Abstract
Luminescent properties are affected by lattice environment of luminescence centers. The lattice environment of emission centers can be effectively changed due to the diversity of lattice environment in multiple site structure. But how precisely control the doped ions enter into different sites is still very difficult. Here we proposed an example to demonstrate how to control the doped ions into the target site for the first time. Alkali metal ions doped ZnMoO4:Bi3+, Eu3+ phosphors were prepared by the conventional high temperature solid state reaction method. The influence of alkali metal ions as charge compensators and remote control devices were respectively observed. Li+ and K+ ions occupy the Zn(2) sites, which impede Eu and Bi enter the adjacent Zn(2) sites. However, Na+ ions lie in Zn(1) sites, which greatly promoted the Bi and Eu into the adjacent Zn(2) sites. The Bi3+ and Eu3+ ions which lie in the immediate vicinity Zn(2) sites set off intense exchange interaction due to their short relative distance. This mechanism provides a mode how to use remote control device to enhance the energy transfer efficiency which expected to be used to design efficient luminescent materials.
Similar content being viewed by others
Introduction
Phosphor-converted white light emitting diodes (pcWLEDs) are treated as next generation lighting source1,2. Currently, the most common pcWLEDs employ blue InGaN LED coated with yellow Y3Al5O12:Ce3+ (YAG:Ce3+) phosphor3,4. It is un-optimized for indoor use due to emission spectrum deficient in the red spectral region. To enhance red emission and raise color rendering index, a blend of YAG:Ce3+ and red emitting phosphor is generally utilized5. However, the current commercial red phosphor like Y2O2S:Eu3+ present chemical instability and low absorption in near ultraviolet (UV) region6. Hence high efficient and stable red phosphors that can be excited in near UV region should be developed.
The luminescence property of phosphor is known to be strongly affected by crystal lattice environment of the host. At present, adjusting the lattice environment of activators is a hotspot for solid state lighting7. In order to improve the luminescence efficiency, site occupation of activators in host lattice has been investigated from different perspectives5,7,8,9. Peng5 found the site occupancy preference of Mn4+ in Sr4Al14O25 is at the Al(4) and Al(5) higher covalent sites rather than the Al(6) site. They believe the high fluorescence intensity and thermal stability are due to the special environment of Mn4+ centers. Wang9 has improved the thermal stability of CaAlSiN3:Eu2+ phosphors through neighboring-cation substitution. Tsai7 has enhanced the luminescent behavior of Eu2+ doped CaAlSiN3 phosphors through adjusting the lattice environment of Eu2+ ions. And the lattice environment of Eu2+ doped CaAlSiN3 phosphor has been modified through cation substitution to induce charge variation and a rearrangement of neighboring nitride clusters.
Bi3+ is a common activator and there are lots of reports on Eu3+ red luminescence enhancement via energy transfer from Bi3+ to Eu3+ in a variety of hosts10,11. A free Bi3+ ion has 6s2 electronic configuration and the absorption band of phosphors doped with Bi3+ might be extended to near UV region due to the influence of the host lattice on the outermost electrons12,13. Lili Wang14 has summarized and established the relationships between the positions of energy levels of Bi3+ and environmental factor he of host materials in dozens of compounds. Recently, Bi3+ and Eu3+ co-doped ZnMoO4 phosphors have been synthesized through solid state reaction and their properties have been discussed12. It has been found that there exists a super energy transfer process from Bi3+ to Eu3+ due to the special S-shaped cluster in ZnMoO4. There are three kinds of Zn sites in ZnMoO4 and every six nearest Zn atoms form an S-shaped cluster. According to the quantitative relationship between energy levels of Bi3+ and host lattice environment established before14, the positions of 1S0→3P1 transition of Bi3+ in three different Zn sites were predicted and then it was concluded that Bi3+ ions prefer to occupy Zn(2) sites. Thus the distance between Bi3+ and Eu3+ ions can be adjusted through their total concentrations. When their total molar concentration is larger than one sixth of that of Zn sites, Bi3+ and Eu3+ will locate in two adjacent Zn(2) sites and therefore extreme efficient energy transfer occurred12.
