Abstract
Diagnostic approaches based on multimodal imaging of clinical noninvasive imaging (eg. MRI/CT scanner) are highly developed in recent years for accurate selection of the therapeutic regimens in critical diseases. Therefore, it is highly demanded in the development of appropriate all-in-one multimodal contrast agents (MCAs) for the MRI/CT multimodal imaging. Here a novel ideal MCAs (F-AuNC@Fe3O4) were engineered by assemble Au nanocages (Au NC) and ultra-small iron oxide nanoparticles (Fe3O4) for simultaneous T1–T2dual MRI and CT contrast imaging. In this system, the Au nanocages offer facile thiol modification and strong X-ray attenuation property for CT imaging. The ultra-small Fe3O4 nanoparticles, as excellent contrast agent, is able to provide great enhanced signal of T1- and T2-weighted MRI (r1 = 6.263 mM−1 s−1, r2 = 28.117 mM−1 s−1) due to their ultra-refined size. After functionalization, the present MCAs nanoparticles exhibited small average size, low aggregation and excellent biocompatible. In vitro and In vivo studies revealed that the MCAs show long-term circulation time, renal clearance properties and outstanding capability of selective accumulation in tumor tissues for simultaneous CT imaging and T1- and T2-weighted MRI. Taken together, these results show that as-prepared MCAs are excellent candidates as MRI/CT multimodal imaging contrast agents.
Similar content being viewed by others
Introduction
The development of personalized therapeutical approaches and the increasing precision of surgical techniques indicate the importance of multimodal imaging to assist physicians in diagnosis and monitoring the response to therapy. In particular, noninvasive imaging (eg. MRI and CT) and minimally invasive in vivo bioimaging techniques are valuable tools in the arsenal of clinical diagnostics1,2,3. The new emergence of MRI/CT scanner (eg. General Electric CT & MRI scanners) allows doctors to get more precise information of tumor localization and boundary identification by combination of MRI and CT imaging. Accompanied with the development of imaging technology, high-performance, especially those all-in-one multimodal contrast agents (MCAs) are highly demanded for accurate diagnosis and therapy. In the past several years, various of MCAs based on Au and Fe3O4 nanoparticles have been developed for in vivo and pre-clinical MRI/CT imaging with the purpose of increasing the contrast of lesion, because these nanoparticles can offer facile thiol modification, enhanced chemical stability, excellent biocompatibility, superparamagnetic capability and strong X-ray attenuation property4,5,6,7,8,9,10,11,12. However, the achievement of these MCAs by using Fe3O4 and Au shell always have low MRI contrast capability, because common Fe3O4 can only provide T2 weight MRI with dark imaging and Au shell coated on the surface of Fe3O4 also prevent the connection with water molecules in tissue resulting in the reduction of the MRI contrast signal.
To address above problem, here we have exploited a new folic acid functionalized MCAs (F-AuNC@Fe3O4), based on Au nanocages (AuNCs) and ultrasmall Fe3O4 nanoparticles, to associate in one signal nanosystem several different properties, such as, tumor targeting, bright T1 and dark T2dual MRI contrast enhancement and X-ray attenuation (Fig. 1). Compared with other multimodal imaging systems, our presented MCAs are the first engineered for simultaneous T1–T2dual MRI and CT contrast. The simultaneous T1 and T2 weight contrast imaging could great enhance the sensitivity of MRI to give the comprehensive high spatial resolution of soft tissue information for tumor contour and localization, while the real-time and three-dimensional high spatial resolution of hard tissue information for tumor contour and localization could be provided by CT imaging. The use of noninvasive imaging like MRI and CT, possible with our MCAs, can provide the complementary information necessary for accurate evaluation of the tumorigenesis areas, which is one of the major challenges of diagnostic imaging.
Results and Discussion
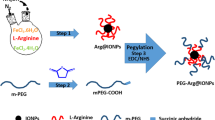
Synthesis and Characterization of F-AuNC@Fe3O4
It have been demonstrated that the size control and further surface functionalization are two key factors in the development of high-performance nanoprobes for tumor targeting13. Because AuNC@Fe3O4 has no selectivity toward tissues and is unable to discriminate between malignant and nonmalignant tumors, conjugation of specific bimolecular (folic acid) with AuNC@Fe3O4 has been employed to improve their lesion targeting selectivity. The final nanoparticles were purified by gel filtration using Sephacryl HR-300 gel medium.
