Abstract
The regulation of pancreatic β cell mass is a critical factor to help maintain normoglycemia during insulin resistance. Nutrient-sensing G protein-coupled receptors (GPCR) contribute to aspects of β cell function, including regulation of β cell mass. Nutrients such as free fatty acids (FFAs) contribute to precise regulation of β cell mass by signaling through cognate GPCRs and considerable evidence suggests that circulating FFAs promote β cell expansion by direct and indirect mechanisms. Free Fatty Acid Receptor 2 (FFA2) is a β cell-expressed GPCR that is activated by short chain fatty acids, particularly acetate. Recent studies of FFA2 suggest that it may act as a regulator of β cell function. Here, we set out to explore what role FFA2 may play in regulation of β cell mass. Interestingly, Ffar2−/− mice exhibit diminished β cell mass at birth and throughout adulthood and increased β cell death at adolescent time points, suggesting a role for FFA2 in establishment and maintenance of β cell mass. Additionally, activation of FFA2 with Gαq/11-biased agonists substantially increased β cell proliferation in in vitro and ex vivo proliferation assays. Collectively, these data suggest that FFA2 may be a novel therapeutic target to stimulate β cell growth and proliferation.
Similar content being viewed by others
Introduction
In response to states of chronic insulin resistance, pancreatic islets employ multiple compensatory responses in an attempt to maintain whole-body glucose homeostasis. These responses consist of enhanced insulin secretion as well as expansion of beta (β) cell mass1. When an individual is unable to sustain these compensatory mechanisms, due to a confluence of genetic, environmental, and/or lifestyle factors, progression to Type 2 diabetes (T2D) can occur. The specific contribution of β cell mass deficits versus impaired β cell function in the progression to de facto T2D remains a matter of some debate2. However, several lines of evidence suggest that initial β cell loss (possibly occurring as early as the pre-diabetic phase) places increased secretory burden on the surviving β cells, leading to chronic β cell stress and further impairments in β cell function as a result of β cell exhaustion3,4,5,6. Along these lines, impaired pre- or postnatal development of β cells is suggested to predispose some individuals to T2D when exposed to aggravating factors such as obesity and insulin resistance2. This possibility is illustrated by studies in which factors such as genetic polymorphisms and fetal malnutrition have been shown to impair β cell mass and result in increased diabetes risk later in life7,8,9,10.
Consistent with these reports, the nutritional status of an individual is thought to be an important regulator of β cell mass. Multiple nutrients such as glucose, amino acids and free fatty acids contribute to maintain precise regulation of β cell mass11. For example, evidence suggests that circulating levels of glucose and free fatty acids (FFA) can promote β cell expansion, although this remains a matter of some debate12. Additionally, nutrient sensing via the gut may indirectly contribute to regulation of β cell mass by promoting GLP-1 secretion from intestinal L-cells, which in turn acts at the β cell to promote cell survival and proliferation. Many nutrients and nutrient-regulated factors exert their influence through G protein-coupled receptors (GPCRs), consistent with the well characterized ability of these receptors to regulate multiple aspects of β cell function and health, including glucose-stimulated insulin secretion (GSIS) and β cell survival and proliferation13. For example, chronic signaling through Gαq/11 and Gαs by designer GPCRs enhanced β cell mass as a result of increased β cell proliferation and β cell hypertrophy14,15. In support of this observation, signaling via the Gαs-coupled GLP-1 receptor by the agonist Exendin-4 improves β cell function, potentiating GSIS and enhancing β cell replication and neogenesis16. Similarly, activation of Gαq/11-coupled receptors such as the M3 muscarinic and long chain free fatty acid receptor FFA1 potentiate GSIS and have been suggested to promote β cell survival and proliferation13,17,18. In contrast, activation of Gαi/o19,20 or Gαz21 pathways inhibits β cell function and proliferation.
In addition to FFA1, multiple other FFA-sensing GPCRs have been identified in the β cell and have garnered considerable interest as potential targets for the treatment of T2D in recent years22,23. Recently, our group and others have reported that islet expression of the short chain fatty acid receptor FFA2 is dynamically regulated in association with multiple models of insulin resistance, including pregnancy and diet-induced and genetic models of obesity and diabetes24,25,26. The endogenous ligands of FFA2, short chain fatty acids, are derived primarily from fermentation of dietary fiber by gut flora27, positioning FFA2 as one possible link between the gut microbiome and its host. These observations have led us to explore and describe a role for FFA2 in affecting crucial aspects of β cell biology. These studies revealed that FFA2 signaling can either stimulate GSIS via the Gαq/11 pathway or inhibit GSIS via Gαi/o28. Specifically, we found that two different classes of previously described FFA2 agonists, small carboxylic acids and phenylacetamide derivatives, demonstrated a bias toward activating Gαq/11 or Gαi/o pathways, respectively. Around the same time, two other groups published their findings, with McNelis et al. reporting dual coupling of FFA2 to Gαi/o and Gαq/11, with potentiation of GSIS mediated primarily by Gαq/1129. By contrast, Tang et al., observed solely Gαi/o signaling via FFA2, leading to inhibition of GSIS30. With respect to β cell mass, Tang et al. and McNelis et al. also reported conflicting findings. Whereas Tang et al. reported no differences in islet morphology between WT and Ffar2−/− mice30, McNelis et al. reported a defect in β cell mass expansion in response to insulin resistance as a result of Ffar2 deletion29. Because of the importance of β cell mass in T2D pathogenesis, here we sought to clarify a potential role for FFA2 in regulating β cell mass and proliferation and to determine how biased signaling of FFA2 through Gαq/11 or Gαi/o may determine its effect on β cell mass.
