Abstract
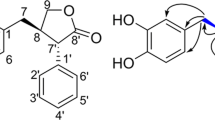
Ligubenzocycloheptanone A (1), a novel tricyclic butenolide with a 6/7/5-membered ring skeleton, was isolated from the rhizome of Ligusticum chuanxiong. Its unusual structure was determined using UV, IR, HRESIMS, 1D and 2D NMR data, X-ray diffraction crystallography and by the comparison of experimental and calculated electronic circular dichroism (ECD) spectra. 1 possessed a benzocycloheptanone core featuring butyrolactone, which is rarely observed in nature. A possible biosynthetic pathway was proposed. Ligubenzocycloheptanone A showed strong radical scavenging activity with an IC50 value of 2.3 μM.
Similar content being viewed by others
Introduction
Ligusticum chuanxiong Hort (Umbelliferae) is mainly distributed in Sichuan province in China. Its dried rhizome is used in traditional Chinese medicine for the treatment of headaches, rheumatic arthralgia, menstrual disorders, swelling pain due to traumatic injury, and cardiovascular and cerebrovascular diseases1,2,3. Phytochemical investigations of this plant have reported the presence of phenolic acids4, alkaloids5, and phthalides6,7,8. Among these compounds, phthalides are usually considered to be the active constituents of Ligusticum chuanxiong with the effects of inhibiting vasoconstriction, antiproliferation, antioxidation, and antiinflammation9,10,11,12. In our search for additional active constituents from this plant, a novel tricyclic butenolide with an uncommon 6/7/5-membered ring skeleton, named ligubenzocycloheptanone A (Fig. 1), was isolated from the rhizome of Ligusticum chuanxiong. Its structure was determined using UV, IR, HRESIMS, 1D and 2D NMR data, X-ray diffraction crystallography, and by comparing experimental and calculated electronic circular dichroism (ECD) spectra. Ligubenzocycloheptanone A may be derived biosynthetically from a phenyl acrylic acid and a hexose diacid. This may be useful for the exploration of a new synthetic approach to benzocycloheptanone derivatives. Ligubenzocycloheptanone A showed strong radical scavenging activity with an IC50 value of 2.3 μM.
Results
Compound 1 was isolated as a light yellow amorphous powder. Its molecular formula, C15H12O8, was established using HRESIMS with m/z 321.0593 [M+H]+ (calcd 321.0605), which requires 10 degrees of unsaturation. Its IR spectrum exhibited absorptions at 3374.4 cm−1 (hydroxy), 1741.4 and 1667.2 cm−1 (carbonyl), and 1587.9 cm−1 (aromatic rings).
The 1H NMR spectrum of 1 displayed two ortho-coupled aromatic protons at δH 7.06 (1H, d, J = 8.0 Hz, H-5) and 7.13 (1H, d, J = 8.0 Hz, H-6) and one olefinic proton with an allylic coupling at δH 7.29 (1H, d, J = 3.0 Hz, H-7). Additionally, a characteristic downfield proton signal at δH 13.31 (1H, s, 3-OH) suggested the presence of an internal hydrogen bond. Furthermore, two oxymethines at δH 4.59 (1H, dd, J = 2.0, 6.5 Hz, H-4′) and 4.34 (1H, d, J = 2.0 Hz, H-5′), one methine at δH 3.41 (1H, m, H-3′), and one methylene at δH 2.93 (1H, d, J = 13.0 Hz, H-2′a) and 3.37 (1H, t, J = 13.0 Hz, H-2′b) were observed in the 1H NMR spectrum.
The 13C NMR (Table 1) and HSQC spectra revealed a total of 15 carbon signals: one methylene, six methines, and eight quaternary carbons. Eight sp2 carbons at δC 153.7 (C-3), 149.3 (C-4), 135.7 (C-7), 128.9 (C-8), 127.8 (C-6), 125.2 (C-1), 119.5 (C-5), and 118.5 (C-2) could be attributed to six aromatic carbons and two olefinic carbons, suggesting the presence of a tetrasubstituted benzene ring and an olefinic moiety. The substitution pattern of the benzene ring was determined mainly by HMBC correlations (Fig. 2). In the HMBC spectrum, one aromatic proton at δH 7.06 (1H, d, J = 8.0 Hz, H-5) showed strong HMBC correlations with two carbons at δC 125.2 (C-1) and 153.7 (C-3), and the other one at δH 7.13 (1H, d, J = 8.0 Hz, H-6) showed correlations with three carbons at δC 118.5 (C-2), 149.3 (C-4), and 135.7 (C-7). In combination with the key correlations from the olefinic proton at δH 7.29 (1H, d, J = 3.0 Hz, H-7) to C-1, C-2, C-6, C-8, and C-9, a 3,4-dihydroxycinnamoyl moiety in 1 was clearly established.
