Abstract
A tight regulation between the pro– and anti–inflammatory immune responses during plasmodial infection is of crucial importance, since a disruption leads to severe malaria pathology. IL-22 is a member of the IL-10 cytokine family, which is known to be highly important in immune regulation. We could detect high plasma levels of IL-22 in Plasmodium falciparum malaria as well as in Plasmodium berghei ANKA (PbA)-infected C57BL/6J mice. The deficiency of IL-22 in mice during PbA infection led to an earlier occurrence of cerebral malaria but is associated with a lower parasitemia compared to wt mice. Furthermore, at an early time point of infection T cells from PbA-infected Il22−/− mice showed an enhanced IFNγ but a diminished IL-17 production. Moreover, dendritic cells from Il22−/− mice expressed a higher amount of the costimulatory ligand CD86 upon infection. This finding can be corroborated in vitro since bone marrow-derived dendritic cells from Il22−/− mice are better inducers of an antigen-specific IFNγ response by CD8+ T cells. Even though there is no IL-22 receptor complex known on hematopoietic cells, our data suggest a link between IL-22 and the adaptive immune system which is currently not identified.
Similar content being viewed by others
Introduction
An important element of immune-regulatory mechanisms is the IL-10 cytokine family, which includes IL-22, a member of the IL-20 subfamily1. The characteristic feature of this subfamily is their ability to link the immune system with epithelial tissues. The IL-20 cytokines are mainly produced by cells of the immune system. However, the expression of the receptors is only shown on non-hematopoietic cells so far2, nevertheless recent studies were able to show an IL-22Rα1 expression on hematopoietic cells in autoimmunity diseases3,4. The IL-22 receptor is heterodimeric and is composed of the IL-22Rα1 chain and the IL-10Rβ chain. The binding of IL-22 to its receptor induces phosphorylation of the kinases Jak1 and Tyk2 followed by the phosphorylation of the transcription factors STAT1, STAT3 and STAT5 5,6 and leads to the maintenance of the homeostasis of epithelial cells at barrier surfaces7,8. The interaction of IL-22 with its receptor can be inhibited by a soluble IL-22 binding protein (IL-22BP or IL-22Rα2). IL-22BP is mainly expressed in mucosal tissues and in the spleen9. The IL-22BP-producing cell type is not exactly identified even though first findings show that CD103+ CD11b+ dendritic cells in the intestinal lamina propria produce IL-22BP under homeostatic conditions in response to retinoic acid9,10. Hence, the function of IL-22 on IL-22Rα1 expressing cells is controlled by the soluble IL-22BP, which has a higher binding affinity to IL-22 than IL-22Rα111. IL-22 is produced by different cells of the immune system including T, B, NK, γδ+ T cells and also ILC 3 cells12,13. IL-6, IL-21 and IL-23 are STAT3 activating cytokines which are able to induce IL-22 production by T cells and ILC 3 cells14,15,16. Other important pathways known to induce IL-22 in T cells are the Notch and aryl hydrocarbon receptor signaling17,18. The function of IL-22 in immunity is controversially discussed. In psoriasis the expression of IL-22 is increased; it promotes inflammation, epidermal hyperplasia and additionally induces chemokines and pro-inflammatory cytokines19. In inflammatory bowel disease IL-22 is highly expressed as well but has both protective and pathogenic effects, depending on the type of colitis20,21,22,23. In the liver, where the IL-22Rα1 chain is highly expressed on hepatocytes, IL-22 has protective effects24. Furthermore, IL-22 is known to play a role concerning the composition of the microbiota25, especially during infection26. Moreover, IL-22 is involved in antimicrobial host defence by the induction of antimicrobial peptides27,28,29.
Since IL-22 plays an elusive role in different immune disease or infection models it is of special interest to elucidate its role in malaria. An important finding concerning IL-22 in malaria was done by Koch et al.30. Since the IL22 gene is located in proximity to the IFNG gene which is highly important for malaria, it was taken into consideration for further investigations regarding the severity of malaria symptoms in a large case-control study. Two haplotypes of IL22 were found to induce either resistance or susceptibility to Plasmodium falciparum (P. falciparum) infection. This study brought attention to IL-22 for further investigations concerning its function in plasmodial infection. Further observations described that IL-22 plays a protective role during Plasmodium chabaudi (P. chabaudi) infection in mice31.
Besides P. chabaudi infection of mice there are three additional murine plasmodial species known to infect rodents: Plasmodium berghei, Plasmodium yoelii and Plasmodium vinckei. A widely established plasmodial model to investigate cerebral malaria symptoms is the infection of C57BL/6 mice with Plasmodium berghei ANKA (PbA)32. The plasmodial life cycle is divided into two stages: the asymptomatic liver stage and the blood-stage during which severe complications such as cerebral malaria can occur. Compared to the other strains, PbA is the most similar to P. falciparum concerning nucleotide identity (70.3%) and protein identity (62.9%)33 and causes similar symptoms in mice compared to the P. falciparum infection in human during the blood-stage32. P. falciparum infections still cause the majority of complicated malaria cases34. Cerebral malaria is the most severe complication of a plasmodial infection and can lead to death35. Especially in sub-Saharan Africa P. falciparum infections are one of the leading causes of morbidity and mortality of the disease. With the murine model of PbA infection in C57BL/6 mice the importance of the immune system for disease progression was highlighted. Cerebral malaria in this model was shown to be highly promoted by CD8+ T cells that adhere to activated brain endothelium as a consequence of TH1-dependent IFNγ production32. Especially IFNγ was shown to play an important role concerning the development of cerebral malaria during PbA infection36,37. To keep a well-balanced immune response to plasmodial infection the induction of regulatory mechanisms during malaria is indispensable, since the disruption of this balance leads to pathology. Important for these regulatory mechanisms are the cytokines TGFβ and IL-1038,39,40,41.
In this study, blood-stage infection of C57BL/6 mice with PbA was used to analyse the impact of IL-22 during malaria. During the blood-stage of PbA infection mice start to develop cerebral malaria symptoms at d6–8 post infection (p.i.). Here we could observe that Il22−/− mice showed a significantly earlier occurrence of cerebral malaria symptoms accompanied by a lower parasitemia compared to wt mice during PbA infection. In addition to this phenotype an early increase of IFNγ and an overall lower IL-17 production was observed in the absence of IL-22 during PbA infection. Taken together IL-22-deficient mice showed a stronger pro-inflammatory immune response during PbA infection resulting in an earlier occurrence of cerebral malaria symptoms but also resulting in lower parasitemia.