Results
Genetic deletion of Ffar2 impairs postnatal β cell mass
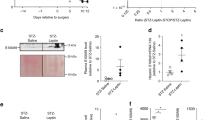
In T2D, the loss of functional β cell mass is an important factor in disease-development31. As GPCRs have been described to contribute to β cell mass through their role in mediating β cell properties such as proliferation and apoptosis13,14,15,16,17,18,19,20, we examined the effect of Ffar2 deletion on β cell mass. Immunohistochemical (IHC) and morphometric analysis of normal chow (NC) fed Ffar2−/− male mice at 26 weeks revealed significantly decreased insulin positive area (relative to total pancreatic area), smaller average β cell area per islet and decreased β cell mass compared to age matched, WT controls (Fig. 1a). Notably, we have also observed this phenotype in a separate study of 10–12 week old Ffar2−/− females32. To examine the influence of FFA2 in regulating adaptive β cell mass expansion in response to insulin resistance, we also assessed β cell mass in WT and Ffar2−/− mice at 26 weeks, following 20 weeks of high fat diet (HFD). Although the overall trend toward diminished β cell mass persisted in HFD-fed Ffar2−/− mice, β cell mass in Ffar2−/− islets did increase in response to HFD, suggesting that mice with deletion of Ffar2 retain some capacity for adaptive β cell mass expansion in response to HFD (Fig. 1a).
Genetic deletion of Ffar2 impairs postnatal β cell mass.
(a) β cell area, average β cell area per islet and β cell mass of WT and Ffar2−/− mice. Adult mice were fed either normal chow (NC) or high fat diet (HFD) as indicated; β cell area is expressed as percent insulin positive area relative to total pancreatic area. (b) β to α cell ratio at each indicated time point. (c) Representative images of WT and Ffar2−/− islets at P21 and 26 weeks. Scale bar = 20 μm. Green: insulin, red: glucagon. For all experiments, n ≥ 3; white bars = WT, black bars = Ffar2−/−. *p < 0.05, **p < 0.01 and ***p < 0.001.
Given the diminished β cell mass in adult Ffar2−/− mice under normal diet conditions, we next examined β cell mass at younger ages, specifically, immediately postnatal (P1) and at 21 days (P21), to determine when the β cell mass phenotype became apparent. Interestingly, we observed significant reductions in the β cell area of Ffar2−/− mice at both ages and marginally decreased β cell mass at P21, suggesting that impairments in β cell mass develop during the prenatal period in Ffar2−/− mice (Fig. 1a). Despite these impairments, overall islet architecture and α to β cell ratio was unaffected at each age examined (Fig. 1b,c).
Ffar2−/− islets demonstrate increased β cell death in vivo
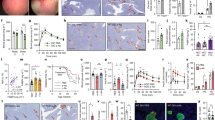
To better understand the mechanism by which Ffar2 contributes to β cell mass, we next assessed β cell proliferation, as this is a major factor regulating postnatal β cell mass and is modulated in part by GPCR signaling33. Using Ki67 immunostaining to assess β cell proliferation at early-postnatal (P1), adolescent (P21) and adult (10 weeks and 26 weeks) ages, we observed dramatically increased β cell proliferation in Ffar2−/− pancreata at P21 and a trend toward increased proliferation at 10 weeks, but found no differences in β cell proliferation between WT and Ffar2−/− mice at P1 or 26 weeks on NC diet (Fig. 2a). Thus, these data do not suggest that impaired β cell proliferation is a primary mechanism leading to diminished β cell mass in Ffar2−/− pancreata.
Ffar2−/− islets do not demonstrate impaired proliferation, but have increased β cell death.
(a) β cell proliferation in WT and Ffar2−/− mice as determined by Ki67 co-localization with insulin-positive cells at P1, P21, 10 weeks and 26 weeks. Bottom: Representative images at P21 shown, scale bar = 20 μm. Green: insulin, red: Ki67. (b) Preproinsulin unmethylation index for WT and Ffar2−/− mice at indicated time points. (c) β cell apoptosis in WT and Ffar2−/− mice as determined by cleaved caspase 3 (CC3) staining at P21 and 10 weeks. Bottom: Representative images at P21 shown. For all experiments, n ≥ 3; white bars = WT, black bars = Ffar2−/−. *p < 0.05, **p < 0.01 and ***p < 0.001.
Given these findings, we suspected that diminished β cell mass may stem from increased rates of β cell death in Ffar2−/− mice. Recent studies have reported that β cell death results in an increase in serum unmethylated preproinsulin DNA, the presence of which may serve as a reliable biomarker of β cell death34,35,36,37. We therefore analyzed the serum of WT and Ffar2−/− mice at P21 and 10 weeks of age by multiplex PCR and found a significantly higher unmethylation index in Ffar2−/− mice at both time points, indicating increased β cell death in Ffar2−/− mice (Fig. 2b); these findings were further supported by observations of increased cleaved caspase 3 staining in Ffar2−/− islets at P21 and 10 weeks (Fig. 2c). Taken together, these data suggest that Ffar2 contributes to the prenatal establishment of β cell mass and that deletion of Ffar2 results in increased postnatal β cell death, suggesting a role for FFA2 in supporting β cell survival.
