Abstract
Ethanol and butanol biosynthesis in Clostridium acetobutylicum share common aldehyde/alcohol dehydrogenases. However, little is known about the relative contributions of these multiple dehydrogenases to ethanol and butanol production respectively. The contributions of six aldehyde/alcohol dehydrogenases of C. acetobutylicum on butanol and ethanol production were evaluated through inactivation of the corresponding genes respectively. For butanol production, the relative contributions from these enzymes were: AdhE1 > BdhB > BdhA ≈ YqhD > SMB_P058 > AdhE2. For ethanol production, the contributions were: AdhE1 > BdhB > YqhD > SMB_P058 > AdhE2 > BdhA. AdhE1 and BdhB are two essential enzymes for butanol and ethanol production. AdhE1 was relatively specific for butanol production over ethanol, while BdhB, YqhD, and SMB_P058 favor ethanol production over butanol. Butanol synthesis was increased in the adhE2 mutant, which had a higher butanol/ethanol ratio (8.15:1) compared with wild type strain (6.65:1). Both the SMB_P058 mutant and yqhD mutant produced less ethanol without loss of butanol formation, which led to higher butanol/ethanol ratio, 10.12:1 and 10.17:1, respectively. To engineer a more efficient butanol-producing strain, adhE1 could be overexpressed, furthermore, adhE2, SMB_P058, yqhD are promising gene inactivation targets. This work provides useful information guiding future strain improvement for butanol production.
Similar content being viewed by others
Introduction
Microbial production of chemicals and fuels from renewable resources is a prospective approach to avoid problems that are usually associated with fossil-based chemical synthesis, such as environmental pollution, unsustainable demand, and increasing costs. Alcohols, such as 1,3-propanediol, butanol, isobutanol, and 1,4-butanediol can be used as important platform chemicals or biofuels. Many efforts have been made to improve production of such target alcohols by engineering aldehyde/alcohol dehydrogenases, as they are commonly the final enzymes used for converting acyl-CoAs or aldehydes to their corresponding alcohols.
The substrate specificity of the aldehyde/alcohol dehydrogenase is critical for the production of target alcohol. Among the multiple alcohol dehydrogenases of Escherichia coli, it was found that the endogenous alcohol dehydrogenase YqhD contributed most to isobutanol production. Exogenous enzymes have also been utilized to increase alcohol production. When exogenous AdhA from Lactococcus lactis was expressed in E. coli, it led to better isobutanol production due to its higher isobutyraldehyde reductase activity than the native enzymes including YqhD1. Expression of AdhE2 from C. acetobutylicum was also more optimal for production of butanol than the native AdhE of E. coli as a result of this enzyme’s higher affinity for butyryl-CoA and lower affinity for acetyl-CoA. Researchers therefore engineered the strain for optimal butanol production by deleting E. coli adhE and expressing the exogenous adhE22,3. The same engineering strategy was also successfully used for 1,4-butanediol production in E. coli4. Notably, in an attempt to obtain an engineered E. coli strain capable of producing a high-titer of 1,3-propanediol, the broad-spectrum hypothetical dehydrogenase (encoded by yqhD) from E. coli showed better performance than the specific 1,3-propanediol dehydrogenase (encoded by dhaT) from Klebsiella pneumoniae5, indicating that exogenous dehydrogenases might not always be the optimal enzymes for target production. In fact, there are many aldehyde/alcohol dehydrogenases in microbial cells, but their physiological functions are not always clear and their relative contributions to alcohols production are unknown. In some species, different aldehyde/alcohol dehydrogenases contribute to the production of one alcohol while in other species, the same aldehyde/alcohol dehydrogenase(s) might contribute to the production of different alcohols.
