Abstract
Traditionally, nisin was produced industrially by using Lactococcus lactis in the neutral fermentation process. However, nisin showed higher activity in the acidic environment. How to balance the pH value for bacterial normal growth and nisin activity might be the key problem. In this study, 17 acid-tolerant genes and 6 lactic acid synthetic genes were introduced in L. lactis F44, respectively. Comparing to the 2810 IU/mL nisin yield of the original strain F44, the nisin titer of the engineered strains over-expressing hdeAB, ldh and murG, increased to 3850, 3979 and 4377 IU/mL, respectively. These engineered strains showed more stable intracellular pH value during the fermentation process. Improvement of lactate production could partly provide the extra energy for the expression of acid tolerance genes during growth. Co-overexpression of hdeAB, murG and ldh(Z) in strain F44 resulted in the nisin titer of 4913 IU/mL. The engineered strain (ABGL) could grow on plates with pH 4.2, comparing to the surviving pH 4.6 of strain F44. The fed-batch fermentation showed nisin titer of the co-expression L. lactis strain could reach 5563 IU/mL with lower pH condition and longer cultivation time. This work provides a novel strategy of constructing robust strains for use in industry process.
Similar content being viewed by others
Introduction
Nisin, as an antimicrobial peptide with 34 residues, is known for its wide range of antimicrobial activity against gram-positive bacteria and also shows antimicrobial activity against gram-negative bacteria especially when combined with a chelating agent, such as disodium EDTA1,2. Therefore it is widely applied in the dairy and meat manufacturing industries for its outstanding characteristics of higher antimicrobial efficiency, less poisonous compared to other chemical preservatives3. Due to its wide commercial application, many methods and technologies have been adopted to improve nisin yield and further reduce production cost, such as intentional mutagenesis4, optimization of fermentation process5, traditional random mutation and genome shuffling6, etc. In previous studies, some approaches was attempted to increase nisin production by immobilization of L. lactis cells and optimization of the nisin pathway for nisin biosynthesis. Expression of chitin-binding domain (ChBD) from Bacillus circulans which was fused with PrtP anchor on the surface of L. lactis for specific binding to chitin could maintain high cell densities and stable nisin production7, the highest nisin production was improved to 10500 IU/mL by the continuous nisin production system CICON-FER8. In Lactococcus lactis subsp. lactis A164, additional copies of nisin self-protection (immunity) genes nisFEG and the regulatory genes nisRK with high copy number plasmid in the producer strain enhanced the 1.5–1.7-fold higher than the nisin yield of the original strain9. Also in another study, provision of additional copies by cloning nisRK and/or nisFEG genes on plasmids in the L. lactis LL27 resulted in producing 45%, 24% and 44% more nisin compared to the original yield 520 IU/mL, respectively10. Furthermore, the bioactive recombinant nisin of the nisin deficient strain L. lactis MG1363 were overproduced with a yield of 1098 IU/mL when additional copies of an entire nisin biosynthesis pathway were present11.
Despite many methods and technologies exploited before, the requirement to construct a manufacturing strain based on fermentation process is unsatisfied. L. lactis, as one of main nisin producers, is inhibited by lactic acid produced during the fermentation process. Hence fermentation condition has to be neutralized in order to ensure the cell growth in traditional fermentation process12. However, nisin, a significant product, showed a relative higher activity in the environment with the pH 2.0 or lower and degraded as the increase of pH value6. To alleviate the degradation rate of nisin during the fermentation process, lowering pH value of the fermentation broth will be an alternative approach. Therefore, it is necessary to construct an acid tolerant strain which can maintain its growth activity in the relative acidic fermentation condition and also alleviate nisin degradation.
Fortunately, recent advances in genome mining methods have provided a potential access to disclose acid tolerant genes and gene clusters13,14. A number of acid tolerant mechanisms in many microorganisms have been elucidated, such as in L. Lactis and E. coli, which make it possible to improve acid tolerance of bacteria by the transformation of acid tolerant genes. The mechanisms of acid tolerance in bacteria can be classified into four categories: (I) Decrease of intracellular protons mainly involved in transmitting obstacle of extracellular H+, exportation of intracellular H+ and consumption of intracellular H+. (Ia) The solidification of cell wall, the change of cell membrane and the protection of trehalose can work by transmitting obstacles of extracellular H+ 15,16,17,18,19,20. (Ib) The mechanism of intracellular H+ consumption mainly includes decarboxylation, the generation of alkali and the protective effect of glutathione and histidine21,22,23,24,25,26,27. (Ic) Proton pumps plays a major role in exportation of intracellular H+. The multi-subunit F1F0-ATPase can generate ATPs which facilitate the extrusion of proton from the cell28,29. (II) Molecular chaperone family proteins, such as 70 kDa DnaK family and 60 kDa GroE family proteins, can play protective roles under acid stress30. In addition, the protection of DNA repair for cells are needed. Studies shown that DNA synthesis and repair increased under acid stress31,32. (III) Regulatory factors include the global regulatory factors, two-component-regulating system and other regulatory factors. (IIIa) EvgS/EvgA is the main two-component signal transduction systems (TCS) in the E. coli, which can endow bacteria the sense ability of acid stress and is involved with the regulation of other genes33,34. (IIIb) The overexpression of some non-coding sRNAs can improve the ability against low pH environment significantly. A novel sRNA gcyB can increase the survival of E. coli by enhancing the expression level of sigma factor RpoS in the stationary phase35. Simultaneous overexpression of the non-coding sRNA, such as DsrA, RprA and ArcZ displayed an 8500-fold higher survival of E. coli to low pH, which also provided a protective effect on carboxylic acid and oxygen stress36. (IIIc) Sigma factors including RpoS belong to the global regulatory factors. Sigma factors ensure RNA polymerase combined to specific region of promoters and RpoS can protect bacterial strains against various stress conditions such as heat shock, acid or alkali stress. The number of regulated genes by RpoS is more than 500 according to the literature37. (IV) Some membrane proteins related with carbon metabolism also could contribute to the acid tolerance of bacterial strains38,39,40,41. In response to low pH, the central carbon metabolism would be strengthened42. More energy can be obtained from the enhanced carbohydrate metabolism, which can be consumed by the bacterial strains against the acid stress.
This study aims to construct an acid tolerant module in L. lactis, hoping to balance the optimal pH of cell growth and nisin activity, so as to obtain higher nisin yield. To reach this goal, some acid tolerant genes were transformed to L. lactis F44 and the nisin production was analyzed.
Acid tolerance tests of these overexpression strains demonstrated that the nisin production was positively correlated with the heterologous acid tolerant genes. Higher nisin yield and growth of strains could be maintained simultaneously. To our knowledge, this is the first report to reveal the relationship between nisin production and acid tolerance during fermentation process. This work represents a new strategy to introduce process-based-design concept into constructing robust industrial strain.
