Abstract
Microalbuminuria is associated with an increased risk of cardiovascular disease (CVD), but not all individuals require treatment. Retinal microvascular abnormalities and microalbuminuria reflect early systemic microvascular changes. We examined the joint effect of retinal abnormalities and microalbuminuria on CVD risk in an Asian cohort. We conducted a prospective, population-based study. Retinal abnormalities were defined as presence of retinopathy and/or retinal venular widening. Microalbuminuria was defined as urinary albumin: creatinine ratio between 30–300 mg/g. Incident CVD was defined as newly diagnosed clinical stroke, acute myocardial infarction or CVD death. Cox regression models were performed to determine the associations between retinal abnormalities and microalbuminuria with risk of CVD, while controlling for established risk factors. 3,496 participants (aged ≥ 40) were free of prevalent CVD. During the follow-up (5.8 years), 126 (3.60%) participants developed CVD. Persons presenting with both retinal abnormalities and microalbuminuria were 6.71 times (95% CI, 2.68, 16.79) as likely to have incident CVD compared with those without either abnormalities. There was a significant interaction effect between retinal abnormalities and microalbuminuria on incident CVD. Assessment of retinal abnormalities in patients with microalbuminuria may provide additional value in identifying persons at risk of developing CVD.
Similar content being viewed by others
Introduction
Microalbuminuria is an increasing well-recognized indicator of cardiovascular disease (CVD) risk, with many studies consistently demonstrating an association between microalbuminuria and incident CVD1,2,3. However, microalbuminuria measurements can be highly variable4,5. For example, short-term hyperglycemia, exercise, urinary tract infections and acute febrile illness can cause transient elevations in urinary albumin excretion4,6. As such, microalbuminuria may result in an “overestimation” of CVD risk.
Retinal vessels, measuring 100 to 300 μm in size, offer a unique and easily accessible “window” to study the health and disease of the human microcirculation7,8. There is increasing evidence showing that retinal microvascular changes, reflecting systemic microcirculation damage, may provide additional value in CVD risk prediction9,10. Specifically, two retinal microvascular abnormalities, retinopathy and retinal venular caliber widening, measured from digital retinal photographs, have been found to be associated with subclinical CVD11,12,13,14 and to predict clinical CVD events10,12,15.
Because both microalbuminuria and retinal microvascular abnormalities are potential microvascular markers of CVD events, it may be possible that a “multiple markers” approach may be useful for risk stratification and identification of patients who are at higher risk of developing CVD. Measurement of retinal microvascular changes have been shown to be accurate and repeatable7 and retinal vascular imaging utilized as an adjunctive test have been shown to increase the precision of a more widely accepted test to benefit a specific subgroup of patients16 For example, Baumann et al. have recently reported that a combination of retinal arteriolar narrowing and albuminuria increased the risk of chronic kidney disease as compared with person who has no evidence of microvascular damage17.
In the present study, we aimed to examine the joint effect of retinal microvascular abnormalities and microalbuminuria on CVD risk in a multi-ethnic Asian cohort.
Results
A total of 3,496 participants were free of prevalent CVD events at baseline. During a median follow-up of 5.8 years (baseline examination [2003 to 2007] and 31 December 2011), 126 (3.6%) participants developed CVD events. Of the CVD events, there were 53 (42.1%) AMI events, 38 (30.2%) stroke events and 35 (27.7%) CVD deaths. Table 1 shows the baseline characteristics of those who developed CVD events and those who did not.