Acetate does not promote FFA2 mediated β cell proliferation
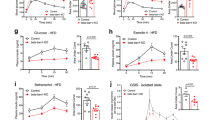
As Gαq/11-coupled GPCRs are well known to mediate β cell proliferation15, we more directly examined whether FFA2 signaling can influence β cell proliferation using ex vivo studies with intact mouse islets from 10–12 week old WT and Ffar2−/− mice. Here, we did not observe any significant differences in the rate of proliferation between WT and Ffar2−/− β cells under untreated conditions, nor in response to the primary endogenous FFA2 ligand, acetate or the GLP-1 receptor agonist, exendin 4, which is known to potently enhance β cell proliferation (Fig. 3a). It is noteworthy, however, that treatment with prolactin, which is also known to stimulate β cell proliferation, did not significantly enhance proliferation in Ffar2−/− β cells (Fig. 3a)16,38. This observation is especially important in light of our recent findings that adaptive β cell mass expansion during pregnancy is compromised in Ffar2−/− female mice32, as this adaptive expansion is regulated in large part by prolactin receptor (PRLR) signaling39. Moreover, a gene expression analysis by our group revealed no difference in PRLR expression in WT and Ffar2−/− islets or commonly associated downstream genes to the PRLR pathway (Supplementary Table 1); thus, the impaired proliferative response to prolactin does not appear to result from impaired gene expression in the PRLR pathway. However, these findings suggest that while there are no apparent differences in proliferation between WT and Ffar2−/− β cells under basal conditions, Ffar2−/− β cells may be impaired in their capacity to respond to some proliferative factors and further investigation into the relationship between FFA2 and PRLR signaling is needed.
Biased agonism of FFA2 promotes β cell proliferation.
(a) β cell proliferation in intact islets from WT and Ffar2−/− mice following treatment with prolactin (PRL, 0.5 μg/ml), Exendin-4 (100 nM) and acetate (1 mM). (b) β cell proliferation in INS1 cells following treatment with prolactin (PRL, 0.5 μg/ml), acetate (AcO, 1 mM), 2-propynoic acid (2-PA, 0.5 mM), 2-butynoic acid (2-BA, 5 mM), cyclopent-1-enecarboxylic acid (CPCA, 0.5 mM), or CPTB (5 μM), as determined by flow cytometry. (c) β cell proliferation in intact WT islets following treatment with placental lactogen (PL, 0.5 μg/ml), acetate (AcO, 1 mM), 2-butynoic acid (2-BA, 5 mM), or cyclopent-1-enecarboxylic acid (CPCA, 0.5 mM). (d) β cell proliferation in intact islets from WT and Ffar2−/− mice following treatment with CPCA (0.5 mM). For all experiments, n = 3. *p < 0.05, **p < 0.01 and ***p < 0.001. For a and d, white bars represent WT, black barsrepresent Ffar2−/−.
Gαq/11-biased agonism of FFA2 promotes β cell proliferation
As noted above, acetate did not appear to influence β cell proliferation. However, we have previously demonstrated that some FFA2-specific agonists demonstrate biased G-protein signaling at mouse FFA2 via either Gαq/11 or Gαi/o pathways28. Because FFA2 couples to both Gαq/11 and Gαi/o, which have been described to either enhance or inhibit β cell proliferation15,20, respectively, we next explored the effect of biased Gαq/11 or Gαi/o signaling via FFA2 on β cell proliferation. Assessing the effect of Gαq/11- and Gαi/o-biased FFA2 agonists on β cell proliferation in vitro in the INS1 rat β cell line, we found that 48 hours treatment with the Gαq/11-biased agonist 2-butynoic acid (2-BA, previously referred to as SCA15), elicited a significant increase in β cell proliferation as determined by BrdU incorporation (Fig. 3b). Further, another Gαq/11-biased agonist, 2-propynoic acid (2-PA, previously referred to as SCA14), elicited a considerable, but non-significant increase in β cell proliferation (Fig. 3b). Conversely, treatment with the Gαi/o-biased agonist CPTB inhibited β cell proliferation, consistent with a model of Gαq/11-mediated potentiation of β cell proliferation and Gαi/o-mediated inhibition of β cell proliferation via FFA2.
To test these observations in a more physiologically relevant model, we conducted an initial screening of the Gαq/11-biased compounds in isolated, intact WT islets and proliferation was assessed by BrdU incorporation. Here we found that treatment with 5 mM 2-BA and 0.5 mM cyclopent-1-enecarboxylic acid (CPCA) resulted in a substantial increase in β cell proliferation relative to untreated islets (Fig. 3c), while 2-PA, which promoted proliferation in INS1 β cells, had no effect in mouse islets (data not shown). Thus, we next tested CPCA, which elicited the largest increase in proliferation in our initial islet studies, in WT and Ffar2−/− islets and observed a 49% increase in proliferation in WT, but not Ffar2−/− islets, demonstrating that this effect is in fact FFA2-dependent (Fig. 3d). We further explored the signaling pathways activated by CPCA using in vitro calcium mobilization and cAMP accumulation assays, which are indicators of Gαq/11 and Gαi/o signaling pathways, respectively. Here, CPCA demonstrated relatively potent calcium mobilization in the BTC3 β cell line, but less potency in the cAMP assay, consistent with Gαq/11-biased signaling (Table 1). These data are compared to acetate, which does not affect β cell proliferation and demonstrates higher potency for cAMP inhibition and less potent calcium mobilization. Taken together, these data suggest that Gαq/11-biased stimulation of FFA2 by CPCA can promote β cell proliferation.