Results
Activity of nisin and the growth of the original strain in acidic condition
Nisin, as a kind of small molecular peptide, showed different activity in diverse pH conditions. For analyzing the activity of nisin in fermentation process, 4000 IU/mL nisin standards were added to the fermentation medium at pH 2.0, 3.0, 4.0, 5.0, 6.0 and 7.0, respectively. Nisin titer was measured every 2 hours during the incubation. As it is shown in Fig. 1, the activity of nisin decreased significantly with the increase of pH value of medium. Nisin could remain stable for 4 h at pH 2.0, but when the pH value reached 5.0, nisin titer fell by 50% in 2 h.
Fermentation process was also greatly affected by the initial pH of broth. The fermentation medium was adjusted to pH 2.0, 3.0, 4.0, 5.0, 6.0 and 7.0, respectively and the starter strain L. lactis F44 was incubated for 12 h in above media respectively. Biomass, pH and nisin titer of the fermentation broth were measured every two hours during fermentation process. As shown in Supplementary Fig. S1a, the growth status of the bacteria was declining with the pH values decreasing. When the pH value of broth was under 4.0, the growth of F44 stopped. During fermentation process, the pH value of the fermentation broth reduced because of lactic acid production (see Supplementary Fig. S1b). Nisin accumulation began from 2 h and reached to its peak of 2810 IU/mL at 8 h and then declined (see Supplementary Fig. S1c). At the end of the fermentation, the nisin titer reduced by 50% compared with the highest. The optical density of F44 reached maximum at 8 h, meanwhile, the pH value was close to 5.0, which was unfavorable for the growth of F44 strain, thus led to the growth decrease and the nisin degradation.
Nisin can be preserved better below pH 2.0 conditions and the activity of nisin reduced with the increase of pH. While the strain F44 can hardly grow below pH 5.0. The optimal pH difference between nisin activity and bacteria growth limited the nisin production. Thus, it would be a good method to solve the problem by improving acid tolerance of L. lactis strains.
Introduction of single acid tolerant gene to improve nisin production
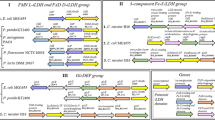
To determine the effects of different acid tolerant proteins on nisin production, various acid tolerant genes with different mechanism were introduced respectively into L. lactis F44 and the effects of these genes on acid tolerance were analyzed. Firstly, genes with different acid tolerant mechanisms were transformed to L. lactis F44 strain individually. It is indicated that the capacity of nisin production increased differently due to the expression of different acid tolerant genes (Fig. 2). Among the engineered strains, the strain harboring murG gene, which is responsible for the transfers of the N-acetylglucosamine moieties onto the carrier lipid in cell wall biosynthesis43, performed best in nisin production. Nisin accumulation was up to 4377 IU/ml, which is 55.7% higher than that of the original strain. Meanwhile, the growth rate of the engineered strains was slightly lower than that of F44 (see Supplementary Table S3). At the late fermentation period, such as 8 h after starting fermentation, F44 stopped growing almost, while the engineered strains can still maintain good growth activity at least till 10 h.
Effect of different acid tolerance genes on nisin titer in 8h (at the peak value).
Original strain F44, strains overexpressing acid tolerant genes and strains overexpressing lactic acid synthetic genes were grown in feed medium at 30 °C and photographed every 2 h. Comparative nisin production in F44 and engineered strains. Error bars, SD from three replicate flasks. *p < 0.05, **p < 0.01, t-test.
Enhancing lactic acid synthesis pathway to improve nisin production
We also cloned two groups of pyk, pfy and ldh genes encoding pyruvate kinase Pyk, phosphofructokinase Pfk and lactate dehydrogenase Ldh, the key enzymes of lactic acid synthesis pathway, from L. lactis F44 and Lactobacillus casei Zhang, respectively (Fig. 2). Then six genes were introduced to L. lactis F44 separately. The results showed that nisin titer of the engineered strains harboring pyk, pfy and ldh all have been improved. Among the engineered strains, the nisin titer of F44 overexpressing ldh gene from L. casei Zhang was highest. The nisin titer was improved from 2810 IU/mL to 3979 IU/mL, increased by 41.6%. As for the cell growth, the six strains performed similarly with the strains harboring single acid tolerant gene, which showed better robustness than F44 in the stationary phase (see Supplementary Fig. S2a,b).
Combined expression of several genes to improve nisin production
To further improve the acid tolerance of the strain, the genes which helped to enhance the nisin yield of L. lactis F44 through single overexpression and involves in different acid tolerant mechanisms and energy metabolism were selected to be co-overexpressed and we constructed the following overexpression strain: L. lactis F44 (ABG) (combined expression of triple genes hdeA, hdeB and murG), F44 (ABGL) (combined expression of tetrad genes, hdeA, hdeB, murG and ldh (Z)), F44 (MLL) (combined expression of murG and ldh (L)), F44 (MLZ) (combined expression of murG and ldh (Z)), F44 (PPL) (combined expression of triple genes, pfk (Z), pyk (Z) and ldh (Z)). The nisin titer of all these engineered strains increased during the fermentation process (Fig. 3a). However, at the exponential time, the growth rate of the engineered strains was significantly lower than F44 strain. This might result from the extra-consumption of energy in over-expression of acid tolerant genes and shortage of energy used in cell growth. When entering the late exponential phase, strain F44 (ABGL) showed remarkable increase in the production of lactate, which could provide bacteria more energy (Fig. 3b). The extra energy supply might be used to maintain the stability of cells. Among the engineered strains, the highest nisin titer was observed for F44 (ABGL), which displayed 4913 IU/mL nisin titer, increased by 74.84% compared with F44 strain. In addition, the transcription levels of hdeA, hdeB, murG and ldh(Z) in the F44 (ABGL) were significantly higher than its corresponding genes in the wild type F44 (Fig. 4a,b). The efficiency of the PCR reached 90.13% (see Supplementary Fig. S4), which meant the results of qRT-PCR analysis showed that the P45 promoter of pLEB124 had high efficiency at different pHs and all the heterogenous genes in the engineered strains could be successfully transcribed to the corresponding mRNAs.
The transcriptional analysis of acid-tolerant genes hdeA, hdeB murG and ldh in wild type F44 and the engineered strain F44 (ABGL) by qRT-PCR.
The total RNA extracted from cells which were incubated at pH 7.0, 6.0, 5.0 and 4.0 in seed medium, respectively. The obtained cDNA was subjected to qRT-PCR. The 16S rRNA gene was used as the internal control gene and hdeA, hdeB murG and ldh in wild type F44 were set as the guide sample.