Tables 2 shows the Cox proportional-hazards regression models of retinal arteriolar caliber, retinal venular caliber, retinopathy and microalbuminuria with CVD risk. Retinal venular widening (per SD increase: HR 1.33, 95% CI, 1.09 to 1.62) was independently associated with increased risk of CVD, after adjusting for age, gender, ethnicity, current smoking, diabetes, total cholesterol, HDL cholesterol, systolic BP, anti-hypertensive medication, eGFR, CRAE and retinopathy. The association was similar when retinal vascular caliber was analyzed as quartiles (HR 2.07; 95% CI, 1.15 to 3.73). The presence of retinopathy (HR 2.05, 95% CI, 1.30 to 3.22) was also significantly associated with increased risk of CVD. No significant associations were observed between retinal arteriolar caliber with incident CVD events. The presence of microalbuminuria was independently associated with increased risk of CVD events (HR 1.68, 95% CI, 1.13, 2.50), after adjusting for age, gender, ethnicity, current smoking, diabetes, total cholesterol, HDL cholesterol, systolic BP, anti-hypertensive medication, eGFR, CRVE, and retinopathy. In the supplementary analysis, further adjustment for hsCRP level in the multivariate models did not alter the associations between widened retinal venular caliber (HR 2.05, 95% CI, 1.12, 3.74), (per SD increase: 1.33, 95% CI, 1.09, 1.63), presence of retinopathy (HR 1.81, 95% CI: 1.12 to 2.92), and presence of microalbuminuria (HR 1.72, 95% CI, 1.15, 2.57) with incident CVD (Supplementary Table 1).
The joint effect of microalbuminuria and retinal microvascular abnormalities (widened retinal venules and presence of retinopathy) on the risk of CVD events is graphically depicted in Fig. 1. Compared with the referent group, those with any retinal microvascular abnormalities and microalbuminuria (Group 4) were 2.83 times (95% CI, 1.65, 4.87) as likely to develop incident CVD. Persons presenting with both widened retinal venules and retinopathy but without microalbuminuria (Group 5) at baseline, were 3.04 times (95% CI, 1.29, 7.17) as likely to develop incident CVD, compared to those with neither retinal microvascular abnormalities nor microalbuminuria (referent). Importantly, persons presenting with the coexistent of widened retinal venules, retinopathy, and microalbuminuria (Group 6) were 6.71 times (95% CI, 2.68, 16.79) as likely to have incident CVD compared to persons without the presence of microalbuminuria and retinal microvascular abnormalities (Table 3). There was a significant interaction effect between retinal microvascular abnormalities and microalbuminuria on incident CVD (p-interaction = 0.025), demonstrating the association between retinal microvascular abnormalities with incident CVD was stronger in participants with microalbuminuria. In the supplementary analysis, we separately explored the joint effect of presence of clinical retinopathy and microalbuminuria on the risk of CVD events. We observed that individuals with microalbuminuria and clinical retinopathy were 3.69 times (HR: 3.69, 95% CI 2.00, 6.81) as likely to develop incident CVD, compared to those with neither presence of retinopathy nor microalbuminuria (referent) (Supplementary Fig. 1).
P-interaction for retinal microvascular abnormalities and microalbuminuria = 0.025 Group 1 (Referent): No retinal microvascular abnormalities and no microalbuminuria Group 2: Presence of microalbuminuria only Group 3: One retinal microvascular abnormality (either retinal venular widening or retinopathy) only Group 4: One retinal microvascular abnormality and presence of microalbuminuria Group 5: Two retinal microvascular abnormalities (retinal venular widening and retinopathy) and no presence of microalbuminuria Group 6: Two retinal microvascular abnormalities and presence of microalbuminuria.
Discussion
In this multi-ethnic Asian study, we found that persons with microvascular changes in the eye (retinal venular widening and/or presence of retinopathy) or in the kidney (manifesting as microalbuminuria) were at higher risk of CVD than those without these abnormalities. Importantly, we observed a significant joint effect of retinal microvascular abnormalities and microalbuminuria on the risk of CVD. Persons with evidence of microvascular damage in both the eyes and kidney were nearly 7 times as likely to develop CVD as those without these changes. Our findings suggest that assessment of retinal vasculature may be utilized as an adjunctive test to further identify persons who are at higher risk of CVD, in addition to the assessment of microalbuminuria and traditional risk factors.