Results
Elevated levels of IL-22 in malaria
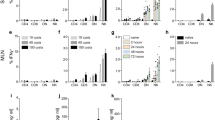
IL-22 is significantly increased in plasma samples of human individuals suffering from acute P. falciparum malaria compared to healthy controls (Fig. 1A). Also in the plasma of PbA-infected C57BL/6 mice, elevated levels of IL-22 at d6 p.i. were observed (Fig. 1B). Since the IL-22 level in plasma is increased in malaria, it was of interest to specify the cell type which is responsible for IL-22 production. To this end, C57BL/6 mice were infected with PbA iRBC and sacrificed at d6 p.i. Spleen cells were stimulated ex vivo, stained intracellularly for IL-22 and were subsequently analysed by flow cytometry. Even though the secretion of IL-22 was significantly increased in CD4+ T cells as well as in NK cells, the highest proportion of IL-22 secreting cells was observed in the γδ+ T cell population (Fig. 1C).
Increased IL-22 levels in plasmodial infections.
Plasma samples of patients with P. falciparum infection were compared to healthy controls (ncontrol = 9; nP. falciparum = 30) (A). Plasma samples of PbA iRBC-infected wt mice were compared to PBS treated control mice at d6 p.i. (ncontrol = 43; nPbA = 36) (B). The concentration of IL-22 in the plasma was analysed by ELISA. IL-22 producing cells were determined by flow cytometry. For this purpose wt mice were infected with 1 × 105 PbA iRBC i.p. or treated with PBS as control. At d6 p.i. mice were sacrificed and 2 × 106 splenocytes were stimulated for 6 h with PMA/Ionomycin, including the last 5 h with Monensin. The percentages of IL-22 secreting cells were determined by flow cytometry and are depicted in relation the their parent cell populations (nnaive = 3; nPbA d6 = 5). Depicted are the means ± SEM.
Lack of IL-22 results in a more severe malaria outcome
The infection with PbA sporozoites in the absence of IL-22 resulted in a lower parasitemia (Fig. 2A). To elucidate if this decreased parasitemia was due to a reduced parasite burden in the liver in the absence of IL-22, wt and Il22−/− mice were infected with 1000 sporozoites i.v. and the liver load of PbA parasites was determined by qPCR 24 h p.i. (Fig. 2B). However, no differences were observed regarding the parasite burden in the liver. Hence, the effect of IL-22 on the parasitemia seems to be restricted to the blood-stage. To further analyse how the absence of IL-22 is influencing the outcome of disease, wt and Il22−/− mice were infected with PbA iRBC and the course of the infection was monitored (Fig. 2C). Interestingly, the lack of IL-22 led to an earlier occurrence of cerebral malaria (CM). A similar result was observed by transiently neutralizing IL-22 with a specific antibody in wt mice compared to IgG treated control mice (Fig. 2D). In order to control if the lack of IL-22 during PbA infection is associated with increased inflammation in the liver ALT (Fig. 2E) and AST (Fig. 2F) levels were analysed in PbA iRBC-infected wt and Il22−/− mice at d6 p.i. Even though an induction of ALT was observed in PbA iRBC-infected wt mice compared to the naive group, there were no differences visible during PbA iRBC infection between wt and Il22−/− mice.
Impact of IL-22 in the course of PbA infection.
Wt and Il22−/− mice were infected with 1 × 105 PbA iRBC i.p.. The parasitemia was analysed at d6 p.i. (nwt = 6;  = 6) (A). Wt and Il22−/− mice were infected with 1000 sporozoites i.v. The parasite burden in the liver was determined 24 h after sporozoite infection by qPCR (nwt = 5;
= 6) (A). Wt and Il22−/− mice were infected with 1000 sporozoites i.v. The parasite burden in the liver was determined 24 h after sporozoite infection by qPCR (nwt = 5;  = 6) (B). Survival of PbA iRBC-infected wt and Il22−/− mice were monitored during the course of infection (nwt = 6;
= 6) (B). Survival of PbA iRBC-infected wt and Il22−/− mice were monitored during the course of infection (nwt = 6;  = 6) (C). Wt mice were infected with 1 × 105 PbA iRBC i.p. and simultaneously treated with 20 μg αIL-22 or control IgG, subsequently the course of infection was monitored (nwt = 5;
= 6) (C). Wt mice were infected with 1 × 105 PbA iRBC i.p. and simultaneously treated with 20 μg αIL-22 or control IgG, subsequently the course of infection was monitored (nwt = 5;  = 5) (D). ALT (E) and AST (F) levels in the plasma were analysed in the control groups and in PbA iRBC-infected wt and Il22−/− mice at d6 p.i. (nwt naive = 2;
= 5) (D). ALT (E) and AST (F) levels in the plasma were analysed in the control groups and in PbA iRBC-infected wt and Il22−/− mice at d6 p.i. (nwt naive = 2;  naive = 2; nwt PbA d6 = 5;
naive = 2; nwt PbA d6 = 5;  PbA d6 = 5). Depicted are the means ± SEM.
PbA d6 = 5). Depicted are the means ± SEM.
Lack of IL-22 induces a decreased parasitemia during PyNL infection in mice
In order to follow the outcome of IL-22-deficiency on the parasitemia for a longer time period, we used Plasmodium yoelii non-lethal (PyNL) infection of C57BL/6 mice. This Plasmodium species led to a transient parasitemia, which is cleared within three weeks without inducing cerebral pathology. As in the PbA model, PyNL-infected Il22−/− mice suffer from a decreased parasitemia at d7 p.i. compared to PyNL-infected wt mice (Fig. 3A). Furthermore, the administration of a neutralizing IL-22 antibody at the time point of infection revealed a significantly decreased parasitemia compared to the control group over the course of infection (Fig. 3B).
Impact of IL-22 on the parasitemia during P. yoelii NL infection.
Wt and Il22−/− mice were infected with 2 × 104 P. yoelii NL iRBC i.p. The parasitemia was analysed at d7 p.i. (nwt = 5;  = 6) (A). Wt mice were infected with 2 × 104 P. yoelii NL iRBC i.p. and simultaneously treated with 20 μg of αIL-22 or PBS as control (ncontrol = 5; nαIL-22 = 5). The parasitemia curve was analysed over a time period of three weeks (B). Depicted are the means ± SD.
= 6) (A). Wt mice were infected with 2 × 104 P. yoelii NL iRBC i.p. and simultaneously treated with 20 μg of αIL-22 or PBS as control (ncontrol = 5; nαIL-22 = 5). The parasitemia curve was analysed over a time period of three weeks (B). Depicted are the means ± SD.