The expression levels of the nisin biosynthesis-related genes between wild type F44 and the engineered strain F44 (ABGL) were examined by qRT-PCR. The cells at mid-log phase (8 h) for the highest gene expression was harvested for qRT-PCR. As shown in Supplementary Fig. S6, the expression levels of most genes related to nisin biosynthesis of F44 (ABGL) were slightly higher than that of the original strain F44. And the change of expression levels between the two strains was less than 2 times, which demonstrated no significant differences were found. Moreover, the expression levels of other nisin related genes nisB and nisR of F44 (ABGL) were lower than that of F44.
At the same time, all engineered strains obtained higher survival rate in low pH condition (Fig. 5a,b). Strain F44 (ABGL) displayed the highest survival rate to low pH compared with the original strain F44 (Fig. 5b). The strong acid-tolerant capacity observed from the F44 (ABGL) should be the result of the overexpression of different proteins related to different mechanisms of acid tolerance. These data suggested that the higher nisin yield of the engineered strains than F44 is the result of a significant increase in acid tolerance capacity.
Higher expression of acid tolerant genes ensures the stability of the intracellular pH
Intracellular pH (pHi) plays a major role in response to acid stress in lactic acid bacteria44,45. In order to further explore the influence of the acidic fermentation environment on the engineered strains and the original strain F44, the pHi of these strains were measured during the fermentation process. Obvious differences in pH between F44 and the engineered strains were observed after 6, 8 and 10 h of fermentation. The engineered strains appeared to have the ability to maintain a higher pHi than the F44 (Fig. 6a,b). This indicated that the engineered strains could avoid the sharp decrease of pHi under acid stress effectively. Significant pHi differences were detected in the engineered strains and the original strain F44 in response to acidic environment, which proved the protection of the acid tolerant genes on the L. lactis strain under acid environment. The data above showed that the increase of nisin yield might be the result of the stabilization of the intracellular pH by the overexpression of some acid tolerant genes.
The intracellular pH of F44 and the engineered strains during fermentation process ranging at 6 h, 8 h and 10 h.
(a) Single acid-tolerant gene over-expression strains; (b) Acid-tolerant genes co-overexpression strains. Samples were taken at every 2 h for 6-10 h. Error bars, SD from three independent experiments. *p < 0.05, **p < 0.01, t-test.
Fed-batch fermentation of the engineered strains helps to promote nisin yields
To further identify the performance of the artificial acid tolerant module, we performed fed-batch fermentation to optimize the fermentation processes. For F44, the consumption rate of sucrose was high in the initial 6 h, while the sucrose concentrations remained unchanged after 12 h6. The sucrose was added during fermentation process as an energy supplement for the cell growth and the nisin production. As shown in Fig. 7a,b, the growth rate of F44 was still higher than other engineered strains in the exponential phase. With the fermentation time extended, the biomass of acid tolerant strains were similar to that of F44. There were no significant differences on the biomass among F44 and other two strains, F44(ABGL) and F44 (MurG). Nisin production of F44 (ABGL) and F44 (MurG) was obviously higher than that of the original strain F44 (Fig. 7c). Compared with the nisin titer of F44, the nisin titer of F44 (MurG) and F44 (ABGL) were improved from 3454 IU/mL to 4774 IU/mL and 5167 IU/mL, increased by 38.2% and 49.6%, respectively. Although the growth conditions of the strains were similar, the pHi of engineered strains should be significantly higher than that of F44 due to the overexpression of the acid tolerant genes which provided the relatively mild intracellular environment for cell growth and nisin production.
Fed-batch culture analysis of cell growth (a), pH of broth (b) and nisin production (c) with F44, transformants with single expression of murG and combinatorial expression of hdeA, hdeB murG and ldh genes, respectively. The culture temperature was maintained at 30 °C. Concentrated sucrose (500 g/L) was fed at a constant rate of 2 mL/h between 6–12 h during fermentation process. Samples were taken at every 2 h.
To increase the nisin production and reduce the restriction of acid stress, the broth was maintained at pH 6.0 from 6th hour by adding NaOH solution (10 M). All strains grew fast initially during the first 6 h. F44 grew faster than the engineered strains during the exponential phase, while the difference between F44 and other strains diminished by the fermentation extension (Fig. 8a). When pH was adjusted to 6.0, the nisin titer achieved the fairly high level and then increased slowly, finally dropped gradually. This indicates that the bacteria require an adaptive period when encountering environmental change. Then strains recovered rapidly, the cell growth continued and nisin was accumulated more. The nisin titer of strain F44 (ABGL) also reaches peak value of 5563 IU/mL which was 1.48 fold that of the original strain and was also higher than that of F44 (MurG) during the whole fermentation process (Fig. 8b).
Similarly, we also measured the OD600 and nisin production through the fed-batch fermentation, of which the pH was adjusted to 5.5, 5.0 and 4.0, respectively (see Supplementary Fig. S7). In the culture at pH 5.5, the growth of all strains is only marginally affected by pH regulation and the nisin yield had a quite similar trend at medium pH from 6.0 to 5.5 (see Supplementary Fig. S7a,b). The nisin titer of F44 (ABGL) was 4674 IU/mL, which increased by 24.2% than that of F44.
The low pH of broth has a great effect on the growth and production of nisin. The growth of all strains was strongly inhibited in acidic environment after adjusting the pH of fermentative broth (see Supplementary Fig. S7c,e). F44 grew faster during the exponential phase, while the difference between F44 and the engineered strains diminished in the condition of pH 5.0 and 4.0, which showed that overexpression of acid tolerant genes indeed protected cells under acid stress.
The activity of nisin is better at low pH, but the growth of all strains was restricted which lead to weakening the nisin production (see Supplementary Fig. S7d). When pH of medium was adjusted to 5.0, the nisin titer decreased at first and then transitorily increased in an adaptive buffer stage, finally dropped gradually. The nisin titer of F44 (ABGL), which appeared a short-term bounce of 3671 IU/mL after pH adjustment, was still significantly higher than F44, but that of strain F44 did only reach the level of 2799 IU/mL before acidification of broth. The nisin titer of engineered strains was always higher than that of F44 during the whole fermentation process. For the culture at pH 4.0 by adding HCl at 6 h, the final biomass levels further decreased. The growth retardation of all strains was observed after the acidifying operation, which illustrated that improving acid tolerance of engineered strains have reached the limitation, which has a great impact on nisin production (see Supplementary Fig. S7f).