We demonstrated that individually, the presence of retinal microvascular abnormalities and microalbuminuria are predictive of CVD events, consistent with current understanding and prior studies. The association between retinal microvascular abnormalities (widened retinal venules and retinopathy) and incident CVD has been previously reported in the general population and in specific populations such as in persons with type 2 diabetes18 and in patients with chronic kidney disease19. While these associations have also been observed in several population-based studies10,20, eGFR was not adjusted for in the multivariable models. This a major limitation in those population-based studies since eGFR is an important modifiable risk factor for CVD events21. In our study, we observed an independent association between retinal abnormalities (retinal venular widening and retinopathy) and incident CVD after adjusting for both diabetic status as well as eGFR level. Our study adds to the current literature by demonstrating that retinal microvascular abnormalities are indeed predictors of incident CVD, independent of eGFR and other traditional risk factors. A few possible reasons may explain this. First, retinal venular widening have been reported to be associated with inflammation and endothelial dysfunction22,23, which are important risk factors of cardiovascular disease (CVD)24. Consistently, it was reported in the Rotterdam Study25, the Beaver Dam Eye Study26, the Singapore Prospective Study Program27 and the Multi-Ethnic Study of Atherosclerosis28 that larger venular caliber was associated with inflammatory markers, including C-reactive protein (CRP) and interleukin-6 concentrations. This suggests that the association between retinal venular widening and CVD event may be mediated through inflammatory processes. Second, retinopathy signs (i.e. retinal exudates, haemorrhage) are pathogenically related to loss of pericytes, increased vascular permeability and retinal tissue ischemia, reflecting similar microvascular changes in other vascular beds29,30 Collectively, these lines of evidence support the concept that retinal microvasculature reflects cumulative microvascular damage that contributes to the progression of CVD. Several studies have also investigated the association between microalbuminuria and incident CVD in high-risk populations such as diabetic and hypertensive cohorts31,32 as well as in the elderly population33. Our current findings, one of the few in Asians, are consistent with those in Caucasian populations1,2.
Importantly, we further reported that persons with coexistent retinal microvascular abnormalities (retinal venular widening and retinopathy) and microalbuminuria had the worst prognosis (close to 7 times as likely to develop CVD), compared with those without either markers of microvascular damage.
Our finding suggests that additional assessment of retinal microvascular abnormalities can further identify patients who are more at risk of developing CVD in future, in particular among people with microalbuminuria. Therefore, retinal vascular imaging, which is readily accessible in routine clinical practice, is useful for targeting a more specific subgroup of patients who could benefit from more intensive investigations or early treatment. Our current finding is consistent with one other study which was conducted in the U.S. (National Health and Nutrition Examination Survey [NHANES])20. The authors reported the joint effect of retinopathy and chronic kidney disease with mortality in a general population20. However, this study did not examine other abnormalities of retinal microvascular damage (e.g. generalized retinal venular widening and retinal arteriolar narrowing) and CVD risk.
While our study is observational, there is increasing evidence to support that microvascular changes in the eye and kidney predict the risk of CVD. It is known that microvascular abnormalities in the kidney and eye have overlapping etiologies and similar pathological pathway (for example: endothelial dysfunction, vascular inflammatory processes, and oxidative stress)34,35, leading to increased capillary permeability and transvascular leakages36. However, it has been reported that presence of microalbuminuria can be detected in seemingly healthy participants37 and also in participants with subclinical vascular damage in the kidneys38. As such, it has been hypothesized that variable amount of microalbuminuria reflects varying degrees of vascular function and thus a person’s inherent susceptibility to subsequent organ damage37. On the other hand, retinal microvascular abnormalities reflect greater generalized vascular dysfunction in CVD-related target organs such as the heart and the brain10,12,15. Altogether, it is possible that among participants presenting with microalbuminuria and jointly presenting with retinal microvascular abnormalities may have a more diffuse systemic microvascular damage and thus, greater susceptibility to increased risk of CVD. Future replication studies providing robust and consistent evidence, as well as additional data about potential long-term benefit for patient outcomes, are needed.