Increased TH1 response in Il22−/− mice during PbA infection
Due to the increased incidence of CM in IL-22-deficient mice and since it is known that the disruption of the tight regulation between pro-inflammatory and anti-inflammatory response during malaria leads to a severe pathology, the secretion of the pro-inflammatory cytokines IFNγ and IL-17 by T cells in Il22−/− mice was analysed. Therefore, mice were infected with PbA iRBC and splenic cells were stimulated ex vivo with PMA/Ionomycin. An increased level of IFNγ producing cells was observed in Il22−/− mice compared to wt mice at d3 p.i. This effect was visible in all analysed cell types including CD3+ (Fig. 4A,H), CD8+ (Fig. 4B,I), CD3+ CD8− (Fig. 4C,J) and γδ+ (Fig. 4D,K) T cells. In contrast, the IL-17 secretion was decreased in CD3+ CD8− (Fig. 4F,J) and γδ+ (Fig. 4G,K) T cells at d3 p.i., which might be due to the antagonistic effect of IFNγ on IL-17 prodcution42. The increased IFNγ secretion at d3 post PbA infection by CD3+ T cells was confirmed in mice in which IL-22 was transiently neutralized compared to the control group (Fig. 4E). Even though the secretion of IFNγ was not different on d6 p.i., the IL-17 secretion of Il22−/− mice was still significantly decreased in CD3+ CD8− (Fig. 4F) and γδ+ (Fig. 4F) T cells. Nevertheless, the IFNγ concentration in the supernatant of antigen specific stimulated splenocytes from PbA-infected Il22−/− mice at d6 p.i. was still significantly increased compared to splenocytes from PbA-infected wt mice (Fig. 5A). To gain a closer look at the mechanism behind this, bone marrow derived dendritic cells (BMDCs) from wt and Il22−/− mice were generated and used as antigen presenting cells (APCs). CD8+ T cells of PbA iRBC-infected wt and Il22−/− mice were incubated with PbA antigen-pulsed BMDCs for 24 h and subsequently the IFNγ secretion in the supernatant was analysed using ELISA. This approach showed that CD8+ T cells from Il22−/− mice secrete more IFNγ after ex vivo antigen specific stimulation and furthermore also BMDCs from Il22−/− mice showed an improved stimulatory effect on wt and Il22−/− CD8+ T cells compared to wt BMDCs regarding the ability to induce IFNγ secretion by CD8+ T cells (Fig. 5B).
IFNγ and IL-17 secretion in PbA infection in the absence of IL-22.
Splenic cells were isolated from PbA-infected wt and Il22−/− mice at d3 (nwt = 5;  = 5) and d6 (nwt = 5;
= 5) and d6 (nwt = 5;  = 4/5) p.i.. These splenocytes were stimulated for 4 h with PMA/Ionomycin including the last 3 h with Monensin. The gated single cell populations of lymphocytes were additionally gated for CD3+ (A,H), CD8+ (B,I), CD3+ CD8− (representing CD4+ T cells, since the CD4 receptor is internalized upon stimulation) (C,F,J) and γδ+ T cells (D,G,K). These populations were subsequently analysed for IFNγ and IL-17 secretion by flow cytometry. The IFNγ secretion by CD3+ T cells was additionally analysed from anti-IL-22 treated and PbA iRBC-infected mice and compared to the IgG treated control mice. Depicted are the means.
= 4/5) p.i.. These splenocytes were stimulated for 4 h with PMA/Ionomycin including the last 3 h with Monensin. The gated single cell populations of lymphocytes were additionally gated for CD3+ (A,H), CD8+ (B,I), CD3+ CD8− (representing CD4+ T cells, since the CD4 receptor is internalized upon stimulation) (C,F,J) and γδ+ T cells (D,G,K). These populations were subsequently analysed for IFNγ and IL-17 secretion by flow cytometry. The IFNγ secretion by CD3+ T cells was additionally analysed from anti-IL-22 treated and PbA iRBC-infected mice and compared to the IgG treated control mice. Depicted are the means.
IFNγ secretion of PbA peptide stimulated splenocytes and CD8+ T cells of PbA-infected mice.
Wt and Il22−/− mice were infected with 1 × 105 PbA iRBC. At d6 p.i. mice were sacrificed and the splenocytes were stimulated with 1 μg/mL PbA peptide for 24 h. The IFNγ concentration in the supernatant was analysed with ELISA (nwt; w/o = 4;  ; w/o = 4; nwt n.inf; PbA Peptide = 4; nwt; PbA Peptide = 4; n
; w/o = 4; nwt n.inf; PbA Peptide = 4; nwt; PbA Peptide = 4; n ; PbA Peptide = 4) (A). Wt and Il22−/− mice were infected with 1 × 105 PbA iRBC. At d6 p.i. mice were sacrificed and splenic CD8+ T cells were purified. CD8+ T cells of Il22−/− or wt mice were subsequently incubated on previously PbA peptide pulsed Il22−/− or wt BMDCs. After an incubation time of 24 h the IFNγ concentration in the supernatant was analysed with an ELISA (nwt CD8+; w/o PbA Peptide; wt BMDC = 8; nwt CD8+; PbA Peptide; wt BMDC = 6;
; PbA Peptide = 4) (A). Wt and Il22−/− mice were infected with 1 × 105 PbA iRBC. At d6 p.i. mice were sacrificed and splenic CD8+ T cells were purified. CD8+ T cells of Il22−/− or wt mice were subsequently incubated on previously PbA peptide pulsed Il22−/− or wt BMDCs. After an incubation time of 24 h the IFNγ concentration in the supernatant was analysed with an ELISA (nwt CD8+; w/o PbA Peptide; wt BMDC = 8; nwt CD8+; PbA Peptide; wt BMDC = 6;  CD8+; PbA Peptide; wt-BMDC = 6; nwt CD8+; PbA Peptide; Il22−/− BMDC = 8;
CD8+; PbA Peptide; wt-BMDC = 6; nwt CD8+; PbA Peptide; Il22−/− BMDC = 8;  CD8+; PbA Peptide; Il22−/− BMDC = 8) (B). Depicted are the means ± SEM.
CD8+; PbA Peptide; Il22−/− BMDC = 8) (B). Depicted are the means ± SEM.
Modified antigen presentation in the absence of IL-22
To elucidate why BMDCs from Il22−/− mice have an improved ability to stimulate IFNγ secretion by CD8+ T cells, the expression of costimulatory ligands on antigen presenting cells from the spleen were analysed during PbA infection. For this purpose wt and Il22−/− mice were infected with PbA iRBC and CD11c+CD11b− dendritic cells were analysed for their CD86 expression at d3 p.i. (Fig. 6A). Dendritic cells from IL-22-deficient mice express significantly more CD86 compared to dendritic cells from wt mice at this early time point of infection (Fig. 6B). This result was confirmed by the restimulation of BMDCs with LPS over 24 h. In this case the expression of CD86 and CD80 was significantly higher after stimulation on BMDCs from Il22−/− mice compared to BMDCs from wt mice (Fig. 6F). However, recombinant IL-22 was unable to inhibit LPS-induced expression of CD86 and CD80 on BMDCs (Fig. 6D). Since T cells are stronger activated in Il22−/− mice during PbA infection it was of interest to analyse if the proliferation of T cells in Il22−/− mice is altered in vivo. Therefore, splenic OT1 T cells were labelled with CFSE and transferred into Il22−/− and wt recipient mice. Subsequently the recipients were infected with a transgenic OVA expressing PbA strain (PbA-OVAtg) or with a transgenic GFP expressing PbA strain (PbA-GFP) as control. Even though the proliferation of CFSE+ CD8+ T cells was highly synchronous in Il22−/− and wt mice (Fig. 7A), the lack of IL-22 resulted in an increased fraction of CD8+ T cells that start to proliferate. Hence this led to a significant increase in the recovery of proliferating OT1 T cells in Il22−/− recipient mice after infection with PbA-OVA on d5 p.i. (Fig. 7B).