In C. acetobutylicum, a typical butanol-producing microorganism, there are many aldehyde/alcohol dehydrogenases for alcohol production. Besides the four known dehydrogenases (biofunctional aldehyde/alcohol dehydrogenase: AdhE1 and AdhE26,7, alcohol dehydrogenases: BdhA and BdhB8), there are other genes annotated as encoding alcohol dehydrogenases in the genomes of C. acetobutylicum ATCC 8249 and C. acetobutylicum DSM 173110, including cap0059 (SMB_P058), cac3392 (yqhD), cac3484, and cac3375. The presence of so many aldehyde/alcohol dehydrogenases increases the complexity of engineering a higher (or homo-) butanol producing strain. The production of butanol is always accompanied by the production of ethanol, a phenomenon that may be due to the low specificity of the aldehyde/alcohol dehydrogenases for their substrate (butyryl-CoA and butyraldehyde). As a consequence, by-product ethanol concentrations often increased as butanol production increases. For example, in a C. acetobutylicum solR gene knockout strain, overexpression of the bifunctional alcohol/aldehyde dehydrogenase gene adhE1 increased butanol production by 21%, however this increased ethanol production also by 62%11. Similarly when the adhE1D485Ggene was overexpressed in a pta-buk double deficient C. acetobutylicum strain, butanol production increased by 160% compared with wild-type, however, ethanol production also increased by 233%12. This phenomenon does not only occur in C. acetobutylicum engineered for butanol production; when E. coli are engineered for butanol biosynthesis via the classic clostridial fermentation pathway or reverse β-oxidation pathway3,13, ethanol is also produced as by-product. Furthermore, the overproduction of aldehyde/alcohol dehydrogenases does not always increase butanol and ethanol by the same relative amounts. Each dehydrogenase contributes differently to butanol or ethanol production, and their specific contributions have, so far, not been characterized.
According to transcriptomic results for C. acetobutylicum ATCC 824, cap0059 (SMB_P058), which encodes for an alcohol dehydrogenase, is transcribed as well as adhE1, adhE2, bdhA and bdhB14. In a previous proteomic analysis, we found that a broad-spectrum hypothetical alcohol dehydrogenase YqhD was highly expressed and AdhE1, BdhA and BdhB were also detected15. In this study, we therefore aimed to determine the contributions of each of these six aldehyde/alcohol dehydrogenase in C. acetobutylicum DSM 1731. The six genes were individually disrupted, and their physiological functions and specific contributions to butanol and ethanol synthesis were investigated.
Results
Generation of aldehyde/alcohol dehydrogenases disruption mutants in C. acetobutylicum DSM 1731
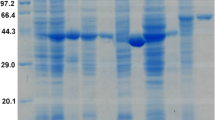
To characterize the contribution of multiple aldehyde/alcohol dehydrogenases in butanol and ethanol production in C. acetobutylicum, the ClosTron system16 with slight modifications17 was used to create aldehyde/alcohol dehydrogenase negative mutants. According to the genome information of C. acetobutylicum DSM 173110, the insertion sites for alcohol dehydrogenase genes: adhE1, adhE2, bdhA, bdhB, SMB_P058, and yqhD were calculated by submitting the corresponding gene sequence into a computer algorithm18. The insertion sites and intron re-targeting PCR primers are described in Additional File1: Table S1. Putative erythromycin-resistant transformants were identified by PCR screening (see Methods). Compared with the specific PCR products from wild-type genes, the mutants yielded enlarged fragments (Fig. 1a). Southern blot hybridization was performed to verify the genomic structure of the mutants. An intron specific probe was used to hybridize the expected fragment. Bands with the expected sizes were detected on the hybridization membrane (Fig. 1b). Positive clones of the alcohol dehydrogenases negative mutants were designated C. acetobutylicum DSM 1731 adhE1::int(807), C. acetobutylicum DSM 1731 adhE2::int(846), C. acetobutylicum DSM 1731 bdhA::int(133), C. acetobutylicum DSM 1731 bdhB::int(669), C. acetobutylicum DSM 1731 SMB_P058::int(150) and C. acetobutylicum DSM 1731 yqhD::int(405), and were used for further studies.
(a) Identification of the mutants by PCR using primers flanking the insertion site. Lane 1, 3, 5, 7, 9, 11, wild-type; lane 2, bdhA mutant; lane 4, bdhB mutant; lane 6, adhE1 mutant; lane 8, yqhD mutant; lane 10, SMB_P058 mutant; lane 12, adhE2 mutant and lane 13, maker. (b) Southern blot confirmation of intron insertion into the aldehyde/alcohol dehydrogenase genes using the intron specific probe 1, wild-type; 2, SMB_P058 mutant; 3, yqhD mutant; 4, bdhB mutant; 5, bdhA mutant; 6, adhE2 mutant (weak band); 7, adhE1 mutant; 8, bdhB mutant (repeated as reference for adhE2 mutant); 9, bdhA mutant (repeated as reference for the adhE2 mutant); 10, adhE2 mutant (repeated).