The strengths of our study include a large population-based sample, quantitative and masked evaluation of retinal vessel diameters, standardized definition of CVD events, and the availability of information on potential confounding factors. Several limitations of this study should be addressed. First, the significant number of participants excluded from the analysis due to missing data, and only a third of the participants had their urine albumin quantified in SiMES, could have resulted in a selection bias. Second, the relatively small number of CVD events limited our ability to perform stratified analyses to examine associations between microvascular damage with stroke and AMI separately. This will require longer follow-up to accrue more CVD end-points. Third, other biomarkers of inflammation (e.g. interleukin-6) were not measured, and we were therefore unable to extensively examine the role of inflammation. Future studies comparing the specific and combined roles of microvascular disease in respect to the role of other inflammatory mediators on CVD development may be warranted.
In conclusion, we have demonstrated that the presence of microvascular changes in the eye (widened retinal venules and presence of retinopathy) and kidney (microalbuminuria) independently predict the risk of CVD. Notably, the risk of CVD is significantly increased in people with coexistent retinal abnormalities and microalbuminuria. Our findings highlight the combined prognostic value of two readily accessible and quantifiable markers of microvascular disease.
Methods
Study population
We conducted a prospective, population-based study in a multi-ethnic Asian cohort to determine the relationship of retinal microvascular abnormalities and microalbuminuria on risk of CVD. We utilized data from the Singapore Prospective Study Program (SP2) and the Singapore Malay Eye (SiMES) study. Details of both study participants and methods have been described elsewhere39,40.
In brief, SP2 included participants from one of the four previous cross-sectional studies. The study sample was selected by the Ministry of Health, Singapore. From 2003 to 2007, 5157 participants attended the clinical examination and 4137 were offered retinal photography. Retinal photographs were available for 4098 participants (Fig. 2A). For the purposes of the current study, we excluded participants younger than 40 years of age, those with ungradable retinal photographs, missing microalbuminuria information, presence of macroalbuminura and with a history of CVD. Ethics approval was obtained from Institutional Review Boards of the National University of Singapore and Singapore General Hospital. Written informed consent was obtained from all participants.
SiMES is a population-based cross-sectional study examining eye diseases in urban Malay adults. In brief, the baseline examination was conducted from August 2004 through to June 2006, and included 3280 participants (Fig. 2B). We excluded participants with ungradable retinal photographs, missing microalbuminuria information, presence of macroalbuminura and with a history of CVD. Written, informed consent was obtained from each participant; the study conducted adhered to the Declaration of Helsinki. Ethical approval was obtained by the Singapore Eye Research Institute Institutional Review Board. Study methods were carried out in accordance with the approved guidelines.
Retinal microvascular changes
Retinal fundus photographs of both eyes were taken after dilating the pupils with 1% tropicamide and 2.5% phenylephrine hydrochloride, using a digital non-mydriatic retinal camera (CR-DGi with a 10D SLR backing; Canon, Tokyo, Japan). Two retinal images of each eye were obtained, one centered on the optic disc and another centered on the fovea.
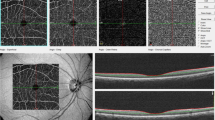
Trained graders, masked to the participants’ characteristics, performed retinal vascular caliber measurements using a computer-based program, Interactive Vessel Analysis software (IVAN) program (University of Wisconsin, Madison, US) (Fig. 3A)41. Retinal vascular caliber was measured through a specified zone of 0.5 to 1 disc diameter from the optic disc margin. Optic disc-centered image of the right eye were analyzed, and in those without gradable right eye images, left eye was analyzed. Based on the revised Knudtson-Parr-Hubbard formula42, the retinal arteriolar and venular calibers were summarized as central retinal arteriolar equivalent (CRAE) and central retinal venular equivalent (CRVE), respectively.
Two hundred randomly selected retinal photographs were re-graded by the same grader to assess intra-grader reliability (intraclass correlation coefficients [95% confidence interval (CI)] 0.99 (0.98–0.99) for CRAE and 0.94 (0.92–0.96) for CRVE)39.
Retinopathy was considered present if any characteristic lesions (microaneurysms, haemorrhages, cotton wool spots, intraretinal microvascular abnormalities, hard exudates, venous beading and new vessels) were found (Fig. 3B)43. For each eye, a retinopathy severity score was assigned accordingly and retinopathy (or clinical retinopathy) was defined as being present if the retinopathy score (a scale modified from the Airlie House classification system) was at level 15 or higher43.
