion
Since its initial discovery in 200237, UVR8 has been the focus of recent attention due to numerous reports of its regulatory effects on UV-B signaling pathway and stress responses in plants50,51. However, the exploration of UVR8 function in vivo is still at an early stage and information about UVR8 is virtually derived from studies with Arabidopsis. Therefore its physiological roles in other diverse species remain poorly understood52. In this study, we explored the cross talk among H2O2, NO and UVR8 in regulating anthocyanin accumulation in the hypocotyls of radish sprouts.
Similar to previous reports in peach, strawberry and lettuce53,54,55, compared with white light and dark, UV-B considerably increased the anthocyanin accumulation in the hypocotyls of radish sprouts (Fig. 1A). Though it has been found that UV-B exposure induces the conversion of UVR8 from a homodimer to a monomeric which will interact with transcription factor COP140,52, the UVR8 expression showed the consistent trend with the anthocyanin accumulation (Fig. 1B), suggesting that as a UV-B photoreceptor, UVR8 is likely to be involved in mediating anthocyanin biosynthesis. This hypothesis is supported by a recent study by Mao et al.56 who found that PeUVR8 in Populus euphratica could increase the anthocyanin accumulation in the Arabidopsis uvr8 mutant.
Apart from UV-B, other abiotic stresses could also induce the biosynthesis of anthocyanins, such as high light intensity57, low temperature58, and nutrient deficiency59. In this study, we also observed that the anthocyanin accumulation was significantly increased by three typical abiotic stresses, cadmium, chilling and salt, regardless of supply with white light or UV-B (Fig. 2A,C). Meanwhile, the expression of UVR8 showed a similar trend (Fig. 2B,D), which was in line with the study of Fasano et al.27, suggesting that UVR8 was involved in abiotic stresses, and there was a potential relationship between anthocyanins and UVR8. By contrast, these increases were not observed under dark (Fig. 3), illustrating that abiotic stress-induced anthocyanin accumulation and increased expression of UVR8 were light-dependent.
Taking into consideration the importance of H2O2 in abiotic stresses and anthocyanin accumulation, we speculated that H2O2 may be a conjunctive point between anthocyanins and UVR8. As expected, compared with control, the concentration of H2O2 under various abiotic stresses (Cd, chilling, NaCl and UV-B) was increased significantly (Fig. 4A), and exogenous addition of H2O2 (0.5–10 mM) enhanced both the anthocyanin accumulation and the expression of UVR8 (Fig. 4B,C). Furthermore, UV-B-induced anthocyanin accumulation, H2O2 concentration and UVR8 expression were all suppressed by addition of DMTU (an inhibitor of H2O2), although DMTU did not affect these factors under white light (Fig. 5), which might be due to the low H2O2 accumulation under normal growth condition. These results imply that UV-B irradiation induces more production of H2O2, which further up-regulates UV-B-induced UVR8 expression to modulate the biosynthesis of anthocyanins.
Several previous studies showed that NO acts as downstream gaseous signal molecule of H2O2 to regulate the stomatal closure40,60, which prompted us to further investigate the role of NO in anthocyanin accumulation and the relationship among NO, H2O2 and UVR8. The endogenous NO concentration in radish sprouts showed an almost linear increase with the concentration of SNP (the donor of NO) (Fig. S1A), with 0.5 mM SNP significantly increased the anthocyanin accumulation (Fig. S1B). Furthermore, we observed that H2O2-induced increases of anthocyanin accumulation, NO concentration and UVR8 expression were all considerably restrained by co-treatment with PTIO (a scavenger of NO) (Fig. 6).