Disruption of adhE1 results in loss of ethanol and butanol production however adhE2 disruption increases butanol production in C. acetobutylicum
AdhE1 and AdhE2 are two bifunctional dehydrogenases, which convert acyl-CoA to aldehyde then to alcohol in two reductive steps using NADH as cofactor6,7. However, their contributions for ethanol and butanol production is different.
Compared with the wild-type strain, mutant C. acetobutylicum DSM 1731 adhE1::int(807) almost lost the ability to produce alcohol with only minor ethanol (0.6 g/L) and butanol (1.5 g/L) production after 48 h fermentation (Fig. 2). Interestingly, C. acetobutylicum DSM 1731 adhE2::int(846) showed a highly different metabolic profile compared with the adhE1 mutant. The adhE2 mutant reached a butanol concentration of 16.3 g/L, an increase of 12.5% compared with the control strain. Meanwhile, the ethanol concentration decreased to 2.0 g/L (Fig. 2), thus there was an increase in the butanol/ethanol ratio from 6.65:1 for the wild-type strain to 8.15:1 for the adhE2 mutant. This mutant consumed 80 g/L glucose after 48 h fermentation with a solvent yield of 31.8% and a butanol yield of 20.3%, both of which were higher than for the wild-type strain (Table 1). Furthermore, the production of another NADH dependent-acid lactate was increased, to 6.3 g/L in adhE1 mutant, compared with 1.0 g/L produced by the control strain, while less lactate (0.2 g/L) accumulated in adhE2 mutant culture medium (Fig. 2).
As to acid production, the adhE1 mutant strain showed a sustained increase in acid accumulation producing 2.2 g/L acetate and 13.6 g/L butyrate compared with 1.7 g/L acetate and 0.5 g/L butyrate for the wild-type strain which re-assimilates acid. The residual acetate decreased to 0.5 g/L in the adhE2 mutant, and there was no remarkable difference in the concentration of residual butyrate between the adhE2 mutant and the wild-type strain, but the peak level of butyrate was much lower for the mutant than the control (Fig. 2). These results indicate that the disruption of adhE1 leads to a failed conversion from acidogenesis to solventogenesis and the ability to re-assimilate acid, while the disruption of adhE2 increases the bacterium’s ability to produce solvent. As the result, there was no visible rebound of the pH value during the fermentation process of the adhE1 mutant strain, due to the lessened acid re-assimilation of this strain. Contrastingly, the pH values of the control strain and the adhE2 mutant rebounded. This can be seen with the final pH of adhE2 mutant being 5.93, a higher value than that of the control (pH 5.88) (Fig. 2).
BdhB is more important for alcohol production than BdhA in C. acetobutylicum
BdhA and BdhB are two NAD(P)H dependent alcohol dehydrogenases that convert aldehydes to alcohols8,19. After 48 h fermentation, the mutant strain C. acetobutylicum DSM 1731 bdhA::int(133) produced similar amounts of butanol to the control strain, however, it also produced more ethanol (2.49 g/L) than the control (Fig. 3) with an overall butanol/ethanol ratio lower than the control (5.63:1 vs 6.65:1). This mutant strain also consumed less glucose (74 g/L) than the control (78 g/L) (Fig. 3), leading to a higher solvent (31.5%) and butanol yield (19.0%) compared with the control (Table 1). However, after disrupting bdhB (SMB_G3335), the alcohol production capability of the strain decreased significantly. The butanol and ethanol titers were 11.7 g/L and 1.1 g/L, respectively, a decrease of 17.5% and 33.3% relative to wild-type strain. Similar to the adhE1 mutant, the bdhB mutant also accumulated lactate, with the maximal titer reaching 3.4 g/L. However, less lactate (0.6 g/L) was accumulated in the bdhA mutant compared with 1.0 g/L for control strain (Fig. 3).
As for acid production, the profile of butyrate production for the bdhA mutant was similar with the control. The mutant strain produced less acetate with approximately 0.7 g/L acetate accumulating in the fermentation broth (Fig. 3). The final pH of the mutant strain culture was higher than that of the control, presumably due to the lower residual acid levels. Especially in the later stage of fermentation, the pH continued to rise, which was coincident with an increase in ethanol production and decreased acetate production (Fig. 3). For the bdhB mutant, there was no remarkable difference in acetate production compared to the control strain, however, the strain accumulated three-fold more butyrate (1.8 g/L) than the control (Fig. 3), suggesting a weaker acid re-assimilation. The pH rebound observed for this mutant was not as significant as that for the wild-type strain (Fig. 3). This was also coincident with the poorer solventogenic performance of the mutant.