Increased induction of costimulatory receptors on dendritic cells in the absence of IL-22.
PbA-infected wt and Il22−/− mice were sacrificed at d3 p.i. and splenic cells were isolated. The exemplary histogram shows the CD86 expression of CD11c+ CD11b− splenocytes from wt and Il22−/− mice in comparison to the fluorescence minus one control for CD86 (FMO-CD86) (A). CD11c+ CD11b− splenocytes from wt and Il22−/− mice were analysed regarding their CD86 expression (nwt = 5;  = 5) (B). Indicated are the means. BMDCs were stimulated with 0.1 μg/mL LPS for 24 h. The induction of CD86 and CD80 expression was determined by comparing the untreated samples with the LPS stimulated samples (C). The mean of four independent experiments is shown. Depicted are the means ± SEM. BMDCs of wt mice were stimulated with 0.1 ng/mL LPS in the presence of 0.05 μg/mL rIL-22 and the expression of CD86 and CD80 on the pre-gated lymphocyte population was determined (n−;− = 5; n −;rIL-22 = 5; nLPS;- = 5; n LPS;rIL-22 = 5) (D). Indicated are the means.
= 5) (B). Indicated are the means. BMDCs were stimulated with 0.1 μg/mL LPS for 24 h. The induction of CD86 and CD80 expression was determined by comparing the untreated samples with the LPS stimulated samples (C). The mean of four independent experiments is shown. Depicted are the means ± SEM. BMDCs of wt mice were stimulated with 0.1 ng/mL LPS in the presence of 0.05 μg/mL rIL-22 and the expression of CD86 and CD80 on the pre-gated lymphocyte population was determined (n−;− = 5; n −;rIL-22 = 5; nLPS;- = 5; n LPS;rIL-22 = 5) (D). Indicated are the means.
Proliferation of CFSE labelled OT1 splenocytes in wt and Il22−/− mice.
Splenocytes were isolated from OT1 mice, labelled with CFSE and subsequently transferred in wt and Il22−/− recipient mice. After 3 hours these mice were either infected with PbA-GFP as a control or PbA-OVAtg. Mice were sacrificed at d5 p.i. and subsequently the proliferation of CFSE+ CD8+ lymphocytes (CFSE dilution) (A) and the relative amount of CFSE+ cells were analysed out of CD8+ lymphocytes (nwt PbA-GFP = 2;  PbA-GFP = 2; nwt PbA-OVAtg = 4;
PbA-GFP = 2; nwt PbA-OVAtg = 4;  PbA-OVAtg = 4) (B). Depicted are the means.
PbA-OVAtg = 4) (B). Depicted are the means.
Discussion
In a previous study Koch et al. discovered two IL-22 haplotypes which influence the severity of malaria in West African patients30. The aim of the present study was to further elucidate the function of IL-22 using experimental malaria models. We find elevated level of IL-22 in the blood plasma of PbA-infected mice. Similar IL-22 levels are present in the blood plasma of P. falciparum-infected human individuals, further supporting the relevance of this cytokine in malaria.
The infection of C57BL/6 mice with PbA is one of the closest models to mimic the pathology of P. falciparum induced cerebral malaria in humans32,43. Hence this murine model was applied to elucidate the effect of IL-22 on the outcome of malaria. Here we could show that the absence of IL-22 in murine malaria exacerbates cerebral malaria symptoms. That IL-22 plays a protective role was already found during P. chabaudi infection31. Also in other diseases like multiple sclerosis44 or hepatitis24,45 IL-22 were shown to exert protective functions. During a P. chabaudi infection CD4+ and CD8+ T cells were shown to produce IL-2231, whereas in a PbA infection γδ+ T cells beside others showed the highest ability to produce IL-22. γδ+ T cells are known to play a crucial role in the development of cerebral malaria46. They expand early during plasmodial infection47 and human γδ+ T cells are the major producers of IFNγ upon in vitro re-stimulation with schizonts antigens48. Similarly we observed an early production of IFNγ by γδ+ T cells. Interestingly the early IFNγ production by γδ+ T cells was significantly increased in IL-22-deficient mice, which might lead to the lower parasitemia in IL-22-deficient mice. The lower parasitemia in the absence of IL-22 signalling was also found using infection with the PyNL strain, which indicate an effect of IL-22 independent of the plasmodial strain used. In the absence of the closely related cytokine IL-10 a similar result was already described49. The infection of IL-10-deficient mice with P. chabaudi lead to a lower parasitemia and an enhanced mortality38,39.
Also the transient neutralization of IL-22 by antibodies induced a similar phenotype as seen in Il22−/− mice. This excludes that compensatory mechanisms in Il22−/− mice are responsible for the observed phenotype. It is already known that IL-22 deficiency may alter the microbiome of mice and therefor may influence the course of malaria indirectly. However it is unlikely that the transient neutralization of IL-22 alters the microbiome till the occurrence of cerebral symptoms25,26.