In animals, NO is synthesized through NO synthase (NOS)61 which has also been detected widely in plants, and inhibitors of mammalian NOS also have the ability to inhibit the generation of NO in plants, though no archetypal NOS-encoding gene(s) have been isolated in higher plants so far62,63. The nitric oxide associated1 (NOA1), reported to encode a protein with NOS activity, has been shown to be a GTPase playing a role in binding RNA/ribosomes64. In addition, it has been reported that NOA1-mediated NO production was involved in plant responses against salinity stress65 and pathogens66. In plants the NO production can also be generated via nitrate reductase (NR), a critical enzyme responsible for nitrate assimilation67,68. In the present study, the enzyme activity and gene expression related to NO biosynthesis were all significantly enhanced by addition of H2O2 (Fig. 7). All these results suggest that irradiation with UV-B enhanced the production of H2O2, which increased the level of NO to further magnify UV-B-induced expression of UVR8 to regulate the anthocyanin biosynthesis. This hypothesis was further verified by the expressions of transcription factors and structural genes responsible for anthocyanin biosynthesis under various treatments (Fig. S3 and 8).
The UVR8-dependent UV-B signaling pathway operates at low fluence rates to initiate classical UV-B photomorphogenic responses, such as the induction of flavonoid biosynthesis69. Therefore, the common pathway of low-intensity-UV-B-induced anthocyanin accumulation may be that UVR8 perceives the UV-B signal, and then transforms it into monomers70,71, which interact with the E3 ubiquitin ligase CONSTITUTIVELY PHOTOMORPHOGENIC1 (COP1)72 to activate gene expression involved in anthocyanin biosynthesis in plants. However, it was reported that both low and high fluence of UV-B could activate UVR873. In this study, we reported that both H2O2 and NO were involved in high-intensity-UV-B-induced anthocyanin accumulation. In detail, high fluence of UV-B or abiotic stress-induced production of H2O2 could regulate the biosynthesis of anthocyanins through the following three pathways: 1) H2O2 directly up-regulates the expression of UVR8, which is light-dependent; 2) H2O2 increases the concentration of NO which magnifies the effects of UV-B on UVR8 transcription; and 3) H2O2 influences the redox which changes the expressions of anthocyanin biosynthesis-related transcription factors, and this process is also light-dependent (Fig. 9). In this study, the UV-B lamps were used as the light source and the spectrum ranges from 280 nm to 360 nm with its peak of 306 nm (Fig. S4), which is a related broad spectrum of light including UV-A. Therefore, the UV-B induced anthocyanin biosynthesis might be partly due to the broad spectrum of light. As the spectrum peak of UV-B lamps is 306 nm, we estimated that in this study, the enhancement of anthocyanin was mainly induced by UV-B, which should be further investigated under a narrow band of UV-B. In addition, the emergence of LED UV-B would facilitate the related researches.
In a identified path, UVR8, the photoreceptor of UV-B, firstly perceives the signal from low-intensity-UV-B irradiation, and then initiates the transcription factors (COP1, MBW complex) which further up-regulates the expression of anthocyanin biosynthesis-related structural genes (PAL, CHS, CHI, F3H, DFR, ANS), finally to enhance the accumulation of anthocyanins. Besides the signal, high-intensity-UVB could also act as an environmental stress which, to some extent, induces the production of H2O2 which could also be induced by abiotic stress. H2O2 may promote the biosynthesis of anthocyanins through three pathways: 1) H2O2 interacts with UVR8, which is light-dependent; 2) H2O2 firstly stimulates the production of NO that interacts with the UVR8; 3) H2O2 up-regulates the expression of transcription factors directly, which is also light-dependent.
Materials and Methods
Plant materials and treatments
Seeds of radish (Raphanus sativus L cv. Yanghua) were soaked in deionized water for about 12 h, and then put on moist gauze to germinate for 24 h. Uniform-sized germinated seeds were selected and sown into cases containing1/4 strength Hoagland’s solution and covered with gauze. These cases were covered with gauze and were maintained in an incubator in dark at 25 °C for 24 or 36 h. Then the sprouts were treated grown in growth chambers equipped with LED white light (Safe Instrument Experimental Factory, Zhejiang, China) or UV-B lamps (Sankyo Denki, Shinagawa, Tokyo, Japan) which emit ultraviolet rays between 280 nm and 360 nm with its peak of 306 nm (Fig. S4). The light intensity of white light was set at 50 ± 5 μmol·m−2·s−1, while the UV-B dose was set at 10 W·m−2 measured with a pocket UV light meter (UV-340 A, Lutron, Taiwan). The temperature was 25 °C and the relative humidity (RH) was 65–75%.