However, there are defects because of charge imbalance in Bi3+, Eu3+ co-doped ZnMoO4 phosphor. Additionally, concentration quenching occurred when the total concentration of Bi3+ and Eu3+ exceed one sixth of Zn sites. To solve the problem of unbalance charge as well as to explore the reasons for luminescence quenching, ZnMoO4:Bi3+, Eu3+, M+ (M = Li, Na, K) phosphors were prepared and their properties were investigated. We found that only Na+ can strongly enhance the luminescence intensity of Eu3+. Based on the previous work as well as the preferred coordination number of Li, Na and K, site occupation of Bi3+ and Eu3+ was further adjusted and controlled. Thus site occupation preference of Bi3+ and Eu3+ and energy transfer between them in ZnMoO4 were discussed in detail. Moreover, the red emission of Eu3+ was further increased after co-doping with charge compensator Na+ ions.
Results
Crystal structure
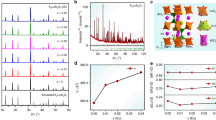
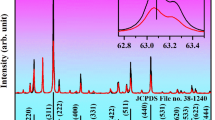
Figure 1 showed the results of the X-ray diffraction of the product after calcination at 700 °C for 3 h. All the diffraction peaks could be indexed to the triclinic wolframite ZnMoO4 phase (JCPDS 35–0765) with space group P-1 and there was no formation of impurity phases. This indicated that the obtained samples were single-phased and the co-doped Eu3+, Bi3+ and alkali metal ions did not lead to any significant change in the host structure.
As can be seen from Fig. 2, every six Zn-O polyhedra form an S-shaped cluster. The completely centro-symmetric S-shaped cluster has three kinds of Zn sites. Each Zn(1) combine with five O ions to form Zn(1)O5 hexahedral. In the center of S-shaped clusters, each two Zn(2)O6 polyhedron connected by sharing edges and their relatively distance is only 3.2202 Å. Zn(2) and Zn(3) ions have six coordination number in an approximately octahedral coordination environment. In our previous work, the host lattice environment of luminescence centres strongly affects the luminescence properties. Covalence of chemical bond, coordination number of central ions and site symmetry appear to be important factors for the luminescence properties of Bi3+ ion15. The environment factor of three kinds of Zn sites were marked in corresponding position in the diagram. The five coordinated Zn(1) site has largest environmental factor value. The environment factor of Zn(2) and Zn(3) sites are very similar with only 5% difference. However, only Na+ ions can form five-coordinate structure. Therefore, Bi3+, Eu3+, Li+ and K+ ions preferentially occupy Zn(2) sites while only Na+ can lie in Zn(1) sites.
The valence state of bismuth in doped samples
When Bi was introduced into phosphors via higher temperature solid state reaction method, it could readily transform into different valence states. In order to ensure the accuracy of the experiments, it is highly essential to identify the valence of bismuth in the samples. Therefore, we examined a representative sample by XPS. The selected spectrum is shown in Fig. 3, which correspond to Zn0.80Eu0.05Bi0.05Na0.10MoO4 sample. The XPS of which shows two characteristic Bi3+ peaks at around 159.0 and 164.3 eV due to 4f7/2 and 4f5/2, respectively. It match well with those of α-Bi2O316,17. It means the dominance of Bi3+ in Bi doped ZnMoO4 samples.