High-resolution transmission electron microscopy (HRTEM) images of the ultra-small sized Fe3O4 nanoparticles and Au nanocage are shown in Figs S1 and S2 (Supporting Information). The average diameter of the Fe3O4 nanoparticles is around 2.2 nm with high uniformity, which could provide simultaneous T1 and T2 weight MRI contrast imaging proved in our previous work9. Au nanoparticles exhibit perfect nanocage structure with outer diameters of around 50 nm14. After assembled together, the HRTEM and SEM of final F-AuNC@Fe3O4 are shown in Fig. 2a,b,d, which clearly show that the prepared F-AuNC@Fe3O4 have hollow structure with rough morphology and they exhibit narrow distribution size around 70 nm. Compared with Au nanocages, it is obviously that the surface of F-AuNC@Fe3O4 was deposited with ultrasmall Fe3O4 nanoparticles. From the inset of Fig. 2b, the HRTEM of the edge of F-AuNC@Fe3O4, we can also clearly see that the small Fe3O4 nanoparticles around 2 nm are conjugated with Au nanocages. The energy-dispersive X-ray (EDX) spectrum of F-AuNC@Fe3O4 (Fig. 2c) also confirms the existence of elements O and Fe from Fe3O4, Au and Ag from the Au nanocages. The structure of F-AuNC@Fe3O4 can give enough space to let Fe3O4 nanoparticles connect with water molecular in tissue to produce high MRI signals due to the Fe3O4 nanoparticles assembled on the outer surface of Au nanocage; and at the other hand, the nanocages structure also gives their high surface to volume ratio, which also could benefit CT signal enhancement15,16. The Dynamic light scattering (DLS) (Fig. 2e) showed that the hydrodynamic diameter of F-AuNC@Fe3O4 was around 110 nm, which indicated that they have smaller size in aqueous without any aggregation and could process reasonable blood circulation time and good lesion accumulation rate13. Inductively coupled plasma mass spectrometry (ICP-MS) study revealed that weight percentages of Au and Fe3O4 encapsulated were 58.2 and 17.2%, respectively.
The UV-vis absorption spectra of the Au nanocage and F-AuNC@Fe3O4 in aqueous suspension are shown in Fig. 2f and the absorption spectra of ultra-small sized Fe3O4 nanoparticles is provided in Fig. S3 (Supporting Information). Here, Au nanocages and F-AuNC@Fe3O4 exhibits very strong and broad absorption from 600 nm to 800 nm with the maximum absorption at around 750 nm. After deposition of ultra-small sized Fe3O4 nanoparticles onto the surface of Au nanocage, a new broad absorption band appears in the spectrum of F-AuNC@Fe3O4, which is contributed by the strong absorption of Fe3O4 nanoparticles in visible spectral region17.
CT, Relaxometric and Biocompatible Properties
CT and MRI images of F-AuNC@Fe3O4 nanoparticles at various concentrations in PBS solution were studied. F-AuNC@Fe3O4 possesses intrinsic advantages for X-ray CT imaging owing to the larger X-ray absorption efficiency of Au element. As known, the higher the atomic number and electron density is, the higher the attenuation coefficient is. Therefore, the developed F-AuNC@Fe3O4 was expected that have the good imaging ability in X-ray CT imaging. The X-ray attenuation (CT) potency of these F-AuNC@Fe3O4 with various concentrations of Au was examined and the pure Au nanoparticles (ca. 50 nm in diameter) were used as a control. It is clearly that the CT signal of F-AuNC@Fe3O4 increase in a concentration dependent manner (Fig. 3b,c). Interestingly, the F-AuNC@Fe3O4 exhibit higher X-ray attenuation potency than that of bare Au NPs at the same concentration. According to previous reports, smaller Au nanoparticles exhibited more pronounced X-ray attenuating capability than the larger one, which caused by the high surface to volume ratio and mainly contributed to X-ray attenuation4,16. Therefore, the enhanced CT contrast of F-AuNC@Fe3O4 compared to Au solid nanoparticle is attributed to their hollow and cage structure.
MRI and CT contrast ability of MCAs (F-AuNC@Fe3O4).
(a) T1 and T2 weighted MR images of MCAs with different Fe ions concentrations. (b) CT imaging with different concentration of Au concentrations. (c) Plot of X-ray attenuation in Housfileld units (HU) as a function of Au concentration of MCAs (black squares), compared to pure gold nanoparticles (red circles). (d,e) Plot of 1/T1 and 1/T2 over Fe concentration of as synthesized MCAs.