Anthocyanin analysis
The total anthocyanin concentration in the radish hypocotyls was determined according to the method developed by Zhou et al.74, which involves measuring the absorbance (530) of extracts. Briefly, samples (0.5 g) were incubated in 5 mL of 1% HCl in methanol at room temperature in the dark for 24 h. Tubes were shaken with a vortex mixer every 6 hours. The anthocyanins in the aqueous phase were then quantified spectrophotometrically (A530 − 0.25 × A657) (UV-5200 spectrophotometer, Shanghai Metash Instruments Co., Ltd, Shanghai, China).
Observation of the hypocotyls cross section
The hypocotyls of radish sprouts were transected with a blade and then observed under a light microscope (Model Stemi 2000-C; Carl Zeiss, Germany) and photographed on a color film (Powershot A620, Canon Photo Film, Japan).
Quantitative and Real-time RT-PCR analysis
Total RNA was isolated from root tissues using Trizol extraction reagent (Invitrogen, Gaithersburg, MD, USA) and the RNA purity was verified by the ratio (>1.9) of 260/280 nm absorbance. DNA-free total RNA (8 μl) from different treatments was used for first-strand cDNA synthesis in a 20 μl reaction volume (Thermo Scientific, MD, Lithuania) according to the manufacturer’s instructions. Real-time quantitative PCR reactions were performed using a Mastercycler® eprealplex real-time PCR system (ABI 7500, MD, USA) with Bestar® SybrGreen qPCR mastermix (DBI, Bioscience Inc., Germany) in a 20 μl reaction volume according to the user manual.
PCR primers targeting actin, UVR8, COP1, NOA1, NR, PAL, CHS, CHI, F3H, DFR, LDOX, ANS, UFGT, PAP1 and PAP2 were designed using Primer Express® version 3.0 (Applied Biosystems). In order to design the UVR8 primer, the UVR8 mRNA of Arabidopsis was blasted against the radish data base (RadishBase), and a similar mRNA (Unigene ID: UN17090, Length: 1690) was obtained. The similarity is 90%. As UVR8 proteins occur widely among plant species and are well conserved72, the products from this prime were thought to be UVR8. All primers (Table S1) were synthesized by Genewiz Bio-engineering Ltd. Company (Suzhou, China). The relative expression levels were presented as values relative to that of corresponding control sample at the indicated time, after normalization to actin transcript levels.
Determination the activities of NR and NOS
The activity of NR was determined as described by Sun et al.75. The hypocotyls (0.5 g) were homogenized with a mortar and pestle on ice with 5 ml of extract buffer containing 50 mM HEPES-KOH (pH 7.5), 5% glycerol (v/v), 10 mM MgCl2, 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), and 10 μM flavin adenine dinucleotide (FAD). The extract was centrifuged at 13 000 g for 20 min at 4 °C.
The activity of NR was measured immediately by mixing 250 μl of supernatant with 250 μl prewarmed (25 °C) assay buffer containing 50 mM HEPES-KOH (pH 7.5), 10 mM MgCl2, 1 mM DTT, 2 mM KNO3 and 200 μM NADH. The reaction was started by adding assay buffer, incubated at 30 °C for 30 min and then stopped by adding 50 μl 0.5 M Zn-acetate. The nitrite produced was measured colorimetrically at 540 nm after adding 1 ml of 1% sulfanilamide in 3 M HCl plus 1 ml of 0.02% N-(1-naphthyl) ethylenediamine in 0.2 M HCl.