Disruption of SMB_P058 and yqhD leads to higher butanol/ethanol ratio however gives different metabolic profiles
SMB_P058 is carried by pSMBa, a megaplasmid of C. acetobutylicum DSM 1731, and is annotated as alcohol dehydrogenase. The mutant strain C. acetobutylicum DSM 1731 SMB_P058::int(150) showed different properties in butanol and ethanol production, producing more butanol (15.4 g/L) and less ethanol (1.52 g/L) than the wild-type (Fig. 4). Thus the butanol/ethanol ratio improved from 6.65:1 to 10.12:1 (Table 1). These data indicate that the enzyme product of gene SMB_P058 favors biosynthesis of ethanol over butanol. yqhD on chromosome was also annotated as an alcohol dehydrogenase. The yqhD mutant strain produced less ethanol (1.46 g/L) than the control without a significant effect on butanol production (Fig. 4), which led to an increased butanol/ethanol ratio (10.17:1) (Table 1). We therefore propose that YqhD contributes to ethanol but not butanol production. These two mutant strains produced less lactate compared with control strain (Fig. 4), however, on other hand, these two strains had a higher butanol yield with 19.2% for SMB_P058 mutant and 19.6% for yqhD mutant relative to the control (Table 1). Similar to the adhE2 mutant, strain SMB_P058::int(150) accumulated less butyrate before acid assimilation with the peak value of butyrate production being much lower. We speculate that this profile is due to an improved ability to assimilate acid by SMB_P058::int(150). For yqhD mutant, more acetate (2.0 g/L) was accumulated compared with the control, reflected in the lower pH observed in the later stages of the fermentation (Fig. 4).
Discussion
In C. acetobutylicum, several different aldehyde/alcohol dehydrogenases are involved in butanol and ethanol production, each with specific order of expression, expression level and substrate preference. In this work, our data show that the adhE1 mutant almost lost the ability to produce alcohol during glucose batch fermentation. adhE1 and adhE2 located in the megaplasmid were the only two genes encoding aldehyde dehydrogenase in C. acetobutylicum. It has been known that AdhE2 is expressed only under alcohologenic conditions7,20,21,22,23 which were not employed here. AdhE1 is the only aldehyde dehydrogenase which provides aldehyde for alcohol production via other alcohol dehydrogenases. Expressing an aldehyde dehydrogenase from C. beijerinckii NCIMB 8052 in the adhE1 mutant partly restored acetone and alcohol production (Figure S1). The transcription level of adhE1 was also the highest among all the aldehyde/alcohol dehydrogenases in C. acetobutylicum14. These data indicated that the aldehyde dehydrogenase activity of adhE1 could not be substituted by AdhE2, suggesting an essential role of AdhE1 in butanol and ethanol generation. This work also corroborates earlier work highlighting the primary role of aldehyde dehydrogenase activity of AdhE1 in C. acetobutylicum24.
AdhE2 is responsible for alcohol production only under alcohologenic conditions which can be defined as growth with high NADH/NAD+ ratio at neutral pH in glucose-limited cultures after addition of neutral red or methyl viologen, or in a more reductive culture medium like glucose-glycerol or glucose-glycerol-pyruvate7,20,21,22,23. A recent study showed that AdeE2 contributed almost 100% for butanol production under alcoholgenic conditions whilst contributing no greater than 9% under solventogenic conditions19. This suggests that the expression of adhE2 is sensitive to culture conditions, which may be one reason why our results differ from those in a previous report where there was no change of fermentation products in adhE2 mutant25. In this study, calcium carbonate was used as a buffering agent in the supplemented Clostridium basal medium (CBM) in an anaerobic cabinet25, whereas here we controlled the pH of the clostridial growth medium (CGM) at ≥5.0 by addition of ammonia to the anaerobic bioreactor (solventogenic condition). In solventogenic conditions, adhE2 was transcribed at a much lower level than adhE114. Perhaps the disruption of adhE2 led to favorable substrate and redox equivalent availability for adhE1, the only enzyme in a adhE2 minus background that could simultaneously convert acetyl-CoA and butyryl-CoA to alcohol and provide substrate aldehyde for other alcohol dehydrogenases. These could therefore explain why butanol production increased in the adhE2 mutant. On other hand, the synergistic effect of an increased acid reutilization and improved cell growth (Figure S2) could be another reason, however, the mechanism behind this is, so far, unclear.