Moreover, we next evaluated the contrast capability of as prepared F-AuNC@Fe3O4 for T1 and T2 weight MRI at different Fe concentrations. The relaxation efficiencies are calculated by measuring the longitudinal and transverse relaxation rates (r1 and r2) of the proton signals of the F-AuNC@Fe3O4. As shown in Fig. 3d,e, F-AuNC@Fe3O4 exhibits a high r1 value of 6.263 and an r2 value of 28.117 mM−1 s−1. The high r1 relaxivity of F-AuNC@Fe3O4 all can be attributed to the large number 5 unpaired electrons of Fe3+ ions on the ultra-small Fe3O4 nanoparticle surface5. The r2/r1 ratio is also an important parameter to evaluate the efficiency of T1 contrast agents. The r2/r1 ratio of F-AuNC@Fe3O4 was 4.48 demonstrating that it can be efficient T1 contrast agents. Although there are other nanomaterials based on Fe3O4-Au for biomedical imaging4,5,6,7, as we know F-AuNC@Fe3O4 is the first example that exhibits high r1 relaxivity value for T1-weight MR enhanced imaging. Figure 3a also demonstrated the T1 and T2 modal MR images of F-AuNC@Fe3O4 with different Fe ion concentrations. The signal enhancement progressively increases with increasing of the F-AuNC@Fe3O4 concentrations, which manifests the potential of F-AuNC@Fe3O4 as powerful multifunctional contrast agent for T1 and T2 MRI.
For biomedical applications, it is necessary to guarantee the hemocompatibility and cytocompatibility7,18. Hemolytic assay and cell viability was used to assess the biocompatibility of the F-AuNC@Fe3O4, the results (Figs S4 and S5, Supporting Information) show synthesized F-AuNC@Fe3O4 have almost negligible damage to the red blood cells (<5% hemolytic activity) and have little toxicity to human lung cancer cell line A549 and normal human umbilical vein endothelial cells.
In vitro cellular uptake assay
The targeting ability of F-AuNC@Fe3O4 to folate receptor-overexpressed cancer cells was studied using A549 cells as an example. To more directly display the targeting capabilities of the F-AuNC@Fe3O4, the fluorescence Cy5-PEG-SH was selected to label the nanoparticls. In order to give clear fluorescence imaging and retain the ability of bio-functionalization, firstly, the mixture of COOH-PEG-SH and fluorescence Cy5-PEG-SH with 9:1 ratio was used to functionalize the surface of AuNC@Fe3O4 and then fluorescent AuNC@Fe3O4 was conjugated with targeting molecular folic acid to form the fluorescent F-AuNC@Fe3O4 for targeting cancer cell. As a control group, the fluorescent AuNC@Fe3O4 without surface folic acid also was used, which showed similar physical properties to those of fluorescent F-AuNC@Fe3O4. Figure 4a,b row show the confocal images of A549 cells after incubation with fluorescent F-AuNC@Fe3O4 and AuNC@Fe3O4 suspensions at a concentration of 0.5 mg/mL for one hour, respectively. The fluorescence image shown in Fig. 4a(i) clearly shows the successful internalization of the fluorescent F-AuNC@Fe3O4 into A549 cells. The much brighter red emission observed in Fig. 4a(i) as compared to that in Fig. 4b(i) (A549 cell incubation with fluorescent AuNC@Fe3O4) indicates that more F-AuNC@Fe3O4 can be internalized into A549 cells via folate receptor-mediated endocytosis and the F-AuNC@Fe3O4 have more capability for cancer targeting recognition. These results confirm the F-AuNC@Fe3O4 can be effectively targeted to the folate receptor expressed cancer cells.
Confocal images of the A549 cells after one hour incubation with the 0.5 mg/mL of fluorescent F-AuNC@Fe3O4 (a); 0.5 mg/mL of fluorescent AuNC@Fe3O4 (b). The horizontal images were in this sequence: bright field (i), the red fluorescence field (ii), the nuclei stained by DAPI (iii), overlayer of DAPI and fluorescence (iv), overlayer of all (v). Scale bars are 100 μm.