To determine NOS activity, the total protein was extracted using the buffer containing 100 mM HEPES-KOH (pH 7.5), 1 mM EDTA, 10% glycerol (v/v), 5 mM DTT, 0.5 mM PMSF, 0.1% Triton X-100 (v/v), 1% polyvinylpyrrolidone (PVP) and 20 μM FAD. NOS activity was then measured after centrifugation at 13 000 g for 20 min at 4 °C according to Gonzalez et al.76. Briefly, NOS activity was detected in1 ml of reaction mixture containing 100 mM phosphate buffer(pH 7.0), 1 mM L-Arg, 2 mM MgCl2, 0.3 mM CaCl2, 4 μM BH4, 1 μM FAD, 1 μM flavin mononucleotide (FMN), 0.2 mM DTT, 0.2 mM NADPH, and 200 μl of protein extract. The decrease in absorbance as a result of NADPH consumption was determined at 340 nm for 5 min. NOS activity was calculated using the extinction coefficient of NADPH (e = 6.22 mM−1 cm−1).
Detection of H2O2 concentration
The H2O2 concentration was measured colorimetrically as described by Hung77. H2O2 was extracted by homogenizing hypocotyl tissue with phosphate buffer (50 mM, pH 6.5) containing 1 mM hydroxylamine. The homogenate was centrifuged at 6,000 g for 25 min. To determine H2O2 concentration, the extracted solution was mixed with 0.1% titanium chloride in 20% (v/v) H2SO4. The mixture was then centrifuged at 6,000 g for 25 min. The absorbance was measured at 410 nm. The NO concentration was calculated by comparing to against a standard curve of H2O2.
Quantification of NO concentration
Nitric oxide concentration was determined using the method described by Hu et al.78 and Zhou et al.74 with slight modifications. Samples (1.0 g) were ground in a mortar and pestle in 8 ml of 50 mM cool acetic acid buffer (pH 3.6, containing 4% zinc diacetate). The homogenates were centrifuged at 10,000 g for 15 min at 4 °C. The supernatant was collected. The pellet was washed by 1 ml of extraction buffer and centrifuged as before. The two supernatants were combined and 0.1 g of charcoal was added. After vortex and filtration, the filtrate was leached and collected. The mixture of 1.5 ml of filtrate with1.5 ml of the Greiss reagent was incubated at room temperature for 30 min. Absorbance was determined at 540 nm. The NO concentration was calculated against a standard curve of NaNO2.
Statistical analyses
Values presented are means ± standard deviation (SD) of three replicates. Data was subjected to analysis of variance (ANOVA), and mean values were compared by Duncan’s multiple range test (p < 0.05). All the statistical analyses were performed using SPSS 19.0 for Windows.
Additional Information
How to cite this article: Wu, Q. et al. Hydrogen peroxide, nitric oxide and UV RESISTANCE LOCUS8 interact to mediate UV-B-induced anthocyanin biosynthesis in radish sprouts. Sci. Rep. 6, 29164; doi: 10.1038/srep29164 (2016).
References
Yoshida, K., Ma, D. W. & Constabel, C. P. The MYB182 protein down-regulates proanthocyanidin and anthocyanin biosynthesis in poplar by repressing both structural and regulatory flavonoid genes. Plant Physiol. 167, 693–710 (2015).
Gould, K. S., Mckelvie, J. & Markham, K. R. Do anthocyanins function as antioxidants in leaves? Imaging of H2O2 in red and green leaves after mechanical injury. Plant Cell. Environ. 25, 1261–1269 (2002).
Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 7, 581–591 (2005).
Schaberg, P., Murakami, P., Turner, M., Heitz, H. & Hawley, G. Association of red coloration with senescence of sugar maple leaves in autumn. Trees. 22, 573–578 (2008).
He, J. & Giusti, M. M. Anthocyanins: natural colorants with health-promoting properties. Annu. Rev. Food. Sci. T. 1, 163–187 (2010).