Isoenzymes BdhB and BdhA showed very different effects on the metabolic profile of C. acetobutylicum. It was shown that BdhB was very important for butanol and ethanol production in C. acetobutylicum second to only AdhE1. Although the ability to produce alcohol was significantly impaired in the bdhB mutant, the butanol production of the bdhA mutant was similar to that of the control. This might result from a difference on the transcription level and substrate specificity of the two alcohol dehydrogenases. The transcription of bdhA occurs earlier than that of bdhB, however the final transcription level of bdhB is significantly higher than that of bdhA8,14. In terms of enzyme activity and substrate specificity: BdhA has only two-fold higher activity with butyraldehyde than with acetaldehyde, whereas BdhB has 46-fold higher activity26. Therefore, BdhA is thought to be functional in low-level butanol production, whereas massive and continuous butanol production can be brought about by BdhB27,28.
The fermentation profiles of the SMB_P058 and yqhD mutants were highly unexpected. Both mutant strains accumulated less ethanol than the wild-type yet did not show loss of butanol production. These two genes correspond to cap0059 and cac3392 in C. acetobutylicum ATCC 824. Both were proposed to be alcohol dehydrogenases for butanol and ethanol production9,10,29. No previous experimental data exist for their specificity in butanol and ethanol formation. SMB_P058 was annotated as an iron-containing alcohol dehydrogenase, however, compared with characterized alcohol dehydrogenases, this enzyme showed its highest identity (38%) with the AdhB from the ethanologenic bacterium Zymomonas mobilis ZM4. AdhB is an important enzyme for ethanol production in Z. mobilis and showed 100% activity towards acetaldehyde and no activity towards butyraldehyde30. The decreased ethanol production by the SMB_P058 mutant in the present work suggests SMB_P058 may be more specific for ethanol production than for butanol. YqhD was annotated as an NAD(P)H dependent butanol dehydrogenase9,10,19. This enzyme showed the highest identity (57%) with the experimentally characterized alcohol dehydrogenase YqhD from E. coli. Overexpression of yqhD in E. coli has previously shown to be more beneficial for synthesis of ethanol than butanol, and deletion of this gene leads to a reduction in ethanol formation without adverse effects on butanol formation13. These results are very similar to our own findings which also indicate that YqhD in C. acetobutylicum favors the synthesis of ethanol over butanol. These results suggest that SMB_P058 and YqhD are very promising candidates for reducing by-product ethanol levels; double deletion of these two genes may therefore lead to a highly optimal butanol-producing strain.
All six aldehyde/alcohol dehydrogenases in C. acetobutylicum are NAD(P)H dependent enzymes. These enzymes play an important role in balancing the cellular redox potential by synthesizing alcohol, regenerating NAD+ for glycolysis. The disruption of these genes may cause redistribution of redox potential. In this work, we found that (NADH dependent) lactate formation was an important outlet for maintaining redox balance in C. acetobutylicum. In the adhE1 and bdhB mutant strains, increased lactate accumulation occurred following a decrease in alcohol production. There are four lactate dehydrogenases in C. acetobutylicum DSM 1731 with one in particular, ldh1 (SMB_G0272), showing to be remarkably upregulated (36-fold) in the adhE1 mutant strain (data not shown). This lactate dehydrogenase is therefore a promising candidate for further metabolic engineering work, to increase the downstream redox potential driving force and further promote butanol production in cells.
Disruption of aldehyde/alcohol dehydrogenases led to a redistribution of redox equivalents between different NAD(P)H dependent enzymes and resulted in an increase in butanol production. However, the increased level is not enough for industrial relevant applications. One reason may be the diversity of aldehyde/alcohol dehydrogenases (enzyme activity, expression level, expression order, and substrate specificity) in C. acetobutylicum. The redox equivalent driving force caused by a single gene disruption may not be strong enough to favor sufficient butanol formation. In C. acetobutylicum, hydrogen production is another crucial regulator of cell redox equivalents. Thus, combinational optimization of the aldehyde/alcohol dehydrogenases system, and lactate dehydrogenases system together with systematic engineering of the hydrogenases may be a way to realize homo-butanol fermentation in C. acetobutylicum.