Photoluminescence properties
In order to investigate the relationship between doping concentrations and luminescence properties, the variation of emission intensity in Zn0.90−yEuyBi0.10MoO4 phosphors with the increasing Eu3+ concentration needs to study. Figure 4(a) showed the excitation spectra of the phosphors monitored at the 5D0→7F2 transition emission (616–619 nm) of Eu3+. In the PLE spectra, the broad band within the range 250–340 nm could be ascribed to the O2−→Mo6+ and O2−→Eu3+ charge transfer band (CTB) transition. The sharp lines were due to the intra configurational 4f-4f transitions of Eu3+ ions, which could be assigned to 7F0-5D4 (363 nm), 7F0-5L7 (385 nm), 7F0-5L6 (395 nm), 7F0-5D3 (417 nm), 7F0-5D2 (466 nm) and 7F0-5D1 (537 nm), respectively. In addition, a strong excitation band peaking at about 354 nm in Zn0.80Eu0.10Bi0.10MoO4 phosphor was observed due to the 1S0→3P1 transition of Bi3+. When the total molar concentration was beyond 1/6, Bi3+ and Eu3+ began to sit two adjacent Zn(2) sites. According to Fig. 4(a), when the Eu3+ content was more than 0.0667, the Bi3+ ions 1S0-3P1 excitation band peaking at about 331 nm decreased suddenly and the peak position of excitation band shifted from 355 nm to 331 nm obviously. The optimal doping concentration of Eu3+ was 0.0667. Moreover, the new super energy transfer from Bi3+ to Eu3+ emerged due to their short distance. According to the inset in Fig. 4(b), the PL intensity increased with increasing Eu3+ concentration within the range from 0.03 to 0.0667. However, when the doping concentration of Eu3+ was beyond 0.0667, the emission intensity decreased with increasing Eu3+ concentration. The concentration quenching occurred due to the short distance between Eu3+ ions. This indicated that the over high concentration of Eu3+ can result in Eu3+ ions occupy two adjacent Zn(2) sites in S-shaped cluster structure.
It is widely reported in the literature that the co-doping of alkali ions in the host lattice can enhance the luminescence significantly due to the strongly affects on the crystal structure. In this paper, Li+, Na+ and K+ ions were added to act as the remote control device to adjust the position and crystal structure environment of Bi3+ and Eu3+ ions in Zn0.90Eu0.05Bi0.05MoO4 phosphors. Because alkali metal ions can modify the local symmetry and the surroundings near the rare earth ions, which can significantly affect the photoluminescence properties. The PL and PLE spectra of Zn0.90Eu0.05Bi0.05MoO4, Zn0.80Eu0.05Bi0.05Li0.10MoO4, Zn0.80Eu0.05Bi0.05Na0.10MoO4 and Zn0.80Eu0.05Bi0.05K0.10MoO4 phosphors at room temperature are shown in Fig. 5. The photoluminescence excitation (PLE) spectra monitored at the 5D0-7F2 transition emission (616–619 nm) of Eu3+ were shown in Fig. 5(a). From Fig. 5(a), the excitation spectra of samples are obviously affected by doping ions. Especially, the introduction of Na+ ions remarkably enhanced the excitation band intensity in the ultraviolet and near ultraviolet region. According to Fig. 5(b,c,d), the shape and positions were similar in the PL spectra for all samples. When excited at about 350 nm, the PL intensity of Zn0.80Eu0.05Bi0.05Na0.10MoO4 phosphor enhanced three times compared to the Zn0.90Eu0.05Bi0.05MoO4 phosphor. The order of emission intensity for the Eu3+ ions with the three alkali metal ions is Na≫Li>K. However, there is no contribution on luminescence enhancement for Li+ ions. Furthermore, the introduction of K+ can significantly reduce the emission intensity of Eu3+. This reflects the impact of the alkali metal ions as remote control device on the energy transfer from Bi to Eu. From Fig. 5(c), when excited at about 394 nm to excite the inner 4f electron transition, the emission spectrum simultaneously affected by energy transfer from Bi to Eu ions due to the extending of Bi3+ ions absorption band. The order of emission intensity for the Eu3+ ions with the three alkali metal ions is Na≫Li>K.