Tsuda, T. Regulation of adipocyte function by anthocyanins; possibility of preventing the metabolic syndrome. J. Agric. Food. Chem. 56, 642–646 (2008).
Holton, T. A. & Cornish, E. C. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell. 7, 1071–1083 (1995).
Forkmann, G. & Martens, S. Metabolic engineering and applications of flavonoids. Curr Opin Biotechnol. 12, 155–160 (2001).
Shirley, B. W. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 126, 485–493 (2001).
Koes, R., Verweij, W. & Quattrocchio, F. Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 10, 236–242 (2005).
Sakuta, M. Diversity in plant red pigments: anthocyanins and betacyanins. Plant Biotechnol.Rep. 8, 37–48 (2014).
Baudry, A. et al. TT2 TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J. 39, 366–380 (2004).
Broun, P. Transcriptional control of flavonoid biosynthesis: a complex network of conserved regulators involved in multiple aspects of differentiation in Arabidopsis. Curr Opin Plant Biol. 8, 272–279 (2005).
Ramsay, N. A. & Glover, B. J. MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 10, 63–70 (2005).
Hichri, I. et al. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J Exp Bot. 62, 2465–2483 (2011).
Gonzalez, A., Zhao, M., Leavitt, J. M. & Lloyd, A. M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 53, 814–827 (2008).
Dixon, R. & Paiva, N. Stress-induced phenylpropanoid metabolism. Plant Cell. 7, 1085–1097 (1995).
Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 70, 1–9 (1999).
Ubi, B. E. et al. Expression analysis of anthocyanin biosynthetic genes in apple skin: effect of UV-B and temperature. Plant Sci. 170, 571–578 (2006).
Yamane, T., Jeong, S., Goto-Yamamoto, N., Koshita, Y. & Kobayashi, S. Effects of temperature on anthocyanin biosynthesis in grape berry skins. Am. J. Enol. Vitic. 57, 54–59 (2006).
Ban, Y. et al. Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant. Cell. Physiol. 48, 958–970 (2007).
Steyn, W. J., Wand, S. J. Jacobs, G., Rosecrance, R. C. & Roberts, S. C. Evidence for a photoprotective function of low-temperatureinduced anthocyanin accumulation in apple and pear peel. Physiol. Plantarum. 136, 461–472 (2009).
Crifo, T., Petrone, G., Lo Cicero, L. & Lo Piero, A. Short cold storage enhances the anthocyanin contents and level of transcripts related to their biosynthesis in blood oranges. J.Agric. Food .Chem. 60, 476–481 (2012).
Heilmann, M. & Jenkins, G. I. Rapid reversion from monomer to dimer regenerates the ultraviolet-B photoreceptor UV RESISTANCELOCUS8 in intact Arabidopsis plants. Plant Physiol. 161, 547–555 (2013).
Wargent, J. J., Gegas, V. C., Jenkins, G. I., Doonan, J. H. & Paul, N. D. UVR8 in Arabidopsis thaliana regulates multiple aspects of cellular differentiation during leaf development in response to ultraviolet B radiation. New Phytol. 183, 315–326 (2009).
Wargent, J. J., Moore, J. P., Ennos, A. R. & Paul, N. D. Ultraviolet radiation as a limiting factor in leaf expansion and development. Photochem. Photobiol. 85, 279–286 (2009).
Fasano, R. et al. Role of Arabidopsis UV RESISTANCE LOCUS 8 in plant growth reduction under osmotic stress and low levels of UV-B. Mol Plant. 7, 773–791 (2014).
Morales, L. O. et al. Multiple roles for UV RESISTANCE LOCUS8 in regulating gene expression and metabolite accumulation in Arabidopsis under solar UV radiation. Plant physiol. 161, 744–759 (2013).
Rizzini, L. et al. Perception of UV-B by the Arabidopsis UVR8 protein. Science. 332, 103–106 (2011).