Discussion
Growing evidence that the loss of functional β cell mass is a primary mechanism of T2D pathogenesis suggests that the identification of novel targets that promote β cell growth, proliferation and survival may be especially desirable targets for early disease intervention. Thus, here we assessed the role of FFA2 in regulating β cell mass. We observed impaired β cell mass at birth and throughout adulthood in Ffar2−/− mice. In vitro assays of β cell proliferation in WT and Ffar2−/− islets did not reveal any intrinsic differences in proliferation at baseline in Ffar2−/− β cells, nor in their response to the GLP1 receptor agonist Exendin 4, but our data did suggest that Ffar2−/− islets may be compromised in their ability to respond to prolactin. This raises the possibility that Ffar2−/− mice may experience a developmental impairment arising from decreased sensitivity to developmental signaling factors. Interestingly, we have previously reported impaired β cell mass expansion and glucose tolerance in Ffar2−/− female mice during pregnancy. As prolactin is a major driver of β cell mass expansion during pregnancy39,40, these data further point to the relationship between PRLR and FFA2 signaling as an important future avenue of inquiry.
Surprisingly, at 21 days of age, Ffar2−/− mice exhibit increased β cell proliferation, which appears to be secondary to a large increase in β cell death, which is detectable until at least 10 weeks of age. These observations suggest that FFA2 also contributes to supporting β cell survival during adolescence and early adulthood. It is noteworthy that despite our observations of diminished β cell mass in Ffar2−/− mice, we have previously reported that these mice do not exhibit any overt metabolic phenotype in vivo28. However, we have observed that isolated Ffar2−/− islets secrete significantly less insulin than WT islets in response to stimuli such as high glucose and the GLP-1 receptor agonist, Exendin-428. Taken together, these data suggest that in our mouse model, sufficient compensation occurs to maintain overall glucose homeostasis in Ffar2−/− mice, despite impaired GSIS in vitro and diminished β cell mass.
Notably, two other groups have recently published their findings regarding the effect of Ffar2 deletion on β cell function29,30. While Tang et al.30 reported no morphological differences in the islets of their WT and Ffar2−/− mice, it is significant that they did not conduct de facto β cell mass or area analysis. More significantly, McNelis et al.29, did not identify any impairments in β cell area from Ffar2−/− mice under normal diet conditions, although they did observe impaired adaptive β cell mass expansion in Ffar2−/− mice on HFD, along with impaired glucose tolerance. In their mouse model, these impairments stemmed from a failure of Ffar2−/− β cells to proliferate. In our studies, we observed a notable, but not significant defect in β cell proliferation following 1 week HFD or 20 weeks of HFD in Ffar2−/− mice (Supplementary Fig. 1). However, our analyses were conducted very early and very late in insulin resistance (1 week and 20 weeks, respectively) and may have missed the window of maximal compensatory β cell proliferation. Moreover, it has been noted that the phenotype elicited by HFD can vary greatly, depending on factors such as the genetic background, the diet’s micro- and macronutrient composition and the timing of high fat-feeding41.
Because expansion of β cell mass is a critical component of islet adaptation to insulin resistance and the maintenance of glucose homeostasis, methods to promote β cell growth and proliferation offer invaluable therapeutic tools for T2D. As we previously reported, some FFA2 agonists demonstrate signaling bias, preferentially signaling through Gαi/o or Gαq/11, which results in differential effects on GSIS28. Likewise, our data here suggest that these differential effects may extend to the regulation of β cell proliferation. Encouragingly, our studies suggest that FFA2 may be a novel target to promote β cell proliferation and survival. As the in vivo metabolism and toxicity of these FFA2 agonists is unknown, the compounds are currently unsuitable for additional experimentation, thus, additional work is underway to develop new Gαq/11-biased FFA2 agonists that are more suitable for in vivo use. However, in vitro and ex vivo testing of these agonists have demonstrated the potential of Gαq/11-biased FFA2 to substantially increase β cell proliferation in intact islets. These studies are in agreement with the recent report by McNelis et al., who observed a statistically significant increase in β cell proliferation following treatment with the phenylacetamide agonist PA1, which they reported to be primarily Gαq/11-mediated. Taken together, these two independent studies point to FFA2 as a potential target to promote β cell proliferation.
It is especially noteworthy that we have previously observed PA1 (referred to herein as CPTB), to be primarily Gαi/o-biased, while the publication by McNelis et al., suggests a Gαq/11-biased signaling mechanism. Accordingly, while McNelis et al. report PA1-mediated increases in β cell proliferation and insulin secretion, we have observed PA1-mediated inhibition of β cell proliferation and insulin secretion. As discussed in recent studies of FFA228,29,30,42,43, these discrepancies may result from the use of different mouse models made by different methods and on different backgrounds. Other factors may contribute to the widely varying phenotypes observed across multiple studies. Specifically, GPCR coupling can be regulated by numerous factors, for example by dimerization with other receptors44 or interaction with modifying proteins45, both of which may be modulated by the physiologic state of the host. Given the apparent complexity of FFA2 signaling and the Ffar2−/− phenotype, it is critical that we develop a deeper understanding of how FFA2 signaling and coupling is regulated. Considering FFA2’s role as a nutrient sensor, future studies aimed at understanding what factors underlie the regulation of FFA2 coupling and signaling in different physiologic and biochemical states will be of particular importance in helping to guide the development of FFA2 into a viable therapeutic target for T2D.