It is known that IL-10-defiency leads to hepatic pathology during murine malaria40. Thus liver damage was analysed in Il22−/− mice. Even though hepatocytes express high levels of the IL-22Rα1 chain29, we observed no different levels of liver transaminases in PbA-infected wt and Il22−/− mice. Moreover IL-10 is known to have a regulatory effect on development of TH1 cell50,51. Thus we analysed if the closely related cytokine IL-225 may have similar effects on the T cell response during murine malaria. One key player during the antimalarial immune response is IFNγ which is the hallmark cytokine of a TH1 dominated immune response. The high IFNγ production of different splenic lymphocyte subsets at an early time point of PbA infection and the enhanced susceptibility for cerebral malaria symptoms in the absence of IL-22 might be due to an enhanced TH1 response during PbA infection52. Even though the IFNγ production is comparable between wt and Il22−/− mice at d6 p.i., at d3 p.i. T cells from Il22−/− mice produce significantly more IFNγ. Interestingly a significantly reduced production of IL-17 is detectable regardless of the time point after infection. The decreased IL-17 production in Il22−/− mice might be a consequence of the early increased IFNγ production by T cells in the absence of IL-22, since IFNγ is known to suppress TH17 differentiation53,54. Hence we assume that the TH1 differentiation is more favoured in IL-22-deficient mice. This increased IFNγ production was also observed in the supernatant of antigen-specifically stimulated splenocytes from PbA-infected Il22−/− mice using PbA-specific peptides55,56. To further identify the reasons for an increased activation of T cells we studied the function of antigen-presenting cells. Interestingly, CD8+ T cells from PbA-infected Il22−/− mice showed already a higher IFNγ production upon re-stimulation with wt BMDCs compared to CD8+ T cells of PbA-infected wt mice. This difference in IFNγ production was even more pronounced if the CD8+ T cells were re-stimulated with BMDCs of Il22−/− mice. Moreover, the re-stimulation of CD8+ T cells by BMDCs generated from Il22−/− mice induced a stronger IFNγ release regardless of the origin of the CD8+ T cells. This finding goes along with the observations that the transient neutralization of IL-22 in the beginning of plasmodial infection leads to the same malaria pathology, concluding that IL-22 has an influence on the activation of the adaptive immune system. The increased ability to stimulate T cells of Il22−/− BMDC might be explained by their increased expression of the costimulatory molecule CD86 on dendritic cells in the spleens of PbA-infected Il22−/− mice compared to wt mice. Thus IL-22 may dampen directly or indirectly the expression of costimulatory ligands of DCs during PbA infection. However, the addition of recombinant IL-22 during LPS stimulation of BMDCs was unable to inhibit the expression of CD86 and CD80, which might be due to an overly strong stimulatory effect of LPS. In addition in was shown recently that several subtypes of DCs produce IL-22BP constitutively10, this might interfere with the direct interaction between IL-22 and BMDCs during this experiment. An alternative explanation would be that IL-22 influences maturation of DCs indirectly by inducing factors on yet unknown cells that interfere with DCs. Interestingly, using the infection with Listeria monocytogenes no increase of the antigen-specific T cell response in Il22−/− mice was observed24. However in this infection model it remains unclear if IL-22 is induced and the absence of any effect of IL-22-deficieny on adaptive immunity might be due the lack of IL-22-induced signalling during Listeria monocytogenes infection.
Considering the enhanced IFNγ release and the increased expression of costimulatory ligands by DCs, it was of interest if the proliferation of PbA-OVAtg specific CD8+ T cells in vivo is improved in the absence of IL-22. Even though the proliferation of CFSE+ CD8+ T cells was highly synchronous in wt and Il22−/− mice at d5 p.i. the fraction of cells that start to proliferate was higher in IL-22-deficient mice leading to the assumption that the lack of IL-22 leads to an increased antigen specific activation of T cells and to a better survival or an altered migration of activated T cells.
Taken together the low parasitemia and the earlier onset of cerebral malaria in the absence of IL-22 might be due to the enhanced expression of costimulatory receptors by DCs followed by an increased activation of antigen specific CD8+ T cells and the earlier IFNγ production of different lymphocyte subsets.
Nevertheless it remains unclear how IL-22 modulates the immune response during PbA infection. In other diseases the expression of the IL-22Rα1 chain was already shown on hematopoietic cells3,4 although it was for long postulated that these cells do not express the IL-22Rα1 chain2,29. Therefore the idea that cells of the immune system are completely absent of an IL-22Rα1 chain expression has to be reconsidered. However, further investigations are needed to elucidate that IL-22 directly acts on hematopoietic cells expressing the IL-22Rα1 chain during malaria. It is also possible that IL-22 interacts with non-hematopoietic cells and indirectly modulates the immune system via yet unidentified factors.
Materials and Methods
Ethics
C57BL/6J (wt), Tg(TeraTerb)1100MjB (OT1), NMRI and Il22−/− mice were bred in the animal facility of the Bernhard Nocht Institute for Tropical Medicine. Il22−/− mice were described previously24 and were used when backcrossed 12 times on C57BL/6 background. For all Il22−/− and wt comparisons co-housed mice were used. For experiments using only wt mice, animals were purchased from Charles River (Sulzfeld, Germany). All animal experiments were performed in agreement with the German animal protection law under the supervision of a veterinarian. The responsible federal health Authorities of the State of Hamburg (Behörde für Gesundheit und Verbraucherschutz) has reviewed and approved the experimental protocol under the permission number 32/15. Mice were sacrificed by cardiocentesis under deep CO2 narcosis. All animals were used at the age of 7 to 10 weeks and were kept in individually ventilated cages under specific pathogen free conditions.
Human samples
Plasma from 30 patients with acute P. falciparum malaria was used. These were obtained from travellers treated at the University Hospital Eppendorf in Hamburg. Samples were obtained after the first and fourth day of the treatment and display a parasitemia ranging from 0.01 to 5%. Diagnosis was performed in the diagnostic department of the Bernhard Nocht Institute for Tropical Medicine. 9 control samples were obtained from healthy donors. Ethical approval was obtained from the Ethikkommission Hamburg and all experiments were carried out in accordance with the relevant guidelines. Written informed consent was obtained from all enrolled participants prior to inclusion in the study.
Plasmodium parasites and infections
PbA and PyNL strains were stored in liquid nitrogen in a solution containing 0.9% NaCl, 4.6% sorbitol and 35% glycerol. Pb-GFP and Pb-OVAtg were a kind gift of W.R. Heath57. To start an experiment, mice were injected with the respective Plasmodium strain intraperitoneal (i.p.) and the blood was collected after 5 to 6 days post infection (p.i.). Infected red blood cells (iRBCs) were subsequently used to infect the experimental mice with either 1 × 105 iRBC PbA or 2 × 104 iRBC PyNL i.p. To maintain the life cycle of plasmodia NMRI mice with a high parasitemia were anesthetized and used for a blood meal of Anopheles stephensi (A. stephensi) mosquito at the mosquito colony of the Bernhard Nocht Institute for Tropical Medicine. After 18 to 25 days the sporozoites were isolated by manual dissection of the salivary glands of mosquitos. For further experiments 1000 sporozoites were injected i.v. into each mouse. PbA-infected C57BL/6 mice develop cerebral malaria symptoms, which include the occurrence of neurological symptoms such as ataxia, convulsion and coma. These symptoms usually appear between day 6 and 8 p.i. and ultimately lead to dead. Mice developing cerebral symptoms were scored daily as described previously (score 0: no symptoms; score 1: deceleration and weight loss <10%; score 2: ruffled fur; score 3: weight loss <20%, strong deceleration, paralysis of one leg; score 4: coma; score 5: dead)58. To avoid unnecessary suffering, mice were euthanized when reaching score 3 and were registered as dead in Kaplan-Meier survival curve statistics, since mice developing score 3 never cleared symptoms in our model and would progress to score 5 within several hours. All experiments were in accordance with the local Animal Ethics Committee Regulations.