Heijde, M. & Ulm, R. Reversion of the Arabidopsis UV-B photoreceptor UVR8 to the homodimeric ground state. Proc. Natl. Acad. Sci. USA. 110, 1113–1118 (2013).
Gruber, H. et al. Negative feedback regulation of UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. Proc. Natl. Acad. Sci. USA. 107, 20132–20137 (2010).
Heijde, M. et al. Constitutively active UVR8 photoreceptor variant in Arabidopsis. Proc.Natl.Acad.Sci.USA. 110, 20326–20331 (2013).
Huang, X., Yang, P., Ouyang, X., Chen, L. & Deng X. W. Photoactivated UVR8-COP1 module determines photomorphogenic UV-B signaling output in Arabidopsis. PLOS Genetics. 10, e1004218 (2014).
Kliebenstein, J. D., Lim, E. J., Landry, G. L. & Last, L. R. Arabidopsis UVR8 regulates ultraviolet-B signal transduction and tolerance and contains sequence similarity to human Regulator of Chromatin Condensation. Plant Physiol. 130, 234–243 (2002).
Casati, P. & Walbot, V. Gene expression profiling in response to ultraviolet radiation in maize genotypes with varying flavonoid content. Plant Physiol. 132, 1739–1754 (2003).
Asada, K. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 50, 601–639 (1999).
Neill, S. J., Desikan, R., Clarke, A., Hurst, R. D. & Hancock, J. T. Hydrogen peroxide and nitric oxide as signaling molecules in plants. J. Exp. Bot. 53, 1237–1247 (2002).
Zhang, J. et al. Reactive oxygen species produced via plasma membrane NADPH oxidase regulate anthocyanin synthesis in apple peel. Planta. 240, 1023–1035 (2014).
Nikkhah, E., Khaiamy, M., Heidary, R. & Azar, A. S. The effect of ascorbic acid and H2O2 treatment on the stability of anthocyanin pigments in berries. Turk. J. Biol. 34, 47–53 (2010).
Tossi, V., Lamattina, L., Jenkins, G. I. & Cassia, R. O. Ultraviolet-B-induced Stomatal Closure in Arabidopsis is regulated by the UV RESISTANCE LOCUS8 photoreceptor in a nitric oxide-dependent mechanism. Plant Physiol. 164, 2220–2230 (2014).
He, J. et al. Role and interrelationship of Gα protein, hydrogen peroxide, and nitric oxide in ultraviolet B-induced stomatal closure in Arabidopsis leaves. Plant Physiol. 161, 1570–1583 (2013).
Barillari, J. et al. Isolation of 4-methylthio-3-butenyl glucosinolate from Raphanus sativus sprouts (kaiware daikon) and its redox properties. J. Agric. Food. Chem. 53, 9890–9896 (2005).
Ciska, E., Honke, J. & Kozlowska, H. Effect of light conditions on the contents of glucosinolates in germinating seeds of white mustard, red radish, white radish, and rapeseed. J. Agric. Food. Chem. 56, 9087–9093 (2008).
Martinez-Villaluenga, C., Frias, J., Gulewicz, P., Gulewicz, K. & Vidal-Valverde, C. Food safety evaluation of broccoli and radish sprouts. Food Chem and Toxicol. 46, 1635–1644 (2008).
Barillari, J. et al. Kaiware Daikon (Raphanus sativus L.) extract: A naturally multipotent chemopreventive agent. J. Agric. Food. Chem. 56, 7823–7830 (2008).
Ippoushi, K., Takeuchi, A., Ito, H., Horie, H. & Azuma, K. Antioxidative effects of daikon sprout (Raphanus sativus L.) and ginger (Zingiber officinale Roscoe) in rats. Food Chem. 102, 237–242 (2007).
Papi, A. et al. Cytotoxic and antioxidant activity of 4-methylthio-3-butenyl isothiocyanate from Raphanus sativus L. (Kaiware Daikon) sprouts. J. Agric. Food. Chem. 56, 875–883 (2008).