In vivo CT, T1 and T2 modal MR Imaging
For in vivo CT imaging, the CT imaging and CT value of the important organ regions were recorded before injection and at different time points post injection (Fig. 5a,b). Compared with the image of pre-injection, a great contrast enhancement was observed in the mouse body, tail vein and tumor region at 0.5 hour after injection, (Fig. 5a), demonstrating that the as synthesized F-AuNC@Fe3O4 can do enhance CT imaging in the circulating system. It is noted that the F-AuNC@Fe3O4 have a clear tendency of accumulation in the tumor tissue, the time-dependent distribution of the F-AuNC@Fe3O4 in the mouse was also tracked by CT signal value after intravenous injection. At timed intervals, the evident enhancement of the signals in different organs could be seen in Fig. 5b, the kidney and tumor imaging were greatly enhanced from 0.5 h to 6 h and HU value of them rose from 95 to 464 and 213 to 730, respectively, while the signal value in other organs shows little fluctuation. A more careful look at the 3D-renderings of CT images, after post injection 6 h, the CT contrast intensity in the body and organs of mouse obviously decrease over time, while the region of the tumor tissue is still labeled and CT imaging of bladder organ are suddenly clear, showing the excellent tumor targeting and renal clearance properties of F-AuNC@Fe3O4. The slow elimination of F-AuNC@Fe3O4 from blood during circulation is attributed to their optimal particle size and surface functionalization. The results demonstrate that the F-AuNC@Fe3O4 have the feasibility to be served as an in vivo CT contrast agent to provide the real-time and 3D-high spatial resolution imaging.
In vivo CT images of nude mice bearing tumor after intravenous injection of Au NPs at different timed intervals (pre-injection, 0.5 h, 1 h and 6 h post-injection), respectively (a) The HU average intensity of some organs (Heart, Liver, Spleen, Kidney, Tumor) after intravenous injection of Au NPs at different timed intervals.
For tumor targeting T1 and T2 modal MR imaging, the F-AuNC@Fe3O4 with higher r1 and r2 relaxivity are expected to enhance the T1 and T2 modal MRI and to ease the toxicity with a decreased dose. T1 and T2 modal MRI of tumor-bearing mice were also recorded at various time intervals: before injection, 0.5, 1 and 6 hours after injection, as shown in Fig. 6a. Compared with pre-injection, we could observe a great contrast enhancement (brighten on the T1-weighted and darken on the T2 weighted MR images) in the mouse body after post injection, demonstrating that as synthesized F-AuNC@Fe3O4 can simultaneously enhance T1 and T2 relaxation in the circulating system. The enhanced signal of blood vessel can be maintained for more than 6 h, which is much longer than that of Gd complex small molecules with a high excretion rate (about several minutes in small animals)9,19. These results show that F-AuNC@Fe3O4 can also be used for long-term blood pool T1 and T2 modal MRI contrast agent, which is very important in clinical MR imaging20,21. The long-term effect is also critical to obtain high-resolution and steady state images. Similar with the CT imaging, after 6 h, the T1 and T2 contrast intensity in the body of mouse obviously decrease over time, while the contrast intensity in the region of tumor increased over time, which confirm the F-AuNC@Fe3O4 have a tendency to be enriched in the tumor tissue.
In general, once injected intravenously, the contrast agents are easily accumulated in liver and spleen tissues22. Therefore, to provide further support of tumor targeting ability as well as information on the biodistribution of the F-AuNC@Fe3O4, both tumor and important organs such as heart, liver, spleen, kidney and tumor were harvested after the in vivo experiments and the MRI with T1 and T2 modal was shown in Fig. 6b, respectively. At first, the much higher T1 and T2 signals in the tumor tissue compared to those from other organs further confirm the specific targeting of the FMNPs to the tumor. And then, the imaging signal of organs might be difference between MRI imaging and CT imaging, which is why we integrate MR imaging with CT imaging for achieving their complementary imaging features.
Histological study
An essential feature of MCAs is their biocompatibility, hence primary organs of tumor bearing mice post the administration of F-AuNC@Fe3O4 were tested via the pathological assay (H&E staining). In Fig. 7, the accumulation of F-AuNC@Fe3O4 was obviously observed in kidney, bladder and tumor (original magnification ×100), but it was little even absence in liver and heart at 6 h post the injection, which is in consistent with our in vivo imaging findings. In addition, no observable morphological changes were seen in these organs, which ruled out the presence of acute injury induced by the injection of F-AuNC@Fe3O4.
In summary, water-dispersible F-AuNC@Fe3O4 nanoparticles-based multimodal contrast agent has been developed for synergistically multimodal imaging of tumor. To the best of our knowledge, this is the first time that ultra-small sized Fe3O4 and Au nanocages were assembled together to provide simultaneous CT imaging and T1 and T2-modal MRI of tumor bearing mice. As a result, due to the small sizes and surface modification, the as-prepared F-AuNC@Fe3O4 preferentially accumulate in the tumor and can be rapidly eliminated from the body via the renal system. The experimental results suggest the potential of F-AuNC@Fe3O4 as a safe and efficient contrast agent for biomedical applications such as multi-modality imaging and molecular diagnostics of diseases in vitro and in vivo, as well as theranostic applications.
Methods
All experimental protocol including any relevant details were approved by the Regional Ethics Committee, Liaoning Medical University, Liaoning Province, China.