Without the influence of Bi3+ excitation band, the emission spectra which were excited at about 465nm reflect the influence of alkali metal ions as charge compensators on the luminescent center of Eu3+ ions. From Fig. 5(d) it can be seen that the influence of doping ions on the fluorescence intensity was different from Fig. 5(b,c). The introduction of Li+, Na+ and K+ ions influenced the luminous intensity of Eu3+ ions obviously. The order of emission intensity for the Eu3+ ions with the three alkali metal ions is Li>Na>K. This reflects the effect of introduce alkali metal ions as charge compensators. However, from Fig. 5(a,b), only Na+ ions can strongly enhance the emission intensity which was different with the effect of charge compensation. Li+ and K+ ions have hindered the energy transfer from Bi to Eu ions. This shows that there is a special enhancement mechanism different with charge compensation. For further investigating the enhancement mechanism, the doping ions were fixed at Na+ ions.
The excitation spectra of Zn0.80−xEu0.05Bi0.05NaxMoO4 phosphors under the excitation wavelength of 614 nm are shown in Fig. 6. From the picture, it can be seen that the introduction of Na+ into the host can significantly enhance the luminescence intensity. Therefore, to maintain the charge balance with Na+ ions will be more advantageous to improve the energy transfer. However, it was found that the position of excitation band blue-shifted clearly, which indicates that the lattice environment of Bi3+ ions has been changed obviously. This is affected by the charge compensation agent. Figure 7 gave the most probable remote control mechanisms.
Remote control mechanism
The introduction of alkali metal ions can not only lower the charge imbalance but also influence the position of Eu3+ and Bi3+ ions. According to the previous work, Bi3+ and Eu3+ ions could occupy preferentially the Zn(2) site12. However, because of the charge imbalance when Bi3+ or Eu3+ seated in the Zn(2) site, alkaline ions will occupy Zn(1) or Zn(2) site which is the nearest dopant neighbor. As reported in literatures, Zn(2) and Zn(3) have six oxygen ligands, while Zn(1) has only five coordination number18,19,20 which can provide more compact space to charge compensation with small ionic radius. Moreover, compared with Zn(2) and Zn(3) site, Zn(1) site has the largest environment factor he12. As far as we know, in alkaline ions (Li+, Na+ and K+), only Na+ ions can form five-coordinated polyhedra21. Therefore, Li+ and K+ ions could only occupy Zn(2) site which is nearest Eu3+ or Bi3+ ions. It is not conductive to shorten the relatively distance between Eu3+ and Bi3+ ions. Thence, the introduction of Li+ and K+ can obstruct the energy transfer from Bi3+ to Eu3+ and then reduce the emission intensity of Eu3+ ions. Table 1 lists the ionic radius under different coordination numbers. It can be seen that only Na+ ions can form five coordination sites. When Na+ ions were added as charge compensators, Zn(1) site was more suitable to be occupied due to the similar ionic radius between Na+ (1.14 Å) and Zn2+ (0.82 Å) for five coordination. In summary, when Na+ was co-doped as charge compensator, it can seat into Zn(1) site and shorten the relative distance between Bi3+ and Eu3+. The possible mechanism was shown in schematic diagram in Fig. 7.
Conclusion
In summary, a series of Eu3+, Bi3+ and alkali metal ions co-doped ZnMoO4 phosphors were prepared by the solid-state method in air atmosphere. Li+, Na+ and K+ ions acted as the remote control device were added to improve the luminescence intensities. The super energy transfer process from Bi3+ to Eu3+ cannot appear only when the total concentration of Bi3+ and Eu3+ ion is very high. Without alkali metal ions, Eu3+ and Bi3+ ions lie in Zn(2) sites. The introduced Li+ and K+ ions seat into Zn(2) site which against the energy transfer from Bi3+ to Eu3+. However, Na+ ions lie in Zn(1) site and then help Bi3+ and Eu3+ ions to seat into the adjacent Zn(2) sites. Compared with Li+ and K+ ions occupied Zn(2) sites, Na+ ions which preferentially occupied Zn(1) site can get closer to the distance between Bi3+ and Eu3+ ions. The results indicated that Na+ ions provided intensity of energy transfer from Bi3+ to Eu3+. This mechanism provides a mode how to use remote control device to enhance the energy transfer efficiency which expected to be used to design efficient luminescent materials.