Wang, P., Du, Y. Y., Li, Yuan, Ren, D. T. & Song, C. P. Hydrogen peroxide–mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell. 22, 2981–2998 (2010).
Tossi, V., Lamattina, L., Jenkins, G. I. & Cassia, R. O. Ultraviolet-B-induced stomatal closure in Arabidopsis is regulated by the UV RESISTANCE LOCUS8 photoreceptor in a nitric oxide-dependent mechanism. Plant Physiol. 164, 2220–2230 (2014).
Brown, B. A. et al. A UV-B-specific signaling component orchestrates plant UV protection. Proc. Natl. Acad. Sci. USA. 102, 18225–18230 (2005).
Favory, J. J. et al. Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J. 28, 591–601(2009).
Jenkins, I. G. The UV-B Photoreceptor UVR8: From Structure to Physiology. Plant Cell. 26, 21–37 (2014).
Sgherri, C., Scattino, C., Pinzino, C., Tonutti, P. & Ranieri, A. M. Ultraviolet-B radiation applied to detached peach fruit: A study of free radical generation by EPR spin trapping. Plant Physiology and Biochemistry. 96, 124–131 (2015).
Josuttis, M. et al. Solar UVB response of bioactives in strawberry (Fragaria ananassa Duch. L.): A comparison of protected and open-field cultivation. J. Agric. Food Chem. 58, 12692–12702 (2010).
Tsormpatsidis, E. et al. UV irradiance as a major influence on growth, development and secondary products of commercial importance in Lollo Rosso lettuce ‘Revolution’ grown under polyethylene films. Environ Exp Bot. 63, 232–239 (2008).
Mao, K., Wang, L., Li, Y. & Wu, R. Molecular cloning and functional analysis of UV RESISTANCE LOCUS 8 (PeUVR8) from Populus euphratica . PLOS ONE. e0132390 (2015).
Page, M., Sultana, N., Paskiewicz, K., Florance, H. & Smirnoff, N. The influence of ascorbate on anthocyanin accumulation during high light acclimation in Arabidopsis thaliana: further evidence for redox control of anthocyanin synthesis. Plant Cell. Environ. 35, 388–404 (2012).
Bai, S. L. et al. An apple B-box protein, MdCOL11, is involved in UV-B- and temperature-induced anthocyanin biosynthesis. Planta. 240, 1051–1062 (2014).
Rubin, G., Tohge, T., Matsuda, F., Saito, K. & Scheible, W. R. Members of the LBD family of transcription factors repress anthocyanin synthesis and affect additional nitrogen responses in Arabidopsis. Plant Cell. 21, 3567–3584 (2009).
Xie, Y. J. et al. Reactive oxygen species-dependent nitric oxide production contributes to hydrogen-promoted stomatal closure in Arabidopsis. Plant Physiol. 165, 759–773 (2014).
Dulak, J. & Jozkowicz, A. Carbon monoxide–a new gaseous modulator of gene expression. Acta Biochim Pol. 50, 31–47 (2003).
Crawford, N. M. et al. Response to Zemojtel et al.: plant nitric oxide synthase: back to square one. Trends in Plant Science. 11, 526–527 (2006).
Zemojtel, T. et al. Plant nitric oxide synthase: a never-ending story? Trends in Plant Science. 11, 524–525 (2006).
Sudhamsu, J., Lee, G. I., Klessig, D. F. & Crane, B. R. The structure of YqeH. An AtNOS1/AtNOA1 ortholog that couples GTP hydrolysis to molecular recognition. J Bio Chem. 283, 32968–32976 (2008).
Zhao, M. G., Tian, Q. Y. & Zhang, W. H. Nitric oxide synthasedependent nitric oxide production is associated with salt tolerance in Arabidopsis. Plant Physiol. 144, 206–217 (2007).
Mandal, M. K. et al. Oleic acid dependent modulation of NITRIC OXIDE ASSOCIATED1 protein levels regulates nitric oxide-mediated defense signaling in Arabidopsis. Plant Cell. 24, 1654–1674 (2012).