Discussion
Many metabolites produced in the industrial fermentation process could seriously inhibit the bacterial growth46, with the example of acidic metabolites, such as lactic acid and some carboxylic acids. These metabolites not only inhibit the cell growth but also reduce the yield of the target products. During the lactate fermentation process, the accumulation of lactic acid produced by lactic acid bacteria acidified the broth, therefore suppressed the cell growth of lactic acid bacteria whose optimal pH was about 6.3–6.9 and also limited the yield of lactic acid47. The traditional method to deal with this problem was to maintain the optimal pH of broth by adding alkali, which not only complicated the fermentation process but also might cause contamination48,49,50. It was reported that one kind of bacteria consuming lactic acid was co-cultured with L. lactis51, which could reduce the influence of lactic acid and pH on the growth of bacteria and nisin production. But the co-cultivation increased the difficulty of the industrial fermentation and the cost of the separation process. Introducing the acid tolerant genes to improve acid resistance of cells is an alternative method, by which, the strains can maintain normal growth rate in the late stationary stage of fermentation and therefore the accumulation of target metabolites can be promoted.
The pH value of broth can decrease to 4.5 due to the accumulation of acidic metabolites (lactic acid) during fermentation, which limits the normal growth of L. lactis, whose optimal pH is about 7.2 and then also affects the production of nisin. At the same time, the fluctuations of intracellular physiological environment under acid stress also lead to the reduction of physiological activity. The improvement of acid tolerance capacity is beneficial to prolong the fermentation period and improve the growth activity of bacteria, which is of great importance to ferment process producing acidic metabolites. In response to acid stress, a large number of acid tolerant proteins were expressed to protect L. lactis cells. In recent years, the development of genome sequencing and various omics approaches have assisted to illuminate many acid tolerant mechanisms. Many genes were found significantly upregulated under acid stress by transcriptional analysis52. Some of these genes were further proved to be involved in various mechanisms under stress conditions53,54. Acid tolerant genes expression in the engineered strains could improve the acid tolerance capacity and maintain good cell growth and therefore might improve nisin yield. We attempted to overexpress 17 acid-tolerant genes and 6 lactic acid synthetic genes in L. lactis F44 (Table 1). It was found that acid tolerance and nisin yield of the engineered strains could be improved in varying degree. Among these engineered strains, overexpression of murG, hdeAB, ldh makes better performance on the robustness and nisin production of cells.
Encoding a kind of glycosyltransferase, murG is involved in the peptidoglycan synthesis of bacterial cell walls by catalyzing the successive transfers of the N-acetylglucosamine moieties onto the carrier lipid43. The cell wall-associated protein MurG might be abundant and accumulated under acidic conditions. The expression of murG lead to higher survival and the enzyme might act as a key role of fixing cell walls under acid stress. HdeA and its structural homologue HdeB could maintain the optimal chaperones activity at pH 2.0 and pH 4.0, respectively, therefore could be used as acid-resistance proteins in bacteria55. These two chaperones can help bacteria to survive in acidic protein-unfolding conditions. It is interesting that the chaperones HdeA and HdeB from E. coli could take effect in L. lactis which belongs to gram-positive bacteria without periplasmic space.
Besides, during the production of lactic acid, as one end product of the glucose metabolism in the lactic acid bacteria, energy can be produced in this process. As the key enzymes involved in the synthesis of lactic acid, pyk, pfy and ldh were confirmed to a higher expression level in lactate fermentation by proteomic analysis52,56. Most acid tolerant mechanisms need to consume energy. Under low pH conditions, the energy from sugar metabolism is mainly used to withstand harsh environment, therefore the cell growth and the production capacity are affected by the energy supply. So enhancing the lactic acid production and energy generation by overexpressing these genes could effectively improve the cell growth, acid tolerance capacity and nisin yield.
It is interesting that the optical density of F44 was higher than that of engineered strains which may result from the faster growth rate of F44 in the exponential phase (see Supplementary Table S3). Indeed, the replication and expression of acid tolerant genes from the vector might cause metabolic burdens in the engineered strains and therefore delayed their growth. After 10 h fermentation, the cell growth and the nisin production of all strains were in stagnation stage with pH close to about 4.5 (see Supplementary Table S3). At the same time, the nisin accumulation dropped greatly and nisin titer of all strains decreased in a same trend, which illustrated that improving acid tolerance of engineered strains has reached the limitation (see Supplementary Fig. S2c and Table S3). These results showed it was difficult to maintain physiological activities of cells as well as normal metabolic process under severe acid stress. However, as expected, nisin yields of the engineered strains were higher than that of the original strain F44, especially in late fermentation stage, which meant these cells could be resistant to acidic environment and remained active and stable (Fig. 2). Although the nisin yields of engineered strains and F44 exist obvious differences, there were no significant differences in the expression levels of nisin gene cluster between them according to the results of qRT-PCR. Thus, it is concluded that improvement of nisin yield is resulted from that overexpression of acid tolerant genes makes the strains more robust and adaptive under acid stress rather than increases the expression of genes related to nisin biosynthesis. The results of combined overexpression of hdeAB, murG and ldh(Z) in F44 showed nisin yield of the engineered strain was improved further and better effects were also achieved by analysis of fed-batch process (Figs 7b and 8b). Although the nisin yield at pH 5.5 did not reach a higher level, the value of 5.5 seemed to be the limit culture pH value enabling growth to proceed and the inhibition of low pH (pH 5.0 and 4.0) on the growth and the ability of nisin production was obvious (see Supplementary Fig. S7). Neither the ΔpH between the cytoplasm and the culture medium nor the pHi were maintained constant when the culture pH value was lower than 5.0 and the efficiency of biomass synthesis relying on the energy supply also decreased57. The lower the pH was, the less the cell concentration was obtained when the strains reached the stationary phase and the nisin production dropped greatly. The nisin titer of all strains decreased in a general trend, which showed it was difficult to maintain physiological activities of cells as well as normal metabolic process due to acidic damage during the cultures performed at medium pH 5.0 or lower. Although the nisin titer of the engineered strains harboring several acid tolerant genes was higher than the single gene expression strains, the overexpression of more acid tolerance genes would consume more energy, therefore, the overload of acid tolerant genes overexpression might affect the higher improvement of the nisin titer. In addition, from the results of acid stress assay, compared with the original strain, the engineered strains constructed in this paper had more survival rates in the environment of lower pH and also had higher nisin yield, which confirmed our hypothesis that overexpressing these genes would increase the production of nisin (Fig. 5a,b).
Maintaining relatively stable intracellular pH is important for microorganisms, which can ensure the normal physiological behavior of cells and guarantee the activity of enzymes58,59. Intracellular pH should be affected by the large changes of environmental pH. Exceeding certain range of environmental pH fluctuation would influence the dynamic balance of the intracellular physiological activities and even affect the survival of cells. The engineered strains showed higher pHi value than that of the original strain (Fig. 6a,b). These engineered strains could maintain a relatively stable pHi and enhance the adaptability of the bacteria in certain acidic environment.