In the 1H-1H COSY experiment (Fig. 2), the correlations of H-3′ with H-2′ and H-4′, and H-4′ with H-3′ and H-5′, suggested the presence of -CH2_CH-CH-CH- in 1. Combined with the HMBC correlations from H-2′ and H-3′ to C-1′ at δC 204.8 and H-4′ and H-5′ to C-6′ at δC 172.7, these results suggested the existence of a hexaric acid moiety. Furthermore, in the HMBC experiment, the correlation of H-2′a with C-2 confirmed that the hexaric acid moiety was connected to C-2 of the tetrasubstituted benzene ring by a C-C bond, while the HMBC correlations of H-3′ with C-7 and C-8 and H-2′ with C-8 suggested that C-3′ was connected to C-8. A benzocycloheptanone skeleton was formed. The absence of other sp or sp2 carbon signals and the remaining 1 degree of unsaturation implied that 1 contained a lactone ring, which was further confirmed by the HMBC correlation of H-4′ with C-9.
The relative configuration of 1 was deduced through the ROESY experiment. In the ROESY experiment (Fig. 3), the key correlations of H-4′ with H-2′a and H-2′b indicated the trans configuration of H-3′ and H-4′.
The absolute configuration of C-3′, C-4′, and C-5′ in 1 was established by comparing the experimental CD spectrum and the calculated ECD data (Supporting Information). Considering the trans configuration of H-3′ and H-4′, 1 has only two pairs of enantiomers (1a: 3′S,4′S,5′R and 1b: 3′R,4′R,5′S; 1c: 3′S,4′S,5′S and 1d: 3′R,4′R,5′R). A systematic conformational analysis was performed for 1a and 1c using a MMFF94 molecular mechanics force field calculation. The optimized conformations of 1a and 1c were obtained using the time-dependent density functional theory (TD-DFT) method at the B3LYP/6-31G(d) level. The overall calculated ECD spectra of 1a, 1b, 1c, and 1d were generated by Boltzmann weighting of their lowest energy conformers. The overall pattern of the calculated ECD spectrum of 1b attributable to the 3′R,4′R,5′S-isomer was consistent with the experimental data for 1 throughout the entire range of wavelengths under investigation (Fig. 4). Accordingly, the dihedral angles of H2′aC2′C3′H3′ (−71°), H2′bC2′C3′H3′ (170°), H3′C3′C4′H4′ (145°), and H4′C4′C5′H5′ (−59°) in the optimized conformation (Fig. 3) favored the coupling constants of H-2′a (d, J = 13.0 Hz), H-2′b (t, J = 13.0 Hz), H-4′ (dd, J = 2.0, 6.5 Hz), and H-5′ (d, J = 2.0 Hz).
Fortunately, the crystal of 1 was obtained in the solvent of MeOH:H2O (9:1). Analysis of single crystal X-ray diffraction unambiguous proved the 3′R,4′R,5′S configurations for 1 (Fig. 5). Based on the above evidence, the structure of 1 was determined to be as shown in Fig. 1 and was named ligubenzocycloheptanone A.
Discussion
Recently, benzotropone and benzocycloheptanone derivatives have attracted substantial attention13,14,15,16,17,18,19. Obviously, 1 represents a novel tricyclic butenolide with a benzocycloheptanone core. Its most intriguing feature from a biosynthetic pathway perspective is that 1 may be derived from a phenyl acrylic acid and a hexose diacid. 2-Deoxy-D-galactose and caffeic acid are considered to be precursors in a plausible biogenetic pathway of 1 (Fig. 6). One molecule of I formed from 2-deoxy-D-galactose by oxidation reacted with caffeic acid in a manner similar to a Friedel-Crafts acylation to give II, followed by dehydration, intramolecular nucleophilic addition, and hydride shift to give V. Finally, a five-membered lactone ring was formed by intramolecular nucleophilic substitution. The key process was the formation of the cycloheptanone moiety by the addition of the double bond in the caffeic acid unit to an α,β-unsaturated ketone. This information may be of considerable interest for the development of a new and efficient synthetic approach to benzocycloheptanone derivatives. In the in vitro bioactivity assays24 (Supporting Information), 1 showed strong radical scavenging activity with an IC50 value of 2.3 μM, using ascorbic acid as a positive control.