Analysis of parasitemia
A small blood drop was collected from the tail vein to determine blood-stage parasitemia in a thin blood smear using WRIGHT stain. The sporozoite liver burden was analysed after 24 h. The livers of sporozoite-infected mice were perfused with PBS and subsequently frozen in liquid nitrogen. The RNA of whole liver was purified using TRIzol reagent (Life Technologies) and reversed transcribed into cDNA using the Maxima First Strand cDNA Kit (Thermo Scientific). The sporozoite liver burden was analysed via an 18S qPCR specific for plasmodial ribosomal RNA (18S_primer fwd: 5′-ggtgtattcdctttatttaatgctt-3, 18S_primer rev: 5′-cacgcgtgcagcctagtat-3′; GAPDH_primer fwd: 5′-ggggcgtgcagcctagtat-3′, GAPDH_primer rev: 5′-ccttccacaatgccaaagtt-3′)59. The quantification of the sporozoite liver load was determined using a SYBR Green qPCR (Thermo Scientific Maxima SYBR Green qPCR Master Mix) with following conditions: Tinitial DNA denaturation = 95 °C, 900 sec; (TDNA denaturation = 95 °C, 15 sec; Tprimer annealing = 50 °C, 20 sec; Telongation = 68 °C, 20 sec) × 35; Tfinal elongation = 72 °C, 30 sec; melting curve.
Analysis of transaminases
PbA-infected mice were sacrificed at d6 p.i., the blood was collected in EDTA coated tubes and centrifuged at 5000 rpm, 15 min at RT. The plasma was stored at −20 °C. ALT and AST levels out from the plasma were analysed in 1 to 3 dilution in PBS with the Reflotron test system (Roche).
Antibodies and flow cytometry
IL-22 neutralization in vivo was performed using 20 μg αIL-22 (R&D, polyclonal goat IgG) i.p. per mouse at d0 p.i. Control mice were injected with the corresponding goat IgG (R&D) or with PBS. Mice were infected with 1 × 105 PbA iRBC i.p. and sacrificed at d3 p.i. For flow cytometry analysis, single-cell suspensions of splenic cells were obtained by mashing spleens through a 70 μm sieve into complete DMEM medium including 10% foetal calf serum, 1% L-glutamine and 0.5% penicillin/streptavidin. Erythrocyte lysis was performed subsequently for 5 min at RT. 2 × 106 cells were stimulated in the presence of 50 ng/mL PMA and 500 ng/mL Ionomycin for four hours including the last three hours with 2 μM Monensin for the detection of IL-17 and IFNγ and for six hours including the last five hours with 2 μM Monensin for the detection of IL-22. Intracellular stainings were performed using the Foxp3 transcription factor staining buffer (eBioscience). The following antibodies were used for flow cytometry analysis: αCD3 PerCP Cy5.5 (clone 145-2C11, Biolegend), αCD8 eFluor 450 (clone 53-6.7, eBioscience), αCD11b V450 (clone M1/70, BD Horizon), αCD11c APC (clone N418, Biolegend), αCD11c PE-Cy7 (clone N418, Biolegend), αCD80 FITC (clone 16-10A1, BD Pharmingen), αCD86 APC (clone GL-1, Biolegend), α γδT PE-Cy7 (clone GL3 Biolegend), αIFNγ PE (clone XMG 1.2, eBioscience), αIL-17 APC (clone 17B7, eBioscience), αIL-22 PE (clone 1H8PWSR, eBioscience), αNK1.1 Alexa Fluor 488 (clone PK 136, BD Pharmingen). Samples were measured on a BD LSRII or a BD FACSAria III. FACS data were analysed using the software FlowJo.
CFSE proliferation assay
Single cell suspension of OT1 splenocytes were incubated with 1 μM CFSE for 2 min 40 sec and subsequently washed twice with PBS. 2 × 107 OT1 splenocytes were transferred i.v. After 4 hours the mice were infected with 1 × 105 PbA iRBC i.p. and sacrificed at d5 p.i. Splenocytes were stained with αCD8 eFluor 450 (clone 53-6.7, eBioscience) and analysed at the LSR II regarding their CFSE dilution.
Cytokine analysis
PBS or PbA-infected mice were sacrificed at d6 p.i. and blood was collected via heart puncture in EDTA coated tubes. The blood plasma was obtained after centrifugation at 5000 rpm 15 min at RT and stored at −20 °C. The blood of P. falciparum-infected human individuals was centrifuged at 5000 rpm for 15 min at RT and stored at −20 °C. The human or murine IL-22 ELISA (eBioscience) was performed according to instructions of the manufacturer. Murine and human plasma samples were used in a 1 to 5 dilution and the OD was measured at 450 nm. Splenocytes of infected mice were stimulated with 1 μg/mL of a mixture of PbA peptides (Pb1: SQLLNAKYL; Pb2: IITDFENL; F4: EIYIFTNI)55,56 (Jerini Biotools) for 24 h at 37 °C and 9% CO2 in complete DMEM medium. The supernatant was stored at −20 °C. The IFNγ concentration in collected supernatants was analysed with the DuoSet ELISA development kit (R&D Systems).
Preparation of bone marrow derived dendritic cells (BMDCs)
Cells were collected out of the femur and tibia. Subsequently, these cells were treated with erythrocyte lysis buffer, the cell number determined and 3 × 106 cells were seeded per 10 cm dish. Bone marrow derived dendritic cells were generated in complete DMEM medium in the presence of granulocyte macrophage colony-stimulating factor (GM-CSF) for 7 days. GM-CSF containing complete DMEM medium was added to the culture each third day additionally. At d7 BMDCs were used for further experiments.
BMDCs stimulation
1 × 105 BMDCs were seeded in uncoated polystyrol well of a 96 well plate and stimulated with 0.1 μg/mL LPS (Sigma-Aldrich) with or without 0.05 μg/mL rIL-22 (PeproTech) for 24 h in complete DMEM medium at 37 °C and 9% CO2. BMDCs were subsequently collected and prepared for FACS staining.
In vitro CD8+ T cell stimulation
2000 BMDCs were seeded per well in a 96 round bottom-plate and pulsed with 1 μg/mL PbA Peptide for 4 h in complete DMEM medium at 37 °C and 9% CO2. After one washing step with PBS, 5 × 104 splenic CD8+ T cells, which were enriched through magnetic cell separation (MACS, Miltenyi) to a purity of >95%, were added to each well. The culture was incubated for 24 h at 37 °C at 9% CO2 in complete DMEM medium. The supernatant was stored at −20 °C for further analysis.