According to what we learnt, it is the first report that the nisin production was improved by over-expressing acid-tolerant genes, which would provide some clues for the construction of other acid-tolerant microorganism. And in the future study, we want to optimize the promoters to regulate the expression level of the genes and reduce the side effects resulted from the excessive expression of heterogenous proteins. In addition, we also plan to construct an acid-tolerant network which can regulate the engineered strains in multi-level and cross joint manner.
Methods
Bacteria strains and growth conditions
The bacterial strains and plasmids used in the study are listed in the Supplementary Table S1. The L. lactis strain F44 was used for the phenotypic examination throughout this study. The E. coli TG1 was used for plasmid preparation. All the E. coli strains were grown at 37 °C, with shaking at 180 rpm in the Luria-Bertani (LB) medium. All L. lactis strains were preserved and cultured in seed medium (wt/vol) containing peptone (1.5%), yeast extract (1.5%), sucrose (1.5%), KH2PO4 (2.0%), NaCl (0.15%), corn steep liquor (0.3%), cysteine (0.26%) and MgSO4·7 H2O (0.015%). The pH value was adjusted to 7.2 with 10 M NaOH before autoclaving at 121 °C for 20 min. The antibiotics erythromycin (Emr) (100 μg/mL for E.coli TG1, 5 μg/mL for L. lactis F44) was used for selection. The tolerance agar plates were supplemented with 1.5% agar after autoclaving. Micrococcus flavus ATCC 10240, preserved in the laboratory, was used as an indicator strain for the bioassay of nisin.
Nisin activity assay
A stock solution of nisin was prepared by mixing 0.1 g of nisin standards (Sigma, USA) in 10 mL of 0.02 M HCl (106 IU/mL) and boiling for 5 min. The stock solution was diluted using 0.02 M HCl to standard nisin solutions. Nisin standards were added into the autoclaved initial fermentation medium at pH 2.0, 3.0, 4.0, 5.0, 6.0 and 7.0, respectively and boiling for 5 min. All the fermentation media containing 4000 U/mL nisin (1 g of nisin in 100 mL media) were incubated at 30 °C. The fermentation broth was sampled every 2 h for nisin titer analysis. 500 μL of fermentation broth was mixed with the same volume of 0.02 M HCl. The mixture was boiled for 5 min and then appropriately diluted with 0.02 M HCl. 1.5% (vol/vol) tween 80 (JiangTian, Tianjin, China) (tween 80 enhances nisin diffusion into the agar medium) and 1% (vol/vol) and 1% (vol/vol) indicator strain M. flavus ATCC 10240 buffer (the final concentration was 107 cfu/mL) was poured into the 26-mL assay medium which was cooled to about 45 °C after autoclaving. The medium was poured into a sterile plate for solidification and precultivation. Standard nisin solutions and test solutions were infused into individual wells (80 μL per well) which had been bored into the assay plate (8 wells per plate) using a 7-mm-diameter metal tube and then the plates were incubated at 37 °C for 24 h. Zones of inhibition were measured and the regression equation was calculated. Each assay of standard sample or the broth sample was performed in triplicate.
Fermentation performance at acidic condition
The seed medium was inoculated with bacterial colonies and incubated overnight. 5% of the overnight culture broth was used to inoculate fermentation medium for 12 h at 30 °C. The initial pH of fermentation medium were adjusted to 2.0, 3.0, 4.0, 5.0, 6.0 and 7.0, respectively. Cell density (OD600), pH values and nisin titer of broth samples were measured every 2 h.
DNA manipulations and genetic construction
The primers of acid tolerant genes used in the study which were designed by primer premier 5 (Premier, Canada) are listed in Supplementary Table S2. These genes were directly amplified from Lactobacillus casei Zhang, L. lactis subsp. lactis F44 or E. coli DH5α via polymerase chain reaction (PCR). The restriction enzyme cutting sites were simultaneously inserted into the amplified gene. Combinations of two, three or more genes were constructed via overlap extension PCR. The resulting fragments were digested with BamHI and HindIII (or SmaI) and then ligated into plasmid pLEB124, cut with BamHI and HindIII (or SmaI) to generate the resulting plasmids. The resulting plasmids were transformed into E.coli TG1 by heat shock transformation for enrichment60. After antibiotics selection, the plasmids were extracted with TIANprep Mini Plasmid Kit (TIANGEN, China) and then were transformed into the L. lactis F44 by electroporation transformation61.
Nisin titer assay
The nisin titer assay was described previously6. Briefly, the nisin standards (Sigma, USA) and the fermentation broth which have removed the cells by centrifugation at 8000 rpm for 5 min, were boiled for 5 min. Then these samples were serially diluted with 0.02 M HCl. After autoclaving, the 26-mL cooled assay medium was inoculated with 1% (vol/vol) M. flavus buffer, with the concentration of 107 cfu/mL. Then the medium was added with 1.5% (vol/vol) tween 80 (JiangTian, Tianjin, China) to enhance nisin diffusion. The mixed medium was quickly poured into sterile plates. After solidification and pre-cultivation, we used a 7-mm-diameter metal tube to drill into the assay plate (8 wells per plate) and removed the agar from the well. Standard nisin solutions and broth solutions were injected into the respective wells (80 μL per well) and the plates were incubated at 37 °C for 24 h. Inhibition zones were measured by calipers. A regression equation was calculated from the measured data. Each assay of the samples including standards and broth was performed in triplicate.
RNA isolation and transcriptional analysis by quantitative real-time PCR
L. lactis strain F44 genome was isolated with the TIANamp Bacteria DNA Kit (TIANGEN). The genome was diluted to concentrations of 10−5, 10−4, 10−3, 10−2, 10−1. After acid shock at pH 7.0, 6.0, 5.0 or 4.0 respectively, total RNAs were isolated with ZR RNA MiniPrep (The Epigenetics company). For qRT-PCR, 0.1~0.5 μg of total RNA were reverse transcribed together with 1 μL corresponding primers in a total reaction volume of 12 μL using TIANScript RT Kit (TIANGEN). The reaction system was harvested by 3000 rpm for 30 s. Each reaction was incubated at 65 °C for 5 min, followed by incubating on ice. Then the reactions were run at 40 °C for 60 min, followed by 70 °C for 5 min to terminate the complementary DNAs synthesis. The generated cDNAs were stored at −70 °C for further qRT-PCR. qRT-PCR was performed with Power SYBR Green PCR Master Mix (Applied Biosystems). Briefly, a 50 μL-reaction solution containing 10~100 ng of cDNA, 200 nM each primer (see Supplementary Table S2), 25 μL 2 × Ultra SYBR Mixture (with ROX) and sterile water was analyzed on a LightCycler 480 Real-Time PCR System (Roche, Switzerland) according to the manufacturer’s instructions. Reactions were run in triplicate in three independent experiments for each condition. qRT-PCR conditions were as follows: 1 cycle at 95 °C for 10 min, 40 cycles of denaturation at 95 °C for 10 s, annealing at 55 °C for 10 s and extension at 72 °C for 20 s. The 16S rRNA gene was used as an internal control to normalize cycle threshold (CT) values. Difference in the relative expression levels were calculated with 2^−(ΔΔCT) method. We performed a 10-fold dilution series experiment using the target assay to establish the standard curve and the slope of the standard curve can be translated into an efficiency value: PCR efficiency = 10−1/slope–162.