Microalbuminuria
Spot untimed urine samples were collected for measurement of albumin and creatinine. Albumin was measured in mg/L and creatinine in mmol/L. The concentration ratio of urine albumin to creatinine expressed in mg/g was used to estimate the total daily albumin excretion. Presence of microalbuminuria was defined as a urinary albumin: creatinine ratio (ACR) of between 30–300 mg/g based on the National Kidney Foundation’s Kidney Disease Outcome Quality Initiative working group definition44.
Assessment of cardiovascular disease events
History of stroke/AMI events was ascertained through self-reporting and/or CVD event documented by National Registry of Diseases Office (NRDO), Singapore.
Incident CVD event was defined as newly diagnosed clinical stroke or acute myocardial infarction (AMI) or CVD death documented by NRDO that occurred during the period between baseline examination and 31 December, 2011, obtained by linking with the stroke, AMI cases and CVD deaths registered by NRDO by record linkage.
MI was defined as either (1) definitive or clinical MI; (2) death cases signed up by pathologists or physicians as MI, with or without necropsy done. Incident MI was identified by linkage with the Singapore Myocardial Infarction Registry (SMIR), a nation-wide registry under the purview of the National Registry of Diseases Office (NRDO). The epidemiological data in SMIR included all MI diagnosed and coded with International Classification of Diseases 9th revision (ICD-9) 410 in all restructured hospital45. Because the Register is electronically captured and is compulsory by law, and because of a unique identifier number for all Singapore citizens and residents (National Registration Identity Card), the Registry is expected to provide good coverage for AMI cases.
Stroke data was obtained from the Singapore Stroke Registry gathered from Mediclaims and case funding from restructured hospitals electronic medical records using ICD-9 codes 430 to 434 and 436 to 437, while excluding 432.1, 435 and 438. Data capture is estimated at 94% since private hospitals were not included46. CVD mortality was defined as CVD as the primary cause of death as stated on the death certificate.
Other variables
Information on participants’ demographic characteristics and medical history was obtained by using a standardized questionnaire administered by trained personnel. Age was defined as the age at the time of clinic examination. Height was measured in cm using a wall-mounted measuring tape and weight was measured in kg using a digital scale. Body mass index (BMI) was calculated as body weight divided by height squared and expressed as kg/m2. Systolic and diastolic blood pressures (BP) were measured twice using a digital BP monitor (Dinamap model Pro Series DP110X-RW, 100V2, GE Medical Systems Information Technologies Inc, Milwaukee, WI). A third measurement was made if the systolic BP differed by >10 mm Hg or the diastolic BP differed by >5 mm Hg. The mean between the 2 closest readings was taken as the BP of that individual.
Venous blood samples were analyzed at the National University Hospital Referral Laboratory for biochemical testing of serum total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein cholesterol, triglycerides, glycosylated hemoglobin (HbA1c), creatinine, glucose and high-sensitivity C-reactive protein (hsCRP). In SP2, diabetes mellitus was defined as fasting plasma glucose ≥7 mmol/L or HbA1c of ≥6.5% or self-reported physician-diagnosed diabetes or use of glucose-lowering medication; In SiMES, diabetes was defined as a casual plasma glucose measurement of ≥11.1 mmol/L or HbA1c of ≥6.5% or self-reported physician-diagnosed diabetes or use of glucose-lowering medication. The estimated glomerular filtration rate (eGFR) was estimated from serum creatinine using the recently developed Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation47.
Statistical Analysis
All statistical analyses were performed using STATA statistical software (Version 12, StataCorp, College Station, Texas). The outcome of interest was incident CVD event and the exposures of interest were retinal vascular caliber (analyzed as quartiles), presence of retinopathy (analyzed as binary) and presence of microalbuminuria (analyzed as binary). Retinal arteriolar (CRAE) and venular (CRVE) calibers were also analyzed as continuous variables (per each standard deviation [SD] increase/decrease). As previous studies have shown that retinal arteriolar narrowing and retinal venular widening are associated with CVD, we used the widest quartile of retinal arteriolar caliber (quartile 4) as the referent category in the retinal arteriolar narrowing analysis, and the narrowest quartile of retinal venular caliber (quartile 1) as referent category in the retinal venular widening analysis7.