Isolation of primary Hepatocytes
Primary Hepatocytes (pHepa) were isolated as described in Burghardt et al.60. Subsequently isolated cells were stored in TRIzol reagent (Life Technologies) at −70 °C.
Statistical analysis
The GraphPad Prism 5 software was used to perform statistical analysis. Statistical differences in survival were analysed using Kaplan-Meier survival curve statistics. Additional statistical differences were identified using an unpaired t-test. For parasitemia testing the Mann-Whitney test was performed, assuming no Gaussian distribution for tested experiments. A regular two way ANOVA with a Bonferroni post-test was performed for pooled data. p-Values were indicated as *p < 0.05; **p < 0.01; ***p < 0.001. All experiments were performed at least twice and indicated error bars are based on SEM values.
Additional Information
How to cite this article: Sellau, J. et al. IL-22 dampens the T cell response in experimental malaria. Sci. Rep. 6, 28058; doi: 10.1038/srep28058 (2016).
References
Rutz, S., Wang, X. & Ouyang, W. The IL-20 subfamily of cytokines–from host defence to tissue homeostasis. Nat Rev Immunol 14, 783–795, doi: 10.1038/nri3766 (2014).
Wolk, K., Kunz, S., Asadullah, K. & Sabat, R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol 168, 5397–5402 (2002).
Justa, S., Zhou, X. & Sarkar, S. Endogenous IL-22 plays a dual role in arthritis: regulation of established arthritis via IFN-gamma responses. PLoS One 9, e93279, doi: 10.1371/journal.pone.0093279 (2014).
Ciccia, F. et al. Interleukin (IL)-22 receptor 1 is over-expressed in primary Sjogren’s syndrome and Sjogren-associated non-Hodgkin lymphomas and is regulated by IL-18. Clin Exp Immunol 181, 219–229, doi: 10.1111/cei.12643 (2015).
Dumoutier, L., Louahed, J. & Renauld, J. C. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol 164, 1814–1819 (2000).
Lejeune, D. et al. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem 277, 33676–33682, doi: 10.1074/jbc.M204204200 (2002).
Aujla, S. J. et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med 14, 275–281, doi: 10.1038/nm1710 (2008).
Sonnenberg, G. F., Fouser, L. A. & Artis, D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol 12, 383–390, doi: 10.1038/ni.2025 (2011).
Martin, J. C. et al. Interleukin-22 binding protein (IL-22BP) is constitutively expressed by a subset of conventional dendritic cells and is strongly induced by retinoic acid. Mucosal Immunol 7, 101–113, doi: 10.1038/mi.2013.28 (2014).
Huber, S. et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491, 259–263, doi: 10.1038/nature11535 (2012).
Jones, B. C., Logsdon, N. J. & Walter, M. R. Structure of IL-22 bound to its high-affinity IL-22R1 chain. Structure 16, 1333–1344, doi: 10.1016/j.str.2008.06.005 (2008).
Ouyang, W., Kolls, J. K. & Zheng, Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28, 454–467, doi: 10.1016/j.immuni.2008.03.004 (2008).
Colonna, M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity 31, 15–23, doi: 10.1016/j.immuni.2009.06.008 (2009).
Mus, A. M. et al. Interleukin-23 promotes Th17 differentiation by inhibiting T-bet and FoxP3 and is required for elevation of interleukin-22, but not interleukin-21, in autoimmune experimental arthritis. Arthritis Rheum 62, 1043–1050, doi: 10.1002/art.27336 (2010).
Siegemund, S. et al. Differential IL-23 requirement for IL-22 and IL-17A production during innate immunity against Salmonella enterica serovar Enteritidis. Int Immunol 21, 555–565, doi: 10.1093/intimm/dxp025 (2009).
Zheng, Y. et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445, 648–651, doi: 10.1038/nature05505 (2007).
Alam, M. S. et al. Notch signaling drives IL-22 secretion in CD4+ T cells by stimulating the aryl hydrocarbon receptor. Proc Natl Acad Sci USA 107, 5943–5948, doi: 10.1073/pnas.0911755107 (2010).
Mukherjee, S., Schaller, M. A., Neupane, R., Kunkel, S. L. & Lukacs, N. W. Regulation of T cell activation by Notch ligand, DLL4, promotes IL-17 production and Rorc activation. J Immunol 182, 7381–7388, doi: 10.4049/jimmunol.0804322 (2009).
Ouyang, W. Distinct roles of IL-22 in human psoriasis and inflammatory bowel disease. Cytokine Growth Factor Rev 21, 435–441, doi: 10.1016/j.cytogfr.2010.10.007 (2010).
Pickert, G. et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med 206, 1465–1472, doi: 10.1084/jem.20082683 (2009).
Sugimoto, K. et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest 118, 534–544, doi: 10.1172/jci33194 (2008).
Zenewicz, L. A. et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 29, 947–957, doi: 10.1016/j.immuni.2008.11.003 (2008).
Kamanaka, M. et al. Memory/effector (CD45RB(lo)) CD4 T cells are controlled directly by IL-10 and cause IL-22-dependent intestinal pathology. J Exp Med 208, 1027–1040, doi: 10.1084/jem.20102149 (2011).
Zenewicz, L. A. et al. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 27, 647–659, doi: 10.1016/j.immuni.2007.07.023 (2007).
Zenewicz, L. A. et al. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J Immunol 190, 5306–5312, doi: 10.4049/jimmunol.1300016 (2013).
Behnsen, J. et al. The cytokine IL-22 promotes pathogen colonization by suppressing related commensal bacteria. Immunity 40, 262–273, doi: 10.1016/j.immuni.2014.01.003 (2014).
Liang, S. C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 203, 2271–2279, doi: 10.1084/jem.20061308 (2006).
Wolk, K. et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol 36, 1309–1323, doi: 10.1002/eji.200535503 (2006).
Wolk, K. et al. IL-22 increases the innate immunity of tissues. Immunity 21, 241–254, doi: 10.1016/j.immuni.2004.07.007 (2004).
Koch, O. et al. Investigation of malaria susceptibility determinants in the IFNG/IL26/IL22 genomic region. Genes Immun 6, 312–318, doi: 10.1038/sj.gene.6364214 (2005).
Mastelic, B. et al. IL-22 Protects Against Liver Pathology and Lethality of an Experimental Blood-Stage Malaria Infection. Front Immunol 3, 85, doi: 10.3389/fimmu.2012.00085 (2012).
Craig, A. G. et al. The role of animal models for research on severe malaria. PLoS Pathog 8, e1002401, doi: 10.1371/journal.ppat.1002401 (2012).
Hall, N. et al. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic and proteomic analyses. Science 307, 82–86, doi: 10.1126/science.1103717 (2005).