Rockel, P., Strube, F., Rockel, A., Wildt, J. & Kaiser, W. M. Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro . J Exp Bot. 53, 103–110 (2002).
Gupta, K. J., Fernie, A. R., Kaiser, W. M. & van Dongen, J. T. On the origins of nitric oxide. Trends in Plant Science. 16, 160–168 (2011).
Jenkins,G. I. The UV-B photoreceptor UVR8: from structure to physiology. Plant Cell. 26, 21–37 (2014).
Christie, J. M. et al. Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges. Science. 335, 1492–1496 (2012).
Wu, D. et al. Structural basis of ultraviolet-B perception by UVR8. Nature. 484, 214–219 (2012).
Rizzini, L. et al. Perception of UV-B by the Arabidopsis UVR8 protein. Science. 332, 103–106 (2011).
Brown, B. A. & Jenkins G. I. UV-B signaling pathways with different fluence-rate response profiles are distinguished in mature Arabidopsis leaf tissue by requirement for UVR8, HY5, and HYH. Plant Physiol. 146, 576–588 (2008).
Zhou, B., Guo, Z. F., Xing, J. P. & Huang, B. R. Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J. Exp. Bot. 56, 3223–3228 (2005).
Sun, C. et al. Nitrate reductase-mediated early nitric oxide burst alleviates oxidative damage induced by aluminum through enhancement of antioxidant defenses in roots of wheat (Triticumaestivum). New Phytol. 201, 1240–1250 (2014).
González, A. et al. Cross talk among calcium, hydrogen peroxide, and nitric oxide and activation of gene expression involving calmodulins and calcium-dependent protein kinases in Ulva compressa exposed to copper excess. Plant Physiol. 158, 1451–1462 (2012).
Hung, K. T., Cheng, D. G., Hsu, Y. T. & Kao, C. H. Abscisic acid-induced hydrogen peroxide is required for anthocyanin accumulation in leaves of rice seedlings. J. Plant Physiol. 165, 1280–1287 (2008).
Hu, X., Fang, J., Cai, W. & Tang, Z. NO-mediated hypersensitive responses of rice suspension cultures induced by incompatible elicitor. Chinese. Sci. Bull. 48, 358–363 (2003).
Acknowledgements
This work was supported by the National Science Foundation of China (31171998) and (31572169).
Author information
Authors and Affiliations
Contributions
Q.W., N.S. and J.C. conceived the study, designed all the experiments. Q.W., N.S., X.Z. and Y.L. performed the experiments and interpreted experimental date. Q.W., N.S., Yongchao L. and J.C. participated in writing the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wu, Q., Su, N., Zhang, X. et al. Hydrogen peroxide, nitric oxide and UV RESISTANCE LOCUS8 interact to mediate UV-B-induced anthocyanin biosynthesis in radish sprouts. Sci Rep 6, 29164 (2016). https://doi.org/10.1038/srep29164
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep29164
This article is cited by
-
Enhancement of phenolic acids accumulation in Salvia abrotanoides (Kar.) Sytsma shoot cultures under elicitation with nitric oxide
Plant Cell, Tissue and Organ Culture (PCTOC) (2022)
-
Short term UV-B radiation mediated modulation of physiological traits and withanolides production in Withania coagulans (L.) Dunal under in-vitro condition
Physiology and Molecular Biology of Plants (2021)
-
The class III peroxidase gene OsPrx24 is important for root Iron plaque formation and benefits phosphorus uptake in Rice plants under alternate wetting and drying irrigation
Plant and Soil (2020)
-
Functional analysis of the UVR8 photoreceptor from the monocotyledonous Zea mays
Plant Growth Regulation (2020)
-
Comparative transcription analysis of photosensitive and non-photosensitive eggplants to identify genes involved in dark regulated anthocyanin synthesis
BMC Genomics (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.