Materials and Instrumentations
All of the chemicals and solvents were purchased from Sigma-Aldrich unless indicated otherwise. Transmission electron microscope (TEM) and energy dispersive X-ray (EDX) analyses were recorded using a FEI TECNAI G20 high-resolution transmission electron microscope operating at 200 kV. The samples were prepared by depositing a drop of a diluted colloidal solution on a carbon grid and allowing the liquid to dry in air at room temperature. Scanning electron microscope (SEM) data was collected by Hitachi Limited S4800. Dynamic light scattering (DLS) measurement was performed on a Malvern Zetasizer NANO ZS. UV-vis spectroscopy was carried out with SpectraMax® M5 Microplate Reader using 96-well plate . The relaxation measurements were performed on a Bruker minispec mq60 NMR analyzer at 40 °C using a magnetic field of 1.41 T. Samples were diluted in MilliQ water to different Fe and Au concentrations in the approximate range and the absolute concentrations were determined afterwards by ICP-MS.
Synthesis of Gold Nanocages (AuNCs)
Herein, Au cages with hollow structures using Ag nanocubes as sacrificial templates have been prepared according to previous methods12. For all of the experiments, we used Au nanocages of mean 50 nm size together with a pore size of 2–3 nm. Briefly, 500 μL of the Ag nanocubes (3 nM) was added to 5 mL of deionized water containing poly (vinyl pyrrolidone) (PVP, 1 mg mL−1) hosted in a 50 mL flask under magnetic stirring and then heated to boil for 10 min. Then around 4 mL of aqueous solution of HAuCl4 (0.1 mM) was added to the flask at a rate of 0.75 mL min−1 until the solution had an optical extinction peak at 755 nm as confirmed by UV spectroscopy. The solution was refluxed for another 10 min until the color of the reaction was stable. Once cooled to room temperature, the sample was centrifuged and washed with saturated NaCl solution to remove AgCl and with water several times to remove PVP and NaCl. For the further experiment, The PVP was replaced on nanocages with 2-aminoethanethiol to obtain AuNC-NH2.
Synthesis of AuNC@Fe3O4
In a typical experiment for the synthesis of 2.2 nm sized ultra-small Fe3O4 particles was prepared according to our previous experiment. First, the polymer poly(acrylic acid) (PAA, 5.56 mmol) was dissolved in Milli-Q water (50 mL) in a 100 mL three-necked flask bubbled with nitrogen air for 40 min to remove oxygen. Then the solution was heated up to 80 °C. Meanwhile, Ferric slat (0.54 mmol) and Ferrous salt (0.279 mmol) as iron precursors was quickly injected into the hot polymer solution under vigorous stirring in a nitrogen atmosphere, followed by drip addition of concentrated ammonia solution (15 mL, 28%) to adjust the pH value to 9–10. After refluxing for one hour, we can get the carboxyl group functionalized ultrasmall Fe3O4 particles.
The resultant carboxyl unit on the surface of Fe3O4 nanoparticels was activated by N-(3-Dimethylaminopropyl)-N–ethylcarbodiimide (EDC) and N-Hydroxysuccinimide (NHS) and subsequently treated with AuNC-NH2 to obtain AuNC@Fe3O4. 10 mg of excessive ultrasmall Fe3O4 nanoparticles was reacted with 10 mg NHS and 20 mg EDC in 5 mL of water, stirring at room temperature for 30 min before adding to the 5 mg of AuNC-NH2 suspension. Then the mixture was stirred at room temperature for another 24 h, followed by centrifugation and washing with ethanol and water and then dispersed in 5 mL of ultra-purified water.
Bio-functionalized of AuNC@Fe3O4 with folic acid to form the F-AuNC@Fe3O4
AuNC@Fe3O4 were first functionalized with COOH-PEG-SH and then conjugated with biomolecular for in vitro and in vivo experiments. Briefly, 5.0 mL of prepared AuNC@Fe3O4 water solution was added to 5.0 mg of COOH-PEG-SH and reacted overnight at 4 °C under stirring. The excess COOH-PEG-SH was removed by centrifugation at 12000 rpm for 8 min and washed five times with water to obtain PEGylated AuNC@Fe3O4. The PEGylated AuNC@Fe3O4 was dispersed in 0.1 M (pH 7.4) phosphate buffer to remove any trace metal.