WHO. World Malaria Report. World Health Organization (2014).
Miller, L. H., Baruch, D. I., Marsh, K. & Doumbo, O. K. The pathogenic basis of malaria. Nature 415, 673–679, doi: 10.1038/415673a (2002).
Grau, G. E. et al. Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci USA 86, 5572–5574 (1989).
Oakley, M. S. et al. The transcription factor T-bet regulates parasitemia and promotes pathogenesis during Plasmodium berghei ANKA murine malaria. J Immunol 191, 4699–4708, doi: 10.4049/jimmunol.1300396 (2013).
Li, C., Corraliza, I. & Langhorne, J. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect Immun 67, 4435–4442 (1999).
Sanni, L. A., Jarra, W., Li, C. & Langhorne, J. Cerebral edema and cerebral hemorrhages in interleukin-10-deficient mice infected with Plasmodium chabaudi. Infect Immun 72, 3054–3058 (2004).
Couper, K. N. et al. IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog 4, e1000004, doi: 10.1371/journal.ppat.1000004 (2008).
Omer, F. M. & Riley, E. M. Transforming growth factor beta production is inversely correlated with severity of murine malaria infection. J Exp Med 188, 39–48 (1998).
Steinman, L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med 13, 139–145, doi: 10.1038/nm1551 (2007).
Li, C., Seixas, E. & Langhorne, J. Rodent malarias: the mouse as a model for understanding immune responses and pathology induced by the erythrocytic stages of the parasite. Med Microbiol Immunol 189, 115–126 (2001).
Perriard, G. et al. Interleukin-22 is increased in multiple sclerosis patients and targets astrocytes. J Neuroinflammation 12, 119, doi: 10.1186/s12974-015-0335-3 (2015).
Radaeva, S., Sun, R., Pan, H. N., Hong, F. & Gao, B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 39, 1332–1342, doi: 10.1002/hep.20184 (2004).
Yanez, D. M., Batchelder, J., van der Heyde, H. C., Manning, D. D. & Weidanz, W. P. Gamma delta T-cell function in pathogenesis of cerebral malaria in mice infected with Plasmodium berghei ANKA. Infect Immun 67, 446–448 (1999).
van der Heyde, H. C., Manning, D. D. & Weidanz, W. P. Role of CD4+ T cells in the expansion of the CD4−, CD8− gamma delta T cell subset in the spleens of mice during blood-stage malaria. J Immunol 151, 6311–6317 (1993).
Goodier, M. R., Lundqvist, C., Hammarstrom, M. L., Troye-Blomberg, M. & Langhorne, J. Cytokine profiles for human V gamma 9+ T cells stimulated by Plasmodium falciparum. Parasite Immunol 17, 413–423 (1995).
Dumoutier, L., Van Roost, E., Ameye, G., Michaux, L. & Renauld, J. C. IL-TIF/IL-22: genomic organization and mapping of the human and mouse genes. Genes Immun 1, 488–494, doi: 10.1038/sj.gene.6363716 (2000).
Couper, K. N., Blount, D. G. & Riley, E. M. IL-10: the master regulator of immunity to infection. J Immunol 180, 5771–5777 (2008).
Specht, S., Ruiz, D. F., Dubben, B., Deininger, S. & Hoerauf, A. Filaria-induced IL-10 suppresses murine cerebral malaria. Microbes Infect 12, 635–642, doi:10.1016/j.micinf.2010.04.006 (2010).
Villegas-Mendez, A. et al. IFN-gamma-producing CD4+ T cells promote experimental cerebral malaria by modulating CD8+ T cell accumulation within the brain. J Immunol 189, 968–979, doi: 10.4049/jimmunol.1200688 (2012).
Harrington, L. E. et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6, 1123–1132, doi: 10.1038/ni1254 (2005).
Park, H. et al. A distinct lineage of CD4+ T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6, 1133–1141, doi: 10.1038/ni1261 (2005).
Howland, S. W., Poh, C. M. & Renia, L. Activated Brain Endothelial Cells Cross-Present Malaria Antigen. PLoS Pathog 11, e1004963, doi: 10.1371/journal.ppat.1004963 (2015).
Chia, W. N., Goh, Y. S. & Renia, L. Novel approaches to identify protective malaria vaccine candidates. Front Microbiol 5, 586, doi: 10.3389/fmicb.2014.00586 (2014).
Lundie, R. J. et al. Blood-stage Plasmodium infection induces CD8+ T lymphocytes to parasite-expressed antigens, largely regulated by CD8alpha+ dendritic cells. Proc Natl Acad Sci USA 105, 14509–14514, doi: 10.1073/pnas.0806727105 (2008).
Lapke, N., Tartz, S., Lee, K. H. & Jacobs, T. The application of anti-Toso antibody enhances CD8(+) T cell responses in experimental malaria vaccination and disease. Vaccine 33, 6763–6770, doi: 10.1016/j.vaccine.2015.10.065 (2015).
McCutchan, T. F. et al. Primary sequences of two small subunit ribosomal RNA genes from Plasmodium falciparum. Mol Biochem Parasitol 28, 63–68 (1988).
Burghardt, S. et al. Hepatocytes contribute to immune regulation in the liver by activation of the Notch signaling pathway in T cells. J Immunol 191, 5574–5582, doi: 10.4049/jimmunol.1300826 (2013).
Acknowledgements
We like to thank Christiane Steeg and Marie-Louise Brunn for technical advice. This work was supported the by grant of the SFB 841 liver inflammation.
Author information
Authors and Affiliations
Contributions
J.S., S.H., D.K. and C.F.A. performed the experiments; J.S. and T.J. were responsible for acquisition of data, analysis and interpretation of data and drafting of the manuscript; T.J. provided the concept and design of the study; M.S.M., D.K. and S.H. provided tools and reagents and developed methods; J.S., S.H. and T.J. revised the manuscript and gave final approval of the submitted manuscript. All authors have read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sellau, J., Alvarado, C., Hoenow, S. et al. IL-22 dampens the T cell response in experimental malaria. Sci Rep 6, 28058 (2016). https://doi.org/10.1038/srep28058
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep28058
This article is cited by
-
Structural diversity, functional aspects and future therapeutic applications of human gut microbiome
Archives of Microbiology (2021)
-
The good and the bad about separation anxiety: roles of IL-22 and IL-22BP in liver pathologies
Seminars in Immunopathology (2021)
-
Tim-3 signaling blockade with α-lactose induces compensatory TIGIT expression in Plasmodium berghei ANKA-infected mice
Parasites & Vectors (2019)
-
Flt3 ligand expands bona fide innate lymphoid cell precursors in vivo
Scientific Reports (2018)
-
Tissue-specific immunopathology during malaria infection
Nature Reviews Immunology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.