First, we performed Cox proportional-hazards regression analysis to examine the relations of CRAE, CRVE, retinopathy, microalbuminuria to the risk of CVD, initially adjusted for age, gender and ethnicity, and additionally for smoking status, total cholesterol, HDL cholesterol, systolic BP, diabetic status, use of anti-hypertensive medication, eGFR and other retinal microvascular abnormalities at baseline. The proportional hazards assumption for each of these models was examined, and there was no evidence that this was violated. In supplementary analysis, we further adjusted for hsCRP level to examine whether the associations of CRAE, CRVE, retinopathy, microalbuminuria and incident CVD events were attenuated.
Second, we determined the joint effect of retinal microvascular abnormalities and microalbuminuria on the risk of CVD events. Retinal microvascular abnormalities were defined as presence of widened retinal venules (quartile 4) and/or retinopathy because these two parameters showed significant associations with incident CVD event in the Cox proportional-hazards regression analysis (Table 2). We categorized the population into 6 groups (i.e., Group 1 (Referent): no retinal microvascular abnormalities and no microalbuminuria; Group 2: no retinal microvascular abnormalities, and presence of microalbuminuria; Group 3: one retinal microvascular abnormality (either widened CRVE or retinopathy), but no microalbuminuria; Group 4: one retinal microvascular abnormality, and presence of microalbuminuria; Group 5: two retinal microvascular abnormalities (widened CRVE and retinopathy) and no presence of microalbuminuria; Group 6: two retinal microvascular abnormalities, and presence of microalbuminuria. We calculated the hazard ratios (HR) for each group vs. the referent group (Group 1) to the risk of incident CVD event. Separately, interaction between retinal microvascular abnormalities and microalbuminuria were evaluated by including a cross-product interaction term as an independent variable (i.e. retinal microvascular abnormalities × microalbuminuria).
In supplementary analysis, we separately explored the joint effect of presence of clinical retinopathy and microalbuminuria on the risk of CVD events.
Additional Information
How to cite this article: Yip, W. et al. Joint Effect of Early Microvascular Damage in the Eye & Kidney on Risk of Cardiovascular Events. Sci. Rep. 6, 27442; doi: 10.1038/srep27442 (2016).
References
Arnlov, J. et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 112, 969–975 (2005).
Hillege, H. L. et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 106, 1777–1782 (2002).
Wachtell, K. et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Annals of internal medicine. 139, 901–906 (2003).
Molitch, M. E. et al. Nephropathy in diabetes. Diabetes care. 27 Suppl 1, S79–83 (2004).
Fink, H. A. et al. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the U.S. Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Annals of internal medicine. 156, 570–581 (2012).
Heathcote, K. L., Wilson, M. P., Quest, D. W. & Wilson, T. W. Prevalence and duration of exercise induced albuminuria in healthy people. Clinical and investigative medicine. Medecine clinique et experimentale. 32, E261–265 (2009).
Cheung, C. Y., Ikram, M. K., Sabanayagam, C. & Wong, T. Y. Retinal microvasculature as a model to study the manifestations of hypertension. Hypertension. 60, 1094–1103 (2012).
Yip, W. et al. Retinal microvascular abnormalities and risk of renal failure in asian populations. PloS one. 10, e0118076 (2015).
McGeechan, K. et al. Meta-analysis: retinal vessel caliber and risk for coronary heart disease. Annals of internal medicine. 151, 404–413 (2009).
Wang, J. J. et al. Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. European heart journal. 28, 1984–1992 (2007).
Cooper, L. S. et al. Retinal microvascular abnormalities and MRI-defined subclinical cerebral infarction: the Atherosclerosis Risk in Communities Study. Stroke. 37, 82–86 (2006).
Sherry, L. M. et al. Reliability of computer-assisted retinal vessel measurementin a population. Clin Experiment Ophthalmol. 30, 179–182 (2002).