The bio-conjugation was performed by EDC-catalyzed peptide bond formation between the carboxyl group on the particle surface and amino group on folic acid. First, 10 mL of freshly prepared EDC solution (10 mg mL−1 in weak acidic MES buffer solution) was mixed with 1 mL HNPs solution with stirring for one hour. Then, 50 mL folic acid solution (containing 0.4% NaOH) was added to the solution and mixed well on a vortex and then the above mixture was left on a rotary shaker overnight at room temperature. After that, the Triton-X 100 (0.25% (w/v), 20 mL) and BSA (2% (w/v), 20 mL) were added. The mixture was then left on the rotary shaker for one hour. Finally, the resulting folic acid functionalized AuNC@Fe3O4 (F-AuNC@Fe3O4) were separated from free biomolecules by gel filtration using Sephacryl HR-300 gel medium.
In vitro cytotoxicity study
Human lung adenocarcinoma A549 cells and human umbilical vein endothelial cells (HUVEC) were cultured in 25 cm2 flasks in Dulbecco’s Modified Eagle’s Medium DMEM (Gibco) containing 10% (v/v) fetal bovine serum (Gibco) at 37 °C in an atmosphere of 5% (v/v) CO2 in air. The media were changed every two days and the cells were passaged by trypsinization before confluence. Before experiment, the cells were pre-cultured until confluence was reached. For studying the cytotoxicity, both A549 and HUVEC cells were seeded in a 96-well plate at a density 104 cells/well for 24 h at 37 °C in 5% CO2. Then, the cells were treated with F-AuNC@Fe3O4 at different concentrations of 100, 200, 300, 400, 500 and even 1000 μg mL−1. After incubation for 24 h, cell viabilities were tested by standard MTT (3-(4,5)-dimethylthiahiazo-2-yl)-2,5diphenyltetrazolium bromide) assay. Cells incubated in the absence of nanoparticles were used as a control. All experiments were performed in triplicate.
Hemolysis assay
In order to detect whether F-AuNC@Fe3O4 will cause damage to red blood cells (RBCs) after being injected into the blood vessels, a hemolysis assay experiment was carried out. The red blood cells were obtained by removing serum from the blood by centrifugation and suction. The 0.5 mL cells suspension was then mixed with: (a) 1 mL of PBS as a negative control; (b) 1 mL deionized water as a positive control; (c) 1 mL nanoparticle PBS solution with different concentrations of 100, 200, 400, 800 and 1000 μg mL−1. The mixtures were then vortexed for 2 h at room temperature. The samples were centrifuged and the absorbance spectrum of the supernatants was measured by UV-Vis characterization.
In vitro targeting cellular imaging
For to evaluate targeting capabilities of the F-AuNC@Fe3O4, the fluorescence Cy5-PEG-SH was used to track the F-AuNC@Fe3O4. Firstly, the mixture of COOH-PEG-SH and fluorescence Cy5-PEG-SH with 9:1 ratio was used to functionalize the surface of AuNC@Fe3O4 and then fluorescence AuNC@Fe3O4 was conjugation with targeting molecular folic acid to form the fluorescence F-AuNC@Fe3O4 for targeting cancer cell. All of the modified procedures can be done as described above. In the next step, the A549 cells (1 × 105 cells per well) were cultured in confocal imaging chambers (NET) at 37 °C for 24 h, then 200 μL of F-AuNC@Fe3O4 and AuNC@Fe3O4 (without bio-conjugation) with a concentration of 0.5 mg mL−1 were added to the chambers. After incubation for one hour, the cells were washed with a large amount of PBS to remove any free nanoparticles attached on the cell membrane and then stained with DAPI (10 μg mL−1) for 20 min, rinsed with PBS at least three times. And then, the Olympus-FluoView FV10 confocal laser-sca nning microscope was used to image morphology of the A549 cells and track the F-AuNC@Fe3O4 location in the A549 cells.
In vivo CT, T1 and T2-weight MRI tri-modal imaging
Male BALB/c nude mice (4–6 weeks old) were purchased from the Cancer Institute & Hospital, Chinese Academy of Medical Sciences, Beijing. The Lung cancer A549 tumor-bearing models were generated by subcutaneous injection of 5 × 106 cells in 50 μL of phosphate buffer solution (PBS) onto the left upper armpit of each mice. All the animal experiments were conducted in accordance with the guidelines of the Regional Ethics Committee, Liaoning Medical University, Liaoning Province, China. Firstly, the mice were anesthetized using 10% chloral hydrate (50 mL). Subsequently, 10 mg of F-AuNC@Fe3O4, dispersion solution was injected through the tail vein into the mouse. In vivo multi-imaging was performed at appropriate time points after tail vein injection.