Measurement of intracellular pH (pHi)
The pHi was measured with the fluorescence method using 5 (and 6-)-carboxyfluorescein succimidyl ester as the fluorescent probe63. Calibration curves were established to exclude artificial bias by different environmental conditions and cell states. pHi values were determined by the radio of the fluorescence signal measurements at excitation wavelengths of 490 nm (pH-sensitive wavelength) and 440 nm (pH-insensitive wavelength) with Fluorescence Spectrophotometer F-2700 (Hitachi High-Tech, Japan). Loading of cells with the fluorescent probe, the establishment of calibration curves and the determination of pHi all followed the procedure which Breeuwer et al. described previously63.
Acid tolerance capacity assay
To investigate the acid tolerance, the wild type strain and the recombination strain after activation culture overnight at 30 °C in the seed medium were harvested in mid-exponential growth phase. Aliquots of cells suspension were diluted to concentrations of 10−6, 10−5, 10−4, 10−3 and 10−2. Cell survival numbers were estimated, where 100 μL of serially diluted samples were spread in triplicate on seed media agar plates with different pH values 4.0, 4.2, 4.4, 4.6, 4.8 and 5.0 and incubated at 30 °C for 48 h. Plates with colonies in the range of 30 to 300 were then used to calculate the average number of CFU/ml.
Fermentation in flasks
The original strain F44 and the engineered strains expressing the acid tolerant genes were inoculated in the seed medium for overnight after the cultivation on seed plates at 30 °C for 48 h. The flask fermentation experiments were carried out in 250-mL Erlenmeyer flasks containing 100 mL of fermentation medium with peptone (1.5%), yeast extract (1.5%), sucrose (1.5%), KH2PO4 (2.0%), NaCl (0.15%), corn steep liquor (0.3%), cysteine (0.26%) and MgSO4·7 H2O (0.015%). Five milliliters of the overnight cultures were inoculated in triplicate into the static flasks and incubated for 14 h at 30 °C. Samples were withdrawn every 2 h for cell density analysis, fermentation broth pH, intracellular pH (pHi), lactic acid concentration and nisin production.
Fed-batch fermentation
The fed-batch fermentation experiment was conducted at 30 °C for 24 h. Initially the fermentation medium was adjusted to pH 7.2 with 10 M NaOH as described above. The medium was inoculated with 5% of the seed culture. After the fermentation of 6 h, the pH of fermentation broth was controlled at 6.0 by the addition of 10 M NaOH. 3 mL sucrose solution (500 g/L) was added to the fermentation broth at 6 h, 8 h, 10 h and 12 h. The fermentation broth was sampled every 2 h for cell density analysis, lactic acid concentration, residual sugar concentration and nisin production.
Statistical analysis
Optical density (OD) was measured at 600 nm with TU-1810 spectrophotometer to monitor the L. lactis cell growth. The pH values of the fermentation broth were measured with FE20 benchtop pH meter (Mettler Toledo, Swiss). To measure the lactic acid concentration and sucrose concentration during the fermentation process, samples were periodically collected for estimating lactate and sucrose concentration in supernatants after removing the cells by centrifugation (8000 rpm, 5 min, 4 °C). The concentration of the residual sugars in the broth was assayed by the dinitrosalicylic acid reagent (DNS) method64. The lactate concentration was quantified by a biosensor SBA-90 (Biology Institute of Shandong Academy of Sciences, China)65. To assess the statistical significance of differences in resistance to acid stress, student’s t tests were used. For the significance of gene expression differences, the statistical t-test was used to identify genes differentially expressed between the wild type strain and the engineered strain. Statistical significance of nisin titer and pHi was estimated using t-test. Statistical analyses of the data were performed using SPSS software version 19.0 (IBM, USA).
Additional Information
How to cite this article: Zhang, J. et al. Enhance nisin yield via improving acid-tolerant capability of Lactococcus lactis F44. Sci. Rep. 6, 27973; doi: 10.1038/srep27973 (2016).
References
Hurst, A. Nisin. Adv. Appl. Microbiol 27, 85–123 (1981).
Stevens, K. A., Sheldon, B. W., Klapes, N. A. & Klaenhammer, T. R. Nisin treatment for inactivation of Salmonella species and other gram-negative bacteria. Appl Environ Microbiol 57, 3613–3615 (1991).
Lee, N. K. et al. Antimicrobial effect of nisin against Bacillus cereus in beef jerky during storage. Korean journal for food science of animal resources 35, 272–276 (2015).
Wu, Z. et al. Mu transposition complex mutagenesis in Lactococcus lactis–identification of genes affecting nisin production. Journal of applied microbiology 106, 41–48 (2009).
Carvalho, A. L., Cardoso, F. S., Bohn, A., Neves, A. R. & Santos, H. Engineering trehalose synthesis in Lactococcus lactis for improved stress tolerance. Appl Environ Microbiol 77, 4189–4199 (2011).
Zhang, Y. F. et al. Genome shuffling of Lactococcus lactis subspecies lactis YF11 for improving nisin Z production and comparative analysis. J Dairy Sci 97, 2528–2541 (2014).
Simsek, O. et al. Immobilization of nisin producer Lactococcus lactis strains to chitin with surface-displayed chitin-binding domain. Applied microbiology and biotechnology 97, 4577–4587 (2013).
Simsek, O. Nisin production in a chitin-including continuous fermentation system with Lactococcus lactis displaying a cell wall chitin-binding domain. J Ind Microbiol Biotechnol 41, 535–543 (2014).
Cheigh, C.-I., Park, H., Choi, H.-J. & Pyun, Y.-R. Enhanced nisin production by increasing genes involved in nisin Z biosynthesis in Lactococcus lactis subsp. lactis A164. Biotechnology letters 27, 155–160 (2005).