Methods
General experimental procedures
The optical rotations were measured on a Jasco P-2000 polarimeter. IR spectra were recorded on an IMPACT 400 (KBr) spectrometer. 1H NMR (500 MHz), 13C NMR (125 MHz), and 2D-NMR spectra were run on INOVA 500 spectrometers. HRESIMS were performed on Agilent 6520 LC-Q-TOF mass spectrometer (Agilent Technologies, Waldbronn, Germany). Column chromatography was performed with macroporous resin (Diaion HP-20, Mitsubishi Chemical Corp., Tokyo, Japan), Rp-18 (50 μm, YMC, Kyoto, Japan), Sephadex LH-20 (Pharmacia Fine Chemicals, Uppsala, Sweden). Preparative HPLC was carried out on a Shimadzu LC-6AD instrument with an SPD-20A detector, using a YMC-Pack ODS-A column (250 mm × 20 mm, 5 μm). HPLC-diode array detection (DAD) analysis was performed on an Agilent 1200 series system with an Apollo C18 column (250 mm × 4.6 mm, 5 μm, Alltech Corp., Kentucky, USA).
Plant material
The rhizomes of Ligusticum chuanxiong Hort. were collected from Pengzhou Town, Sichuan Province in People’s Republic of China, in Jun 2013. The plant material was identified by Ma Lin (Institute of Materia Medica, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100050, P R. China). A voucher specimen (ID-S-2594) was deposited at the Institute of Materia Medica, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100050, People’s Republic of China.
Extraction and isolation
Air-dried powder rhizome of Ligusticum chuanxiong Hort. (100.0 kg) were exhaustively extracted with 80% EtOH under refluxed conditions. After the solvent was evaporated under reduced pressure, the residue (23.1 kg) was suspended in water (50 L) and partitioned successively with EtOAc and n-BuOH. The n-BuOH-soluble portion (1300 g) was chromatographed on macroporous adsorption resins (HP-20) column, eluting with H2O, 15% ethanol, 30% ethanol, 50% ethanol, and 95% ethanol to give fractions A–E, respectively. Fr. C (103.0 g) was chromatographed over reversed phase silica gel column eluting with H2O-MeOH (from 100:0 to 0:100) to give 16 fractions (Fr. C-1–C-16). Fr. C-14 was further purified by Sephadex LH-20 column chromatography and preparative HPLC to yield compound 1 (19 mg).
ligubenzocycloheptanone A (1):  44.5° (c 0.10, MeOH); IR νmax 3374, 2955, 2708, 1741, 1667, 1618, 1588, 1435, 1376, 1302, 1198, 1021 cm−1; UV (MeOH) λmax (log ε): 200 (4.04), 247 (4.18), 307 (3.81), 398 (3.82) nm; For 1H and 13C NMR spectroscopic data, see Table 1; HRESIMS m/z: 321.0593 (calcd for C15H12O8, 321.0605).
44.5° (c 0.10, MeOH); IR νmax 3374, 2955, 2708, 1741, 1667, 1618, 1588, 1435, 1376, 1302, 1198, 1021 cm−1; UV (MeOH) λmax (log ε): 200 (4.04), 247 (4.18), 307 (3.81), 398 (3.82) nm; For 1H and 13C NMR spectroscopic data, see Table 1; HRESIMS m/z: 321.0593 (calcd for C15H12O8, 321.0605).
Additional Information
How to cite this article: Han, B. et al. Ligubenzocycloheptanone A, a Novel Tricyclic Butenolide with a 6/7/5 Skeleton from Ligusticum chuanxiong. Sci. Rep. 6, 28783; doi: 10.1038/srep28783 (2016).
References
Chinese Pharmacopeoia Committee. Pharmacopoeia of the People’s Republic of China Vol. 1, 38 (China Medical Science Press, 2010).
Peng, C., Xie, X. F., Wang, L., Guo, L. & Hu, T. L. Pharmacodynamic action and mechanism of volatile oil from Rhizoma Ligusticum chuanxiong Hort. Phytomedicine 16, 25–34 (2009).