Methods
Animals
Heterozygous Ffar2 knockout mice (Ffar2+/−) on a C57BL/6J background were crossed to produce Ffar2 wild-type (WT) and Ffar2 knockout (Ffar2−/−) mice. Genotypes were determined as previously described42. Mice were housed in a temperature-controlled facility with 12-hour light-dark cycle and given ad libitum access to water and normal (LM-485, Harlan Laboratories, Indianapolis, IN) or high fat chow (TD.06414, Harlan Laboratories, started at 6 or 10 weeks of age). All experiments described in this report were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee at Northwestern University.
Tissue processing, immunolabeling and morphometric analysis
Mouse pancreata were immediately removed from euthanized mice, weighed and fixed in 4% paraformaldehyde in 1X PBS for 1 hour and kept in 30% sucrose in PBS overnight. The next day, pancreata were cryopreserved by embedding in optimal-cutting-temperature compound and stored at −80 °C until further processing. Pancreata were sectioned at 7 μm using a microtome-cryostat. For morphometric analysis, three to four non-overlapping sections from each pancreas (spanning the width of the pancreas) were used for each analysis. All analyses were conducted on at least 3 animals per age, genotype and treatment condition.
For determination of β cell mass, sections were immunolabeled with guinea pig anti-insulin and rabbit anti-glucagon primary antibodies and imaged by epifluorescence at 4X and 40X magnification. The total pancreatic area and insulin positive area of each section was measured using ImageJ software (http://imagej.nih.gov/ij/). β cell mass was estimated as the product of the relative β cell area and the wet weight of the pancreas.
β cell proliferation was examined by immunolabeling with guinea pig anti-insulin and rabbit anti-Ki67 primary antibodies. Islets were imaged at 40X and the number of insulin-positive and insulin- and Ki67-positive cells counted and proliferation was calculated as the percentage of Ki67-positive β cells.
β cell apoptosis was examined by immunolabeling with guinea pig anti-insulin and rabbit anti-cleaved caspase 3 primary antibodies. Islets were imaged at 40X and the number of insulin-positive cells counted. Cleaved caspase 3-positive cells were counted as apoptotic islet cells if they co-localized with insulin, or were located within the insulin-positive islet area.
The following primary antibodies were used, all diluted in antibody diluent buffer (Dako, Carpinteria, CA): guinea pig anti-insulin (1:200; Abcam, Cambridge, MA), rabbit anti-glucagon (1:200; Cell Signaling, Danvers, MA), rabbit anti-Ki67 (1:100; Cell Signaling), rabbit anti-cleaved caspase 3 (1:2000, Abcam, Cambridge, MA). Goat-derived secondary antibodies conjugated to Fluorescein or Texas Red were used at 1:200 dilution (Vector Laboratories, Burlingame, CA). All slides were mounted with Vectashield with DAPI (Vector Laboratories).
Islet isolation and culture
Islets were isolated by collagenase digestion as previously described28. Following isolation, islets were handpicked under a dissection microscope and left to recover overnight at 37 °C in RPMI 1640 supplemented with 10% FBS, 1% L-glutamine and 1% penicillin/streptomycin.
In vitro β cell proliferation assay
INS1 cells were cultured and maintained as previously described46. Cells were seeded at a density of 106 in a 60 mm dish and incubated overnight at 37 °C. The following day, cells were either maintained in culture media (control) or treated with the indicated agonist for 48 hours prior to analysis by flow cytometry. After the first 24 hour treatment period, BrdU was added to culture media at a concentration of 0.1 mg/ml. Following 48 hours of agonist treatment, cells were fixed, permeabilized and labeled with anti-BrdU antibody using a FITC BrdU Flow Kit purchased from BD Pharmingen (San Jose, CA), according to the manufacturer’s protocol. Cells were counted by flow cytometry, with a minimum of 30,000 events counted at a rate of no more than 400 events per second. Dead and apoptotic cells were excluded from counts by Annexin V and propidium iodide staining.
Ex vivo β cell proliferation assay
β cell proliferation was assessed by two separate methods. First, a blinded, small-scale ex vivo assay was performed on intact islets isolated from WT mice as previously described47. Compounds were initially screened at multiple concentrations (0.1–5 mM) to assess dose-response. Subsequent experiments were carried out with acetate, 2-BA and CPCA at the indicated concentrations, as determined by dose response curve. Placental lactogen (PL) at 0.5 ug/ml served as a positive control for β cell proliferation38. β cell proliferation was visualized by insulin and Ki67 immunolabeling in mildly dissociated islets. Slides were scanned using an Aperio ScanScope FL scanner and images processed as previously described48.