Şimşek, Ö., Çon, A., Akkoç, N., Saris, P. & Akçelik, M. Influence of growth conditions on the nisin production of bioengineered Lactococcus lactis strains. Journal of industrial microbiology & biotechnology 36, 481–490 (2009).
Kong, W. & Lu, T. Cloning and optimization of a nisin biosynthesis pathway for bacteriocin harvest. ACS synthetic biology 3, 439–445 (2014).
Cavanagh, D., Fitzgerald, G. F. & McAuliffe, O. From field to fermentation: the origins of Lactococcus lactis and its domestication to the dairy environment. Food microbiology 47, 45–61 (2015).
Mizoguchi, H., Sawano, Y., Kato, J. & Mori, H. Superpositioning of deletions promotes growth of Escherichia coli with a reduced genome. DNA Res 15, 277–284 (2008).
Delaye, L. et al. Blueprint for a minimal photoautotrophic cell: conserved and variable genes in Synechococcus elongatus PCC 7942. BMC Genomics 12, 25 (2011).
Royce, L. A., Liu, P., Stebbins, M. J., Hanson, B. C. & Jarboe, L. R. The damaging effects of short chain fatty acids on Escherichia coli membranes. Appl Microbiol Biotechnol 97, 8317–8327 (2013).
Jarboe, L. R., Liu, P. & Royce, L. A. Engineering inhibitor tolerance for the production of biorenewable fuels and chemicals. Current Opinion in Chemical Engineering 1, 38–42 (2011).
Cotter, P. D. & Hill, C. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol Mol Biol Rev 67, 429–453 (2003).
Mizoguchi, T. et al. Cyclopropane-ring formation in the acyl groups of chlorosome glycolipids is crucial for acid resistance of green bacterial antenna systems. Bioorganic & medicinal chemistry 21, 3689–3694 (2013).
Kandror, O., DeLeon, A. & Goldberg, A. L. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. Proc Natl Acad Sci USA 99, 9727–9732 (2002).
Termont, S. et al. Intracellular accumulation of trehalose protects Lactococcus lactis from freeze-drying damage and bile toxicity and increases gastric acid resistance. Applied and environmental microbiology 72, 7694–7700 (2006).
Cusumano, Z. T. & Caparon, M. G. Citrulline protects Streptococcus pyogenes from acid stress using the arginine deiminase pathway and the F1F0-ATPase. Journal of bacteriology 197, 1288–1296 (2015).
Toledo, H., Valenzuela, M., Rivas, A. & Jerez, C. A. Acid stress response in Helicobacter pylori. FEMS microbiology letters 213, 67–72 (2002).
Nilsson, H.-O. et al. Effect of cold starvation, acid stress and nutrients on metabolic activity of Helicobacter pylori. Appl Environ Microbiol 68, 11–19 (2002).
Zhang, J., Fu, R. Y., Hugenholtz, J., Li, Y. & Chen, J. Glutathione protects Lactococcus lactis against acid stress. Appl Environ Microbiol 73, 5268–5275 (2007).
Broadbent, J. R., Larsen, R. L., Deibel, V. & Steele, J. L. Physiological and transcriptional response of Lactobacillus casei ATCC 334 to acid stress. J Bacteriol 192, 2445–2458 (2010).
Calles-Enriquez, M. et al. Sequencing and transcriptional analysis of the Streptococcus thermophilus histamine biosynthesis gene cluster: factors that affect differential hdcA expression. Appl Environ Microbiol 76, 6231–6238 (2010).
Rossi, F. et al. Quantitative analysis of histidine decarboxylase gene (hdcA) transcription and histamine production by Streptococcus thermophilus PRI60 under conditions relevant to cheese making. Appl Environ Microbiol 77, 2817–2822 (2011).
Even, S., Lindley, N. D. & Cocaign-Bousquet, M. Transcriptional, translational and metabolic regulation of glycolysis in Lactococcus lactis subsp. cremoris MG 1363 grown in continuous acidic cultures. Microbiology 149, 1935–1944 (2003).
O’Sullivan, E. & Condon, S. Relationship between acid tolerance, cytoplasmic pH and ATP and H+-ATPase levels in chemostat cultures of Lactococcus lactis. Appl Environ Microbiol 65, 2287–2293 (1999).
Reuner, A. et al. Stress response in tardigrades: differential gene expression of molecular chaperones. Cell Stress and Chaperones 15, 423–430 (2010).
Cox, M. M. et al. The importance of repairing stalled replication forks. Nature 404, 37–41 (2000).
Kim, J. et al. Fundamental structural units of the Escherichia coli nucleoid revealed by atomic force microscopy. Nucleic Acids Res 32, 1982–1992 (2004).
Itou, J., Eguchi, Y. & Utsumi, R. Molecular mechanism of transcriptional cascade initiated by the EvgS/EvgA system in Escherichia coli K-12. Biosci Biotechnol Biochem 73, 870–878 (2009).
Ma, Z., Masuda, N. & Foster, J. W. Characterization of EvgAS-YdeO-GadE branched regulatory circuit governing glutamate-dependent acid resistance in Escherichia coli. J Bacteriol 186, 7378–7389 (2004).
Jin, Y., Watt, R. M., Danchin, A. & Huang, J. D. Small noncoding RNA GcvB is a novel regulator of acid resistance in Escherichia coli. BMC Genomics 10, 165 (2009).
Gaida, S. M., Al-Hinai, M. A., Indurthi, D. C., Nicolaou, S. A. & Papoutsakis, E. T. Synthetic tolerance: three noncoding small RNAs, DsrA, ArcZ and RprA, acting supra-additively against acid stress. Nucleic Acids Res 41, 8726–8737 (2013).
Weber, H., Polen, T., Heuveling, J., Wendisch, V. F. & Hengge, R. Genome-wide analysis of the general stress response network in Escherichia coli: σS-dependent genes, promoters and sigma factor selectivity. J Bacteriol 187, 1591–1603 (2005).
Yu, Z. et al. Activators of the glutamate-dependent acid resistance system alleviate deleterious effects of YidC depletion in Escherichia coli. J Bacteriol 193, 1308–1316 (2011).
Smith, C. B. & Graham, D. E. Outer and inner membrane proteins compose an arginine-agmatine exchange system in Chlamydophila pneumoniae. J Bacteriol 190, 7431–7440 (2008).
Erkens, G. B. & Slotboom, D. J. Biochemical characterization of ThiT from Lactococcus lactis: a thiamin transporter with picomolar substrate binding affinity. Biochemistry 49, 3203–3212 (2010).
Madeo, M. et al. Thiamine plays a critical role in the acid tolerance of Listeria monocytogenes. FEMS Microbiol Lett 326, 137–143 (2012).