Chan, S. S. K., Choi, A. O. K., Jones, R. L. & Lin, G. Mechanisms underlying the vasorelaxing effects of butylidenephthalide, an active constituent of Ligusticum chuanxiong, in rat isolated aorta. Eur J Pharmacol 537, 111–117 (2006).
Li, S. L. et al. Simultaneous Analysis of Seventeen Chemical Ingredients of Ligusticum chuanxiong by On-Line High Performance Liquid Chromatography-Diode Array Detector-Mass Spectrometry. Planta Med 69, 445–451 (2003).
Kim, M. et al. Tetramethylpyrazine, a natural alkaloid, attenuates pro-inflammatory mediators induced by amyloid β and interferon-γ in rat brain microglia. Eur J Pharmacol 740, 504–511 (2014).
Naito, T. et al. Two phthalides from Ligusticum chuanxiong . Phytochemistry 31, 639–642 (1992).
Naito, T., Niitsu, K., Ikeya, Y., Okada, M. & Mitsuhashi, H. A phthalide and 2-famesyl-6-metyl benzoquinone from Ligusticum chuanxiong . Phytochemistry 31, 1787–1789 (1992).
Naito, T., Ikeya, Y., Okada, M., Mitsuhashi, H. & Maruno, M. Two phthalides from Ligusticum chuanxiong . Phytochemistry 41, 233–236 (1996).
Liang, M. J., He, L. C. & Yang, G. D. Screening, analysis and in vitro vasodilatation of effctive components from Ligusticum chuanxiong . Life Sci 78, 128–133 (2005).
Lee, T. F., Lin, Y. L. & Huang, Y. T. Studies on antiproliferative effects of phthalides from Ligusticum chuanxiong in hepatic stellate cells. Planta Med 73, 527–534 (2007).
Qi, H. Y. et al. Senkyunolides reduce hydrogen peroxide-induced oxidative damage in human liver HepG2 cells via induction of heme oxygenase-1. Chem Bio Interact 183, 380–389 (2010).
Huang, J. et al. Anti-inflammatory ligustilides from Ligusticum chuanxiong Hort. Fitoterapia 91, 21–27 (2013).
Aïssa, C. & Fürstner, A. A Rhodium-Catalyzed C-H Activation/Cycloisomerization Tandem. J Am Chem Soc 129, 14836–14837 (2007).
Braga, D. et al. 1,4-Hydroxybiradical Behavior Revealed through Crystal Structure-Solid-State Reactivity Correlations. J Am Chem Soc 126, 3511–3520 (2004).
Satoh, T., Kimura, T., Sasaki, Y. & Nagamoto, S. Synthesis 44, 2091–2101 (2012).
Yadagiri, B. et al. Synthesis of novel building blocks of benzosuberone bearing coumarin moieties and their evaluation as potential anticancer agents. Eur J Med Chem 79, 260–265 (2014).
Zhang, T. X., Huang, X., Xue, J. & Sun, S. Ring expansion reaction of α-sulfonyl cyclic ketones via insertion of arynes into C–C: a facile and mild access to medium- and large-sized benzannulated carbocycles. Tetrahedron Lett 50, 1290–1294 (2009).
Beletskiy, E. V., Sudheer, C. & Douglas, C. J. Cooperative Catalysis Approach to Intramolecular Hydroacylation. J Org Chem 77, 5884–5893 (2012).
Blois, M. S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 181, 1199–1200 (1958).
Acknowledgements
The authors are grateful to Professor Lin Ma (Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College) for the plant identification and Professor Ying-Hong Wang (Analytical and Testing Center, Institute of Materia Medica, Peking Union Medical College and Chinese Academy of Medical Sciences) for conducting the NMR experiments. This project was supported by the Nation Science and Technology Project of China (No. 2012ZX09301002-002).
Author information
Authors and Affiliations
Contributions
P.-C.Z. and Y.-N.Y. conceived and designed the study. B.H.and X.Z. isolated and identified these compounds. Z.-M.F., L.L. and J.-S.J. participated on the analysis these structures. Y.-N.Y. and P.-C.Z. drafted the paper with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Han, B., Zhang, X., Feng, ZM. et al. Ligubenzocycloheptanone A, a Novel Tricyclic Butenolide with a 6/7/5 Skeleton from Ligusticum chuanxiong. Sci Rep 6, 28783 (2016). https://doi.org/10.1038/srep28783
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep28783
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.