Second, proliferation was assessed by 5-bromo-2-deoxyuridine (BrdU) incorporation as previously described with slight modifications49. WT and Ffar2−/− islets were cultured for 4 days with media supplemented with or without prolactin (500 ng/mL); Exendin-4 (100 nM) or acetate (1 mM). The media was changed every second day. 500 ng/mL BrdU (Sigma) was added to the culture media during the last 24 hours. Islets were dispersed with 0.05% trypsin and fixed on poly-L-lysine coated coverslips with 4% paraformaldehyde. Epitopes were retrieved by treatment with 1N hydrochloric acid for 25 minutes at 37 °C. Immunostaining of BrdU and insulin was performed on processed islets and β-cell proliferation rate was determined by quantifying percentage of BrdU-positive insulin-positive cells. Following antibodies were used: primary-rat anti-BrdU (1:100, Abcam), guinea pig anti-insulin (1:100, Abcam); secondary-FITC goat anti-guinea pig IgG (1:200) (Vector Laboratories), AlexaFluor-594 goat anti-rat IgG (1:200, Life Technologies, Madison, WI).
Differential methylation analysis
Differentially methylated insulin DNA was measured by multiplex PCR analysis using terminal serum collected from 4–5 animals per age and genotype as previously described34. Unmethylation index is the ratio of unmethylated preproinsulin DNA to methylated preproinsulin DNA in serum.
Assessment of Ca2+ mobilization and cAMP accumulation by FFA2
Calcium mobilization was assessed in the βTC3 mouse β cell line, a cell line that does not express FFA3, a related receptor28. Cells were seeded overnight and serum starved in a 96-well black wall/clear bottom plate. On the day of the experiment, cells were loaded with 5 μM Fluo-8 (AAT Bioquest, Sunnyvale, CA) and incubated at 37 °C for 60 minutes. Compounds were next titrated at multiple concentrations and fluorescence was measured using a fluorometric imaging plate reader (FLIPR). For cAMP assays, CHO-K1 cells were transfected with an expression vector containing full-length human FFA2 cDNA (GenBank Accession Number NM_005306) with FLAG tag sequence at N-terminus and assays performed as previously described50. This assay was performed at Multispan, Inc. In each experiment, each compound was tested in quadruplicate at multiple concentrations. The pEmax and pEC50 were obtained for each compound based on concentration-response curves generated using GraphPad Prism.
RNA-seq and data analysis
Total cellular RNA was extracted from isolated islets (pooled from 2 mice) using an RNeasy Mini kit (QIAGEN, Venlo, Linburg). Experiments were run in triplicate for each genotype. RNA seq and data analyses were carried out by the Next Generation Sequencing Core, Center for Genetic Medicine, Northwestern University. Briefly, alignment and expression analysis were performed using TopHat (v2.0.8b) and Cufflinks (v2.1.1). Differential expression was determined by cuffdiff using an FDR cutoff value of 0.05. The results of the differential expression analysis were processed with the R package, cummerbund (http://compbio.mit.edu/cummeRbund/) to obtain up-and down-regulated genes.
Additional Information
How to cite this article: Villa, S. R. et al. Loss of Free Fatty Acid Receptor 2 leads to impaired islet mass and beta cell survival. Sci. Rep. 6, 28159; doi: 10.1038/srep28159 (2016).
References
Prentki, M. & Nolan, C. J. Islet beta cell failure in type 2 diabetes. The Journal of clinical investigation 116, 1802–1812, doi: 10.1172/JCI29103 (2006).
Meier, J. J. & Bonadonna, R. C. Role of reduced beta-cell mass versus impaired beta-cell function in the pathogenesis of type 2 diabetes. Diabetes care 36 Suppl 2, S113–119, doi: 10.2337/dcS13-2008 (2013).
Kendall, D. M., Sutherland, D. E., Najarian, J. S., Goetz, F. C. & Robertson, R. P. Effects of hemipancreatectomy on insulin secretion and glucose tolerance in healthy humans. The New England journal of medicine 322, 898–903, doi: 10.1056/NEJM199003293221305 (1990).
Menge, B. A. et al. Metabolic consequences of a 50% partial pancreatectomy in humans. Diabetologia 52, 306–317, doi: 10.1007/s00125-008-1219-1 (2009).
Butler, A. E. et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52, 102–110 (2003).
Meier, J. J. Beta cell mass in diabetes: a realistic therapeutic target? Diabetologia 51, 703–713, doi: 10.1007/s00125-008-0936-9 (2008).
Freathy, R. M. et al. Type 2 diabetes risk alleles are associated with reduced size at birth. Diabetes 58, 1428–1433, doi: 10.2337/db08-1739 (2009).
Hattersley, A. T. & Tooke, J. E. The fetal insulin hypothesis: an alternative explanation of the association of low birthweight with diabetes and vascular disease. Lancet 353, 1789–1792, doi: 10.1016/S0140-6736(98)07546-1 (1999).
Garofano, A., Czernichow, P. & Breant, B. In utero undernutrition impairs rat beta-cell development. Diabetologia 40, 1231–1234, doi: 10.1007/s001250050812 (1997).
Breant, B., Gesina, E. & Blondeau, B. Nutrition, glucocorticoids and pancreas development. Hormone research 65 Suppl 3, 98–104, doi: 10.1159/000091513 (2006).
Nielsen, J. H. et al. Regulation of beta-cell mass by hormones and growth factors. Diabetes 50 Suppl 1, S25–29 (2001).
Oh, Y. S. Mechanistic insights into pancreatic beta-cell mass regulation by glucose and free fatty acids. Anatomy & cell biology 48, 16–24, doi: 10.5115/acb.2015.48.1.16 (2015).
Ahren, B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nature reviews. Drug discovery 8, 369–385, doi: 10.1038/nrd2782 (2009).
Guettier, J. M. et al. A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proceedings of the National Academy of Sciences of the United States of America 106, 19197–19202, doi: 10.1073/pnas.0906593106 (2009).