Even, S., Lindley, N. D., Loubiere, P. & Cocaign-Bousquet, M. Dynamic response of catabolic pathways to autoacidification in Lactococcus lactis: transcript profiling and stability in relation to metabolic and energetic constraints. Mol Microbiol 45, 1143–1152 (2002).
Bouhss, A., Trunkfield, A. E., Bugg, T. D. & Mengin-Lecreulx, D. The biosynthesis of peptidoglycan lipid-linked intermediates. FEMS Microbiol Rev 32, 208–233 (2008).
Siegumfeldt, H., Bjorn Rechinger, K. & Jakobsen, M. Dynamic changes of intracellular pH in individual lactic acid bacterium cells in response to a rapid drop in extracellular pH. Appl Environ Microbiol 66, 2330–2335 (2000).
O’Sullivan, E. & Condon, S. Intracellular pH is a major factor in the induction of tolerance to acid and other stresses in Lactococcus lactis. Appl Environ Microbiol 63, 4210–4215 (1997).
Nicolaou, S. A., Gaida, S. M. & Papoutsakis, E. T. A comparative view of metabolite and substrate stress and tolerance in microbial bioprocessing: From biofuels and chemicals, to biocatalysis and bioremediation. Metab Eng 12, 307–331 (2010).
Hutkins, R. W. & Nannen, N. L. pH homeostasis in lactic acid bacteria. J Dairy Sci 76, 2354–2365 (1993).
Pongtharangkul, T. & Demirci, A. Effects of fed-batch fermentation and pH profiles on nisin production in suspended-cell and biofilm reactors. Appl Microbiol Biotechnol 73, 73–79 (2006).
Parente, E., Ricciardi, A. & Addario, G. Influence of pH on growth and bacteriocin production by Lactococcus lactis subsp. lactis 14ONWC during batch fermentation. Appl Microbiol Biotechnol 41, 388–394 (1994).
Zheng, Z.-Y. et al. A new polysialic acid production process based on dual-stage pH control and fed-batch fermentation for higher yield and resulting high molecular weight product. Appl Microbiol Biotechnol 97, 2405–2412 (2013).
Shimizu, H., Mizuguchi, T., Tanaka, E. & Shioya, S. Nisin production by a mixed-culture system consisting of Lactococcus lactis and Kluyveromyces marxianus. Appl Environ Microbiol 65, 3134–3141 (1999).
Wu, C. et al. A combined physiological and proteomic approach to reveal lactic-acid-induced alterations in Lactobacillus casei Zhang and its mutant with enhanced lactic acid tolerance. Appl Microbiol Biotechnol 93, 707–722 (2012).
Abdullah Al, M., Sugimoto, S., Higashi, C., Matsumoto, S. & Sonomoto, K. Improvement of multiple-stress tolerance and lactic acid production in Lactococcus lactis NZ9000 under conditions of thermal stress by heterologous expression of Escherichia coli DnaK. Appl Environ Microbiol 76, 4277–4285 (2010).
Wu, C., Zhang, J., Du, G. & Chen, J. Heterologous expression of Lactobacillus casei RecO improved the multiple-stress tolerance and lactic acid production in Lactococcus lactis NZ9000 during salt stress. Bioresour Technol 143, 238–241 (2013).
Dahl, J. U. et al. HdeB functions as an acid-protective chaperone in bacteria. J Biol Chem 290, 65–75 (2015).
Wilkins, J. C., Homer, K. A. & Beighton, D. Altered protein expression of Streptococcus oralis cultured at low pH revealed by two-dimensional gel electrophoresis. Applied and environmental microbiology 67, 3396–3405 (2001).
Mercade, M., Lindley, N. D. & Loubiere, P. Metabolism of Lactococcus lactis subsp. cremoris MG 1363 in acid stress conditions. Int J Food Microbiol 55, 161–165 (2000).
Slonczewski, J. L., Fujisawa, M., Dopson, M. & Krulwich, T. A. Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Adv Microb Physiol 55, 1–79, 317 (2009).
Krulwich, T. A., Sachs, G. & Padan, E. Molecular aspects of bacterial pH sensing and homeostasis. Nat Rev Microbiol 9, 330–343 (2011).
Swords, W. E. Chemical transformation of E. coli. Methods in molecular biology (Clifton, N.J.) 235, 49–53 (2003).
Taketo, A. DNA transfection of Escherichia coli by electroporation. Biochimica et biophysica acta 949, 318–324 (1988).
Bustin, S. A. et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clinical chemistry 55, 611–622 (2009).
Breeuwer, P., Drocourt, J., Rombouts, F. M. & Abee, T. A novel method for continuous determination of the intracellular pH in bacteria with the internally conjugated fluorescent probe 5 (and 6-)-carboxyfluorescein succinimidyl ester. Appl Environ Microbiol 62, 178–183 (1996).
Adney, B. & Baker, J. Measurement of cellulase activities. Laboratory analytical procedure 6, 1996 (1996).
Yao, Y. F., Wang, C. S., Qiao, J. & Zhao, G. R. Metabolic engineering of Escherichia coli for production of salvianic acid A via an artificial biosynthetic pathway. Metabolic engineering 19, 79–87 (2013).
Acknowledgements
This program was financially supported by the National Key Technology Support Program (2014BAD02B00), the National Natural Science Foundation of China (31270142, 31540001, 32570089). Dr. Jianjun Qiao was supported by The New Century outstanding talent support program, Education Ministry of China.
Author information
Authors and Affiliations
Contributions
J.Q.: Conceived and designed the experiments and wrote the paper. J.Z.: Conducted the experiments. J.Z. and Q.C.: Analysed the data, validated the results of profile and wrote the paper. W.F. and X.Z.: Performed the experiments. B.Q. and G.Z.: Contributed reagents/materials/analysis tools. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, J., Caiyin, Q., Feng, W. et al. Enhance nisin yield via improving acid-tolerant capability of Lactococcus lactis F44. Sci Rep 6, 27973 (2016). https://doi.org/10.1038/srep27973
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep27973
This article is cited by
-
Acid-tolerant bacteria and prospects in industrial and environmental applications
Applied Microbiology and Biotechnology (2023)
-
Microbial response to acid stress: mechanisms and applications
Applied Microbiology and Biotechnology (2020)
-
Higher nisin yield is reached with glutathione and pyruvate compared with heme in Lactococcus lactis N8
Brazilian Journal of Microbiology (2020)
-
Systemic understanding of Lactococcus lactis response to acid stress using transcriptomics approaches
Journal of Industrial Microbiology and Biotechnology (2019)
-
Improved acid-stress tolerance of Lactococcus lactis NZ9000 and Escherichia coli BL21 by overexpression of the anti-acid component recT
Journal of Industrial Microbiology and Biotechnology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.