CT imaging was acquired using Mediso nanoScan SPECT/CT produced by Mediso Ltd. Imaging parameters were as follows: slice thickness, medium; tube energy, 50 kVp, 670 μA; CTDIvol, 7279.9 cGy; semicircular parameters, full scan; Number of projections, 480; In-plane voxel size, medium. All animals were scanned in the cranial to caudal direction from the lower chest to the pelvis. CT data were analyzed using the Hounsfield units (HU) for regions.
In vivo MR imaging experiments were performed on a 3.0 T small animal MRI instrument (US Varian 3.0 T) and the pulse sequence used was a T1-weighted SE-XL/90 sequence with the following parameters: TR = 482 ms, TE = 16 ms: field of view [FOV]: 40 × 90 cm; matrix: 256 × 256; number of excitations (NEX): 5; slice thickness = 1 mm; FOV: 40 × 90 cm; coil: VOLUME; T2-weighted FSE-XL/90 sequence with the following parameters: TR = 3000 ms, TE = 48 ms: field of view [FOV]: 40 × 90 cm; matrix: 256 × 256; number of excitations (NEX): 5; slice thickness = 1 mm; FOV: 40 × 90 cm; coil: VOLUME.
Biodistributionof F-AuNC@Fe3O4
To assess the specific in vivo tumor targeting of F-AuNC@Fe3O4, After injected with 10 mg F-AuNC@Fe3O4, the tumor bearing mice were sacrificed at 6 h post-administration and the tissues including heart, kidney, liver, spleen and tumor were harvested for isolated organ imaging.
Histological study
Potential acute cytotoxicity and bio-distribution in major organs (heart, liver, lung, kidney, bladder and tumor) of tumor bearing mice post intravenous injection were assessed by dissection. Typical organs were harvested from the rats and immobilized in 4% paraformaldehyde at 4 °C for 48 h and then embedded into paraffin. Sections from the organs were stained with hematoxylin and eosin (H&E) and observed under a light microscope at 100× magnification by an experienced physician and representative images were provided.
Additional Information
How to cite this article: Wang, G. et al. Au Nanocage Functionalized with Ultra-small Fe3O4 Nanoparticles for Targeting T1–T2 Dual MRI and CT Imaging of Tumor. Sci. Rep. 6, 28258; doi: 10.1038/srep28258 (2016).
References
Chen, W., Zhuang, H., Cheng, G., Torigian, D. A. & Alavi, A. Comparison of FDG-PET, MRI and CT for post radiofrequency ablation evaluation of hepatic tumors. Ann Nucl Med 27, 58–64 (2013).
Zhang, Y. M., Jeon, M., Rich, L. J., Hong, H., Geng, J. M. et al. Non-invasive, multimodal functional imaging of the intestine with frozen micellar naphthalocyanines. Nat Nanotechnol 9, 631–638 (2014).
Weissleder, R. & Pittet, M. J. Imaging in the era of molecular oncology. Nature 452, 580–589 (2008).
Zhao, H. Y., Liu, S., He, J., Pan, C. C., Li, H. et al. Synthesis and application of strawberry-like Fe3O4-Au nanoparticles as CT-MR dual-modality contrast agents in accurate detection of the progressive liver disease. Biomaterials 51, 194–207 (2015).
Huang, J., Guo, M., Ke, H., Zong, C., Ren, B. et al. Rational design and synthesis of γFe2O3@Au magnetic gold nanoflowers for efficient cancer theranostics. Adv Mater 27, 5049–5056 (2015).
Amendola, V., Scaramuzza, S., Litti, L., Meneghetti, M., Zuccolotto, G. et al. Magneto-plasmonic Au-Fe alloy nanoparticles designed for multimodal SERS-MRI-CT imaging. Small 10, 2476–2486 (2014).
Zhu, J., Lu, Y. J., Li, Y. G., Jiang, J., Cheng, L. et al. Synthesis of Au–Fe3O4 heterostructured nanoparticles for in vivo computed tomography and magnetic resonance dual model imaging. Nanoscale 6, 199–202 (2014).
Liu, G., Gao, J. H., Ai, H. & Chen, X. Y. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small 9, 1533–1545 (2013).
Wang, G. N., Zhang, X. J., Skallberg, A., Liu, Y. X., Hu, Z. J. et al. One-step synthesis of water-dispersible ultra-small Fe3O4 nanoparticles as contrast agents for T1 and T2 magnetic resonance imaging. Nanoscale 6, 2953–2963 (2014).
He, X. X., Liu, F. Y., Liu, L., Duan, T. C., Zhang, H. M. et al. Lectin-conjugated Fe2O3@Au core@shell nanoparticles as dual mode contrast agents for in vivo detection of tumor. Mol. Pharm. 11, 738–745 (2014).