Materials and Methods
Strains, plasmids and culture conditions
A list of bacterial strains and plasmids used in this study is presented in Table 2. C. acetobutylicum DSM 1731, as used in our previous studies15,31, was used as the wild-type strain. E. coli JM109 was used for vectors construction. E. coli TOP10 bearing the methylating plasmid pAN2 was used for methylation of plasmids. E. coli strains were grown aerobically at 37 °C with shaking at 220 rpm in liquid LB medium or on LB medium supplemented with 1.5% agar. C. acetobutylicum strains were grown anaerobically at 37 °C in reinforced clostridial medium (RCM)32. pMTL00817 was used as the backbone to construct different gene insertion plasmids. Ampicillin, erythromycin and chloromycetin were added at concentrations of 100, 50 and 30 μg/mL, respectively, when necessary. All strains were stored in 15% glycerol at −80 °C. Cell growth was monitored by measuring the absorbance at 600 nm (OD600) with a UV/Vis 2802PC spectrophotometer (Unico, NJ, USA).
DNA manipulation
All primers used in this study are listed in Additional File1: Table S1. All DNA manipulations including PCR product purification, plasmids DNA purification and total genomic DNA extraction were performed using kits from E.Z.N.A (Omega Biotek Inc., Guangzhou, China). PCR polymerase, restriction enzymes and T4 ligase were obtained from New England Biolabs (Beijing, China), and used according to the manufacturer’s instructions. Before transformation into C. acetobutylicum, all plasmids were methylated in E. coli TOP10(pAN2) to protect them from the restriction system33.
Construction of group II intron based gene insertion plasmids
To obtain alcohol dehydrogenase negative mutants, the group II intron based ClosTron system was used16,34. A computer algorithm18 was used for designing intron re-targeting primers (Supplementary file: Table S1). PCR was performed according to the Targetron Gene Knockout System Kit Protocol (http://www.sigmaaldrich.com). The generated 350-bp DNA fragment was ligated into the vector pMTL00817 after digestion with HindIII and BsrGI. Positive clones were screened using specific primers after transformation into E. coli JM109. Electrotransformation of C. acetobutylicum was performed according to the method developed by Mermelstein35. The transformants were first selected on RCM plates containing 30 μg/mL chloromycetin for 48 h. Colonies were then suspended in liquid RCM containing 30 μg/mL chloromycetin overnight. Cells (100 μL) were plated at different dilutions at RCM plates containing 50 μg/mL erythromycins. The positive mutants were selected by colony PCR with primers gene-1 and gene-2. The expected mutation resulted in an enlarged PCR product compared with that obtained from the wild-type. DNA sequencing was used to confirm the correct insertion of the intron into the target site.
Southern blotting
To verify that only one copy of the intron was inserted into the genome, Southern blot hybridization was performed as described in previous work17 with slight modifications. Primers intron-probe-1 and intron-probe-2 were used to generate specific II intron probes with plasmid pMTL008 as the template. Genomic DNA of wild-type strain DSM 1731 and the mutants was digested with HindIII. No DNA fragment released from the wild-type strain hybridized to the probe. Whereas, restriction fragments of the expected size derived from the mutants gave positive bands on the hybridization membrane.
Batch fermentation and analytical method
To analyze the effect of the aldehyde/alcohol dehydrogenases on cellular metabolites, especially ethanol and butanol production, batch fermentation of C. acetobutylicum DSM 1731 and the mutant strains was performed in CGM in BioFlo 110 fermenters (New Brunswick Scientific, Edison, NJ, USA), according to the fermentation method described in previous work36. Cell growth, glucose consumption, and the main fermentation products (butanol, ethanol, acetone, butyrate, acetate and lactate) were determined with the same methods used in previous work36.
Additional Information
How to cite this article: Dai, Z. et al. Elucidating the contributions of multiple aldehyde/alcohol dehydrogenases to butanol and ethanol production in Clostridium acetobutylicum. Sci. Rep. 6, 28189; doi: 10.1038/srep28189 (2016).
References
Atsumi, S. et al. Engineering the isobutanol biosynthetic pathway in Escherichia coli by comparison of three aldehyde reductase/alcohol dehydrogenase genes. Appl Microbiol Biotechnol 85, 651–657 (2010).
Atsumi, S. et al. Metabolic engineering of Escherichia coli for 1-butanol production. Metab Eng 10, 305–311 (2008).
Shen, C. R. et al. Driving forces enable high-titer anaerobic 1-butanol synthesis in Escherichia coli . Appl Environ Microbiol 77, 2905–2915 (2011).
Yim, H. et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol. Nat. Chem. Biol. 7, 445–452 (2011).
Emptage, M., Haynie, S. L., Laffend, L. A., Pucci, J. P. & Whited, G. Process for the biological production of 1, 3-propanediol with high titer. US patent 6, 514, 733 (2003).