Jain, S. et al. Chronic activation of a designer G(q)-coupled receptor improves beta cell function. The Journal of clinical investigation 123, 1750–1762, doi: 10.1172/JCI66432 (2013).
Xu, G., Stoffers, D. A., Habener, J. F. & Bonner-Weir, S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 48, 2270–2276 (1999).
Zhang, Y. et al. The role of G protein-coupled receptor 40 in lipoapoptosis in mouse beta-cell line NIT-1. Journal of molecular endocrinology 38, 651–661, doi: 10.1677/JME-06-0048 (2007).
Ruiz de Azua, I., Gautam, D., Guettier, J. M. & Wess, J. Novel insights into the function of beta-cell M3 muscarinic acetylcholine receptors: therapeutic implications. Trends in endocrinology and metabolism: TEM 22, 74–80, doi: 10.1016/j.tem.2010.10.004 (2011).
Regard, J. B. et al. Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. The Journal of clinical investigation 117, 4034–4043, doi: 10.1172/JCI32994 (2007).
Berger, M. et al. Galphai/o-coupled receptor signaling restricts pancreatic beta-cell expansion. Proceedings of the National Academy of Sciences of the United States of America 112, 2888–2893, doi: 10.1073/pnas.1319378112 (2015).
Kimple, M. E., Neuman, J. C., Linnemann, A. K. & Casey, P. J. Inhibitory G proteins and their receptors: emerging therapeutic targets for obesity and diabetes. Experimental & molecular medicine 46, e102, doi: 10.1038/emm.2014.40 (2014).
Ichimura, A., Hasegawa, S., Kasubuchi, M. & Kimura, I. Free fatty acid receptors as therapeutic targets for the treatment of diabetes. Frontiers in pharmacology 5, 236, doi: 10.3389/fphar.2014.00236 (2014).
Talukdar, S., Olefsky, J. M. & Osborn, O. Targeting GPR120 and other fatty acid-sensing GPCRs ameliorates insulin resistance and inflammatory diseases. Trends in pharmacological sciences 32, 543–550, doi: 10.1016/j.tips.2011.04.004 (2011).
Keller, M. P. et al. A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome research 18, 706–716, doi: 10.1101/gr.074914.107 (2008).
Rieck, S. et al. The transcriptional response of the islet to pregnancy in mice. Molecular endocrinology 23, 1702–1712, doi: 10.1210/me.2009-0144 (2009).
Layden, B. T. et al. Regulation of pancreatic islet gene expression in mouse islets by pregnancy. The Journal of endocrinology 207, 265–279, doi: 10.1677/JOE-10-0298 (2010).
Layden, B. T., Angueira, A. R., Brodsky, M., Durai, V. & Lowe, W. L., Jr. Short chain fatty acids and their receptors: new metabolic targets. Translational research : the journal of laboratory and clinical medicine 161, 131–140, doi: 10.1016/j.trsl.2012.10.007 (2013).
Priyadarshini, M. et al. An acetate-specific GPCR, FFAR2, regulates insulin secretion. Molecular endocrinology, me20151007, doi: 10.1210/me.2015-1007 (2015).
McNelis, J. C. et al. GPR43 potentiates beta cell function in obesity. Diabetes, doi: 10.2337/db14-1938 (2015).
Tang, C. et al. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nature medicine 21, 173–177, doi: 10.1038/nm.3779 (2015).
Weir, G. C. & Bonner-Weir, S. Islet beta cell mass in diabetes and how it relates to function, birth and death. Ann N Y Acad Sci 1281, 92–105, doi: 10.1111/nyas.12031 (2013).
Fuller, M. et al. The short-chain fatty acid receptor, FFA2, contributes to gestational glucose homeostasis. American journal of physiology. Endocrinology and metabolism 309, E840–851, doi: 10.1152/ajpendo.00171.2015 (2015).
Winzell, M. S. & Ahren, B. G-protein-coupled receptors and islet function-implications for treatment of type 2 diabetes. Pharmacology & therapeutics 116, 437–448, doi: 10.1016/j.pharmthera.2007.08.002 (2007).
Fisher, M. M., Perez Chumbiauca, C. N., Mather, K. J., Mirmira, R. G. & Tersey, S. A. Detection of islet beta-cell death in vivo by multiplex PCR analysis of differentially methylated DNA. Endocrinology 154, 3476–3481, doi: 10.1210/en.2013-1223 (2013).
Akirav, E. M. et al. Detection of beta cell death in diabetes using differentially methylated circulating DNA. Proceedings of the National Academy of Sciences of the United States of America 108, 19018–19023, doi: 10.1073/pnas.1111008108 (2011).
Husseiny, M. I. et al. Development of a quantitative methylation-specific polymerase chain reaction method for monitoring beta cell death in type 1 diabetes. PloS one 7, e47942, doi: 10.1371/journal.pone.0047942 (2012).
Lebastchi, J. et al. Immune therapy and beta-cell death in type 1 diabetes. Diabetes 62, 1676–1680, doi: 10.2337/db12-1207 (2013).
Nielsen, J. H., Svensson, C., Galsgaard, E. D., Moldrup, A. & Billestrup, N. Beta cell proliferation and growth factors. Journal of molecular medicine 77, 62–66 (1999).