Cheung, N. et al. Retinal arteriolar narrowing and left ventricular remodeling: the multi-ethnic study of atherosclerosis. J Am Coll Cardiol. 50, 48–55 (2007).
Wong, T. Y. et al. Relation of retinopathy to coronary artery calcification: the multi-ethnic study of atherosclerosis. American journal of epidemiology. 167, 51–58 (2008).
Cheung, C. Y. et al. Retinal microvascular changes and risk of stroke: the Singapore Malay Eye Study. Stroke; a journal of cerebral circulation. 44, 2402–2408 (2013).
Wong, T. Y. Improving the prediction of hypertensive target organ damage using novel markers: lessons from retinal vascular imaging research. Hypertension. 64, 233–234 (2014).
Baumann, M., Burkhardt, K. & Heemann, U. Microcirculatory marker for the prediction of renal end points: a prospective cohort study in patients with chronic kidney disease stage 2 to 4. Hypertension. 64, 338–346 (2014).
Roy, M. S., Klein, R. & Janal, M. N. Relationship of retinal vessel caliber to cardiovascular disease and mortality in African Americans with type 1 diabetes mellitus. Arch Ophthalmol. 130, 561–567 (2012).
Grunwald, J. E. et al. Association between retinopathy and cardiovascular disease in patients with chronic kidney disease (from the Chronic Renal Insufficiency Cohort [CRIC] Study). The American journal of cardiology. 110, 246–253 (2012).
Ricardo, A. C. et al. Retinopathy and CKD as Predictors of All-Cause and Cardiovascular Mortality: National Health and Nutrition Examination Survey (NHANES) 1988-1994. Am J Kidney Dis. 64, 198–203 (2014).
Lim, C. C. et al. Chronic kidney disease, cardiovascular disease and mortality: A prospective cohort study in a multi-ethnic Asian population. Eur J Prev Cardiol. (2014).
Tamai, K. et al. Lipid hydroperoxide stimulates leukocyte-endothelium interaction in the retinal microcirculation. Experimental eye research. 75, 69–75 (2002).
Chester, A. H. et al. Induction of nitric oxide synthase in human vascular smooth muscle: interactions between proinflammatory cytokines. Cardiovascular research. 38, 814–821 (1998).
Willerson, J. T. & Ridker, P. M. Inflammation as a Cardiovascular Risk Factor. Circulation. 109, II-2-II-10 (2004).
Ikram, M. K. et al. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Investigative ophthalmology & visual science. 45, 2129–2134 (2004).
Klein, R., Klein, B. E., Knudtson, M. D., Wong, T. Y. & Tsai, M. Y. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol. 124, 87–94 (2006).
Yim-Lui Cheung, C. et al. C-reactive protein and retinal microvascular caliber in a multiethnic asian population. American journal of epidemiology. 171, 206–213 (2010).
Wong, T. Y. et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA). Investigative ophthalmology & visual science. 47, 2341–2350 (2006).
Hadi, H. A., Carr, C. S. & Al Suwaidi, J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 1, 183–198 (2005).
Dokken, B. B. The Pathophysiology of Cardiovascular Disease and Diabetes: Beyond Blood Pressure and Lipids. Diabetes Spectrum. 21, 160–165 (2008).
Gerstein, H. C. et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 286, 421–426 (2001).
Jager, A. et al. Microalbuminuria and peripheral arterial disease are independent predictors of cardiovascular and all-cause mortality, especially among hypertensive subjects: five-year follow-up of the Hoorn Study. Arterioscler Thromb Vasc Biol. 19, 617–624 (1999).
Cao, J. J. et al. The association of microalbuminuria with clinical cardiovascular disease and subclinical atherosclerosis in the elderly: the Cardiovascular Health Study. Atherosclerosis. 187, 372–377 (2006).
Stehouwer, C. D. & Smulders, Y. M. Microalbuminuria and risk for cardiovascular disease: Analysis of potential mechanisms. J Am Soc Nephrol. 17, 2106–2111 (2006).