Nair, R. V., Bennett, G. N. & Papoutsakis, E. T. Molecular characterization of an aldehyde/alcohol dehydrogenase gene from Clostridium acetobutylicum ATCC 824. J Bacteriol 176, 871–885 (1994).
Fontaine, L. et al. Molecular characterization and transcriptional analysis of adhE2, the gene encoding the NADH-dependent aldehyde/alcohol dehydrogenase responsible for butanol production in alcohologenic cultures of Clostridium acetobutylicum ATCC 824. J Bacteriol 184, 821–830 (2002).
Walter, K. A., Bennett, G. N. & Papoutsakis, E. T. Molecular characterization of two Clostridium acetobutylicum ATCC 824 butanol dehydrogenase isozyme genes. J Bacteriol 174, 7149–7158 (1992).
Nolling, J. et al. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum . J Bacteriol 183, 4823–4838 (2001).
Bao, G. et al. Complete genome sequence of Clostridium acetobutylicum DSM 1731, a solvent-producing strain with multireplicon genome architecture. J Bacteriol 193, 5007–5008 (2011).
Harris, L. M., Blank, L., Desai, R. P., Welker, N. E. & Papoutsakis, E. T. Fermentation characterization and flux analysis of recombinant strains of Clostridium acetobutylicum with an inactivated solR gene. J Ind Microbiol Biotechnol 27, 322–328 (2001).
Jang, Y.-S. et al. Enhanced butanol production obtained by reinforcing the direct butanol-forming route in Clostridium acetobutylicum . mBio 3, e00314–00312 (2012).
Dellomonaco, C., Clomburg, J. M., Miller, E. N. & Gonzalez, R. Engineered reversal of the beta-oxidation cycle for the synthesis of fuels and chemicals. Nature 476, 355–359 (2011).
Alsaker, K. V. & Papoutsakis, E. T. Transcriptional program of early sporulation and stationary-phase events in Clostridium acetobutylicum . J Bacteriol 187, 7103–7118 (2005).
Mao, S. et al. Proteome reference map and comparative proteomic analysis between a wild type Clostridium acetobutylicum DSM 1731 and its mutant with enhanced butanol tolerance and butanol yield. J Proteome Res 9, 3046–3061 (2009).
Heap, J. T., Pennington, O. J., Cartman, S. T., Carter, G. P. & Minton, N. P. The ClosTron: a universal gene knock-out system for the genus Clostridium . J Microbiol Methods 70, 452–464 (2007).
Dong, H. J., Zhang, Y. P., Dai, Z. J. & Li, Y. Engineering Clostridium strain to accept unmethylated DNA. PLoS One 5, e9038 (2010).
Perutka, J., Wang, W. J., Goerlitz, D. & Lambowitz, A. M. Use of computer-designed group II introns to disrupt Escherichia coli DExH/D-box protein and DNA helicase genes. J Mol Biol 336, 421–439 (2004).
Yoo, M. et al. A Quantitative System-Scale Characterization of the Metabolism of Clostridium acetobutylicum . MBio 6, e01808–01815 (2015).
Girbal, L., Vasconcelos, I., Saintamans, S. & Soucaille, P. How neutral red modified carbon and electron flow in Clostridium acetobutylicum grown in chemostat culture at neutral pH. FEMS Microbiol Rev 16, 151–162 (1995).
Peguin, S. & Soucaille, P. Modulation of carbon and electron flow in Clostridium acetobutylicum by iron limitation and methyl viologen addition. Appl Environ Microbiol 61, 403–405 (1995).
Girbal, L., Croux, C., Vasconcelos, I. & Soucaille, P. Regulation of metabolic shifts in Clostridium acetobutylicum ATCC 824. FEMS Microbiol Rev 17, 287–297 (1995).
Honicke, D., Janssen, H., Grimmler, C., Ehrenreich, A. & Lutke-Eversloh, T. Global transcriptional changes of Clostridium acetobutylicum cultures with increased butanol:acetone ratios. N Biotechnol 29, 485–493 (2012).
Nair, R. V. & Papoutsakis, E. T. Expression of plasmid-encoded aad in Clostridium acetobutylicum M5 restores vigorous butanol production. J. Bacteriol. 176, 5843–5846 (1994).
Cooksley, C. M. et al. Targeted mutagenesis of the Clostridium acetobutylicum acetone-butanol-ethanol fermentation pathway. Metab Eng 14, 630–641 (2012).