Huang, C., Snider, F. & Cross, J. C. Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology 150, 1618–1626, doi: 10.1210/en.2008-1003 (2009).
Parsons, J. A., Brelje, T. C. & Sorenson, R. L. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology 130, 1459–1466, doi: 10.1210/endo.130.3.1537300 (1992).
Linnemann, A. K., Baan, M. & Davis, D. B. Pancreatic beta-cell proliferation in obesity. Advances in nutrition 5, 278–288, doi: 10.3945/an.113.005488 (2014).
Bjursell, M. et al. Improved glucose control and reduced body fat mass in free fatty acid receptor 2-deficient mice fed a high-fat diet. American journal of physiology. Endocrinology and metabolism 300, E211–220, doi: 10.1152/ajpendo.00229.2010 (2011).
Tolhurst, G. et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371, doi: 10.2337/db11-1019 (2012).
Park, S., Jiang, H., Zhang, H. & Smith, R. G. Modification of ghrelin receptor signaling by somatostatin receptor-5 regulates insulin release. Proceedings of the National Academy of Sciences of the United States of America 109, 19003–19008, doi: 10.1073/pnas.1209590109 (2012).
Maudsley, S., Martin, B. & Luttrell, L. M. The origins of diversity and specificity in g protein-coupled receptor signaling. The Journal of pharmacology and experimental therapeutics 314, 485–494, doi: 10.1124/jpet.105.083121 (2005).
Asfari, M. et al. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 130, 167–178, doi: 10.1210/endo.130.1.1370150 (1992).
Mosser, R. E. & Gannon, M. An assay for small scale screening of candidate beta cell proliferative factors using intact islets. BioTechniques 55, 310–312, doi: 10.2144/000114115 (2013).
Golson, M. L. et al. Activated FoxM1 attenuates streptozotocin-mediated beta-cell death. Molecular endocrinology 28, 1435–1447, doi: 10.1210/me.2014-1024 (2014).
Yamamoto, T. et al. Prolactin supplementation to culture medium improves beta-cell survival. Transplantation 89, 1328–1335, doi: 10.1097/TP.0b013e3181d98af1 (2010).
Whittle, B. J., Silverstein, A. M., Mottola, D. M. & Clapp, L. H. Binding and activity of the prostacyclin receptor (IP) agonists, treprostinil and iloprost, at human prostanoid receptors: treprostinil is a potent DP1 and EP2 agonist. Biochemical pharmacology 84, 68–75, doi: 10.1016/j.bcp.2012.03.012 (2012).
Acknowledgements
We thank Laura C. Alonso (Univ. of Massachusetts Medical School) for her advice on the islet proliferation assay. B.T.L. is supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Career Development (grant no. 1IK2BX001587-01), the National Institutes of Health under award number, R01DK104927-01A1 and The University of Chicago DR&TC (P30DK020595). S.R.V. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (F31DK102371), the Northwestern University Program in Endocrinology, Diabetes and Hormone Action (NIH T32 DK007169) and the Northwestern University Cellular and Molecular Basis of Disease training grant (NIH T32 GM08061). M.P. is supported by an American Heart Association Postdoctoral Fellowship (#15POST22410016). M.F. is supported by an American Heart Association pre-doctoral award (#15PRE25750015). M.G. is supported by a Merit Review award (1BX000990-01A1) from the Department of Veteran Affairs Tennessee Valley Health Authority and a grant from the Juvenile Diabetes Research Foundation (17-2012-26). R.E.M. was supported by a Mentor-based Postdoctoral award from the American Diabetes Association (7-10-BETA-03). B.A.C. was supported by the Vanderbilt University Training Program in Stem Cell and Regenerative Developmental Biology (T32 HD05702). R.G.M. is supported by NIH grants R01 DK060581 and UC4 DK104166 and S.A.T. is supported by a Junior Faculty Award from the American Diabetes Association. This study utilized core services of the Diabetes Research Center grant P30 DK097512 to Indiana University School of Medicine.
Author information
Authors and Affiliations
Contributions
Experiments were conceived and designed by B.T.L., S.R.V. and M.P. S.R.V. and B.T.L. wrote and edited the manuscript. M.G. and R.G.M. edited the manuscript. S.R.V. and M.P. conducted ex vivo β cell proliferation assays. M.H.F. performed in vitro β cell proliferation assays. S.R.V., T.B., M.R.B. and A.R.A. performed β cell mass and β cell proliferation analysis by IHC. R.E.M., B.A.C. and M.G. performed intact islet proliferation assays and beta cell analysis at P1. S.A.T. and R.G.M. conducted differential methylation analysis. H.M. conducted cAMP accumulation assays. A.G. conducted Ca2+ mobilization assays.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Villa, S., Priyadarshini, M., Fuller, M. et al. Loss of Free Fatty Acid Receptor 2 leads to impaired islet mass and beta cell survival. Sci Rep 6, 28159 (2016). https://doi.org/10.1038/srep28159
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep28159
This article is cited by
-
STAT3 dictates β-cell apoptosis by modulating PTEN in streptozocin-induced hyperglycemia
Cell Death & Differentiation (2020)
-
FFA2-, but not FFA3-agonists inhibit GSIS of human pseudoislets: a comparative study with mouse islets and rat INS-1E cells
Scientific Reports (2020)
-
The crosstalk of gut microbiota and chronic kidney disease: role of inflammation, proteinuria, hypertension, and diabetes mellitus
International Urology and Nephrology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.