Wong, T. Y. & Mitchell, P. Hypertensive retinopathy. N Engl J Med. 351, 2310–2317 (2004).
Granger, D. N., Rodrigues, S. F., Yildirim, A. & Senchenkova, E. Y. Microvascular responses to cardiovascular risk factors. Microcirculation. 17, 192–205 (2010).
de Zeeuw, D., Parving, H. H. & Henning, R. H. Microalbuminuria as an early marker for cardiovascular disease. J Am Soc Nephrol. 17, 2100–2105 (2006).
Weir, M. R. Microalbuminuria and cardiovascular disease. Clin J Am Soc Nephrol. 2, 581–590 (2007).
Sabanayagam, C. et al. Retinal arteriolar narrowing increases the likelihood of chronic kidney disease in hypertension. Journal of hypertension. 27, 2209–2217 (2009).
Foong, A. W. et al. Rationale and methodology for a population-based study of eye diseases in Malay people: The Singapore Malay eye study (SiMES). Ophthalmic Epidemiol. 14, 25–35 (2007).
Wong, T. Y. et al. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology. 111, 1183–1190 (2004).
Knudtson, M. D. et al. Revised formulas for summarizing retinal vessel diameters. Current eye research. 27, 143–149 (2003).
Wong, T. Y. et al. Prevalence and risk factors for diabetic retinopathy: the Singapore Malay Eye Study. Ophthalmology. 115, 1869–1875 (2008).
K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 39, S1–266 (2002).
Singapore Myocardial Infarction Registry, NRDO, Ministry of Health, Singapore., Singapore Myocardial Infarction Registry Report No 2 Trends in Acute Myocardial Infarction Singapore 2007–2012., http://www.nrdo.gov.sg/docs/librariesprovider3/Publications—AMI/sinmyocardinfreg2007-2012.pdf?sfvrsn=0, (2014) Date of access: 12/08/2015.
Singapore Stroke Registy, NRDO, Ministry of Health, Singapore., Singapore Stroke Registry Report No 3 Trends in Stroke in Singapore 2005–2012., http://www.nrdo.gov.sg/docs/librariesprovider3/Publications—Stroke/trends-in-stroke-in-singapore-2005–2012.pdf?sfvrsn=0, (2014) Date of access: 12/08/2015.
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 150, 604–612 (2009).
Acknowledgements
The authors wish to thank all the staff from the Singapore Epidemiology of Eye Disease (SEED) program and Singapore Advanced Imaging Laboratory for Ocular Research (SAILOR) for their work in data collection. The original data sets were derived from studies funded by National Medical Research Council Grants Nos 0796/2003, 0863/2004 and STaR/0003/2008, and Biomedical Research Council Grant No. 501/1/25-5. This study was supported by Singapore Ministry of Health’s National Medical Research Council under its Talent Development Scheme R927/36/2012 (CS) but was conducted independently of all funding organizations.
Author information
Authors and Affiliations
Contributions
W.F.Y., P.G.O., E.S.T., T.Y.W. and C.Y.-l.C. conceived and designed the study. W.F.Y., C.S., E.S.T., T.Y.W. and C.Y.-l.C. collected the data. W.F.Y., C.S., P.G.O., E.S.T., T.Y.W. and C.Y.-l.C. analysed and interpreted the data. W.F.Y., C.S., U.P., K.Y.C., E.S.T., L.H.L., T.Y.W. and C.Y.-l.C. drafted the manuscript and revised it critically for important intellectual content. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yip, W., Sabanayagam, C., Ong, P. et al. Joint Effect of Early Microvascular Damage in the Eye & Kidney on Risk of Cardiovascular Events. Sci Rep 6, 27442 (2016). https://doi.org/10.1038/srep27442
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep27442
This article is cited by
-
Review and comparison of retinal vessel calibre and geometry software and their application to diabetes, cardiovascular disease, and dementia
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Hypertensive eye disease
Nature Reviews Disease Primers (2022)
-
Diabetes, cardiovascular disease and the microcirculation
Cardiovascular Diabetology (2018)
-
Eyeing cardiovascular risk factors
Nature Biomedical Engineering (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.