Experiment
Materials and Synthesis
A series of ZnMoO4:1/6Eu3+, 1/6Bi3+, xM+ (M = Li, Na, K) and ZnMoO4:0.05Eu3+, 0.05Bi3+, 0.1M+ (M = Li, Na, K) phosphors with various concentrations and alkali metal ions were synthesized through the solid state reaction method in air atmosphere. The raw materials were ZnO (99%), Eu2O3 (99.99%), Bi2O3 (99.9%), Li2CO3 (99%), Na2CO3 (99%), K2CO3 (99%) and MoO3 (99%). The starting materials were weighed according to the stoichiometric ratio and well mixed in agate mortar. The mixtures were put into alumina crucible and calcined in muffle furnace at 700 oC for 3 h and then the white powder phosphor was obtained. All samples were ground into a powder with an agate mortar and pestle for further analysis.
Materials Characterization
The crystal structure of the samples was determined by the Bruker D8 Advance X-ray diffractometer (Cu Kα1 radiation, λ = 0.15406 nm) with radiation at a 0.02o (2θ) /0.05 s scanning step. The photoluminescence excitation (PLE) and emission (PL) spectra were recorded with a Hitachi F-4600 spectrophotometer equipped with a 150 W xenon lamp as an excitation source. All the measurements were performed at room temperature.
Additional Information
How to cite this article: Ran, W. et al. Remote Control Effect of Li+, Na+, K+ Ions on the Super Energy Transfer Process in ZnMoO4:Eu3+, Bi3+ Phosphors. Sci. Rep. 6, 27657; doi: 10.1038/srep27657 (2016).
References
Ye, S., Xiao, F., Pan, Y., Ma, Y. & Zhang, Q. Phosphors in phosphor-converted white light-emitting diodes: Recent advances in materials, techniques and properties. Materials Science & Engineering R-Reports 71, 1–34 (2010).
Feldmann, C., Thomas, J., Cees, R. R. & Peter, J. S. Inorganic luminescent materials: 100 years of research and application. Advanced Functional Materials 13, 511–516 (2003).
Zhang, R. et al. A new-generation color converter for high-power white LED: transparent Ce3+:YAG phosphor-in-glass. Laser & Photonics Reviews 8, 158–164 (2014).
Nakmura, S. & Fasol, G. The blue laser diode-GaN based light emitters and lasers (Springer, Berlin, 1997).
Peng, M., Yin, X., Tanner, P. A., Brik, M. G. & Li, P. The site occupancy preference, the enhancement mechanism and the thermal resistance of Mn4+ red luminescence in Sr4Al14O25:Mn4+ for warm WLEDs. Chemistry of Materials 27, 2938–2945 (2015).
Yan, X., Li, W. & Sun, K. A novel red emitting phosphor CaIn2O4:Eu3+, Sm3+ with a broadened near-ultraviolet absorption band for solid-state lighting. Materials Research Bulletin 46, 87–91 (2011).
Tsai, Y.-T. et al. Structural Ordering and Charge Variation Induced by Cation Substitution in (Sr, Ca)AlSiN3:Eu Phosphor. Journal of the American Chemical Society 137, 8936–8939 (2015).
Clabau, F. et al. Mechanism of Phosphorescence Appropriate for the Long-Lasting Phosphors Eu2+-Doped SrAl2O4 with Codopants Dy3+ and B3+. Chemistry of Materials 17, 3904–3912 (2005).
Wang, S.-S. et al. Neighboring-Cation Substitution Tuning of Photoluminescence by Remote-Controlled Activator in Phosphor Lattice. Journal of the American Chemical Society 135, 12504–12507 (2013).