Li, J. C., Hu, Y., Yang, J., Wei, P., Sun, W. J. et al. Hyaluronic acid-modified Fe3O4@Au core/shell nanostars for multimodal imaging and photothermal therapy of tumors. Biomaterials 38, 10–21 (2015).
Skrabalak, S. E., Au, L., Li, X. D. & Xia, Y. N. Facile synthesis of Ag nanocubes and Au nanocages. Nature Protocols 2, 2182–2190 (2007).
Bao, F., Yao, J. L. & Gu, R. A. Synthesis of magnetic Fe2O3/Au core/shell nanoparticles for bioseparation and immunoassay based on surface-enhanced raman spectroscopy. Langmuir 25, 10782–10787 (2009).
Shi, P., Li, M., Ren, J. S. & Qu, X. G. Gold Nanocage-based dual responsive “caged metal chelator” release system: noninvasive remote control with near infrared for potential treatment of alzheimer’s disease. Advanced Functional Materials 43, 5412–5419 (2013).
Xie, J. P., Zhang, Q. B., Lee, J. Y. & Wang, D. I. C. The synthesis of SERS-active gold nanoflower tags for in vivo applications. ACS Nano 2, 2473–2480 (2008).
Cole, L. E., Ross, R. D., Tilley, J. M., Vargo-Gogola, T. & Roeder, R. K. Gold nanoparticles as contrast agents in x-ray imaging and computed tomography. Nanomedicine 10, 321–341 (2015).
Sathe, T. R., Agrawal, A. & Nie, S. M. Mesoporous silica beads embedded with semiconductor quantum dots and iron oxide nanocrystals: dual-function microcarriers for optical encoding and magnetic separation. Anal Chem 78, 5627–5632 (2006).
Xiao, Q., Bu, W., Ren, Q., Zhang, S., Xing, H. et al. Radiopaque fluorescence-transparent TaOx decorated upconversion nanophosphors for in vivo CT/MR/UCL trimodal imaging. Biomaterials 33, 7530–7539 (2012).
Feng, Y., Zong, Y. D., Ke, T. Y., Jeong, E. K., Parker, D. L. et al. Pharmacokinetics, biodistribution and contrast enhanced MR blood pool imaging of Gd-DTPA cystine copolymers and Gd-DTPA cystine diethyl ester copolymers in a rat mode. Model. Pharm. Res. 23, 1736–1742 (2006).
Tombach, B., Reimer, P., Bremer, C., Allkemper, T., Engelhardt, M. et al. First-pass and equilibrium-MRA of the aortoiliac region with a superparamagnetic iron oxide blood pool MR contrast agent (SHU 555C): results of a human pilot study. NMR Biomed. 17, 500–506 (2004).
Bjørnerud, A. & Johansson, L. The utility of superparamagnetic contrast agents in MRI: theoretical consideration and applications in the cardiovascular system. NMR Biomed. 17, 465–477 (2004).
Linderoth, S., Hendriksen, P. V., Bødker, F., Wells, S., Davies, K. et al. On spin-canting in maghemite particles. J Appl. Phys. 75, 6583–6585 (1994).
Acknowledgements
We gratefully acknowledge support from National Natural Science Foundation of China (No. 81401499) and Start-up grant of University of Macau (SRG2015-00007-FHS).
Author information
Authors and Affiliations
Contributions
G.N.W. and X.M. conceived the idea and designed the experiments. G.N.W. and G.W. fabricated the samples and carried out in vitro and in vivo experiments. X.Z. and X.M. performed new contrast agent measurements and analyzed the results. G.N.W., X.Z. and X.M. discussed the results and wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, G., Gao, W., Zhang, X. et al. Au Nanocage Functionalized with Ultra-small Fe3O4 Nanoparticles for Targeting T1–T2Dual MRI and CT Imaging of Tumor. Sci Rep 6, 28258 (2016). https://doi.org/10.1038/srep28258
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep28258
This article is cited by
-
Multifunctional modification of Fe3O4 nanoparticles for diagnosis and treatment of diseases: A review
Frontiers of Materials Science (2021)
-
Facile laser synthesis of multimodal composite silicon/gold nanoparticles with variable chemical composition
Journal of Nanoparticle Research (2019)
-
Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics
3 Biotech (2018)
-
Ultrasensitive multiplexed immunoassay of autophagic biomarkers based on Au/rGO and Au nanocages amplifying electrochemcial signal
Scientific Reports (2017)
-
Intravascular contrast agents in diagnostic applications: Use of red blood cells to improve the lifespan and efficacy of blood pool contrast agents
Nano Research (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.