Welch, R. W., Rudolph, F. B. & Papoutsakis, E. T. Purification and characterization of the NADH-dependent butanol dehydrogenase from Clostridium acetobutylicum ATCC 824. Arch Biochem Biophys 273, 309–318 (1989).
Durre, P. et al. Solventogenic enzymes of Clostridium acetobutylicum: catalytic properties, genetic organization, and transcriptional regulation. FEMS Microbiol Rev 17, 251–262 (1995).
Durre, P. Fermentative butanol production: bulk chemical and biofuel. Ann N Y Acad Sci 1125, 353–362 (2008).
Papoutsakis, E. T. Engineering solventogenic clostridia. Curr Opin Biotechnol 19, 420–429 (2008).
Kinoshita, S., Kakizono, T., Kadota, K., Das, K. & Taguchi, H. Purification of two alcohol dehydrogenases from Zymomonas mobilis and their properties. Appl Microbiol Biotechnol 22, 249–254 (1985).
Wang, S. H. et al. Formic acid triggers the “acid crash” of acetone-butanol-ethanol fermentation by Clostridium acetobutylicum . Appl Environ Microbiol 77, 1674–1680 (2011).
Hirsch, A. & Grinsted, E. Methods for the growth and enumeration of anaerobic spore-formers from cheese, with observations on the effect of nisin. J Dairy Res 21, 101–110 (1954).
Mermelstein, L. D. & Papoutsakis, E. T. In vivo methylation in Escherichia coli by the Bacillus subtilis phage phi 3T I methyltransferase to protect plasmids from restriction upon transformation of Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 59, 1077–1081 (1993).
Heap, J. T. et al. The ClosTron: Mutagenesis in Clostridium refined and streamlined. J Microbiol Methods 80, 49–55 (2010).
Mermelstein, L. D., Welker, N. E., Bennett, G. N. & Papoutsakis, E. T. Expression of cloned homologous fermentative genes in Clostridium acetobutylicum ATCC 824. Nat Biotechnol 10, 190–195 (1992).
Dai, Z. et al. Introducing a single secondary alcohol dehydrogenase into butanol-tolerant Clostridium acetobutylicum Rh8 switches ABE fermentation to high level IBE fermentation. Biotechnol Biofuels 5, 44 (2012).
Acknowledgements
This work was supported by National High Technology Research and Development Program of China (863 program, 2011AA02A208), and National Basic Research Program of China (973 program, 2011CBA00800). Yin Li is supported by the Hundreds of Talents Program of the Chinese Academy of Sciences. Yanping Zhang is supported by the Knowledge Innovation Program of the Chinese Academy of Sciences (No. KSCX2-EW-Q-14) and Youth Innovation Promotion Association CAS (No. 2014076). Many thanks Professor Nigel Peter Minton and Dr. John Heap (University of Nottingham, UK) for gifting pMTL007 and pAN2. Many thanks Dr. Kate Campbell for language editing.
Author information
Authors and Affiliations
Contributions
Z.D., H.D., Y.Z. and Y.L. conceived and designed the study. Z.D. and H.D. performed the experiments. Z.D. analyzed the data. Z.D. wrote the manuscript. H.D., Y.Z. and Y.L. revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Dai, Z., Dong, H., Zhang, Y. et al. Elucidating the contributions of multiple aldehyde/alcohol dehydrogenases to butanol and ethanol production in Clostridium acetobutylicum. Sci Rep 6, 28189 (2016). https://doi.org/10.1038/srep28189
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep28189
This article is cited by
-
Metabolic engineering of Escherichia coli for efficient biosynthesis of butyl acetate
Microbial Cell Factories (2022)
-
Genome-scale metabolic modelling enables deciphering ethanol metabolism via the acrylate pathway in the propionate-producer Anaerotignum neopropionicum
Microbial Cell Factories (2022)
-
Enforcing ATP hydrolysis enhanced anaerobic glycolysis and promoted solvent production in Clostridium acetobutylicum
Microbial Cell Factories (2021)
-
Production of butanol from distillers’ grain waste by a new aerotolerant strain of Clostridium beijerinckii LY-5
Bioprocess and Biosystems Engineering (2021)
-
Analysis of indigenous plasmid sequences of A. baumannii DS002 reveals the existence of lateral mobility and extensive genetic recombination among Acinetobacter plasmids
Journal of Genetics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.