Zhou, H., Jin, Y., Jiang, M., Wang, Q. & Jiang, X. A single-phased tunable emission phosphor MgY2Si3O10: Eu3+, Bi3+ with efficient energy transfer for white LEDs. Dalton Transactions 44, 1102–1109 (2015).
Qiang, S., Barthou, C., Denis, J. P., Pelle, F. & Blanzat, B. Luminescence and energy transfer in Y2O3 co-doped with Bi3+ and Eu3+. Journal of Luminescence 28, 1–11 (1983).
Ran, W. et al. A super energy transfer process based S-shaped cluster in ZnMoO4 phosphors: theoretical and experimental investigation. Journal of Materials Chemistry C, 8344–8350 (2015).
Ran, W. et al. Luminescence properties and energy transfer of CdWO4:Sm3+,Bi3+,M+ (M = Li, Na, K) phosphors for white LEDs. Ceramics International 41, 4301–4307 (2015).
Wang, L. L., Sun, Q., Liu, Q. Z. & Shi, J. S. Investigation and application of quantitative relationship between sp energy levels of Bi3+ ion and host lattice. Journal of Solid State Chemistry 191, 142–146 (2012).
Van Steensel, L. I., Bokhove, S. G., Van der Craats, A. M., De Blank, J. & Blasse, G. The Luminescence of Bi3+ In LaInO3 and some other perovskites. Materials Research Bulletin 30, 1359–1362 (1995).
Kang, F. et al. Processing-dependence and the nature of the blue-shift of Bi3+-related photoemission in ScVO4 at elevated temperatures. Journal of Materials Chemistry C 2, 9850–9857 (2014).
Kang, F. et al. Broadly Tunable Emission from CaMoO4:Bi Phosphor Based on Locally Modifying the Microenvironment Around Bi3+ Ions. European Journal of Inorganic Chemistry 2014, 1373–1380 (2014).
Abrahams, S. C. In Acta Crystallographica A40 (MUNKSGAARD INT PUBL LTD: 35 NORRE SOGADE, PO BOX 2148, DK-1016 COPENHAGEN, DENMARK,, 1966).
Reichelt, W., Weber, T., Söhnel, T. & Däbritz, S. Mischkristallbildung im System CuMoO4/ZnMoO4 . Zeitschrift für anorganische und allgemeine Chemie 626, 2020–2027 (2000).
Meullemeestre, J. & Penigault, E. Les molybdates neutres de zinc. Bulletin de la Société chimique de France 868, 3669–3674 (1972).
Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica Section A: Crystal Physics, Diffraction, Theoretical and General Crystallography 32, 751–767 (1976).
Acknowledgements
This work was supported by the Research Fund for Technology Upgrading of Large Scientific Instruments and Equipment in Shandong Province (2013SJGZ01) and Science and Technology Development Plan of Shandong Province, China (2014GNC110013).
Author information
Authors and Affiliations
Contributions
W.R. designed the whole research and completed the writing of the manuscript. L.W. and L.T. performed the sample preparation and all the experimental tests. D.Q. analyzed the data. J.S. designed the whole research, revised the articles and proposed many good suggestions. All the authors discussed the results and commented on the manuscript at all stages.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ran, W., Wang, L., Tan, L. et al. Remote Control Effect of Li+, Na+, K+ Ions on the Super Energy Transfer Process in ZnMoO4:Eu3+, Bi3+ Phosphors. Sci Rep 6, 27657 (2016). https://doi.org/10.1038/srep27657
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep27657
This article is cited by
-
Tunable photoluminescence and energy transfer of Eu3+,Ho3+-doped Ca0.05Y1.93-xO2 nanophosphors for warm white LEDs applications
Scientific Reports (2022)
-
Visible light-driven photocatalytic degradation of methylene blue dye over bismuth-doped cerium oxide mesoporous nanoparticles
Environmental Science and Pollution Research (2021)
-
Break the Interacting Bridge between Eu3+ Ions in the 3D Network Structure of CdMoO4: Eu3+ Bright Red Emission Phosphor
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.