Abstract
The aim of this meta-analysis was to comprehensively compare the safety and efficacy of robotic-assisted rectal cancer surgery (RRCS) and open rectal cancer surgery (ORCS). Electronic database (PubMed, EMBASE, Web of Knowledge and the Cochrane Library) searches were conducted for all relevant studies that compared the short-term and long-term outcomes between RRCS and ORCS. Odds ratios (ORs), mean differences and hazard ratios were calculated. Seven studies involving 1074 patients with rectal cancer were identified for this meta-analysis. Compared with ORCS, RRCS is associated with a lower estimated blood loss (mean difference [MD]: −139.98, 95% confidence interval [CI]: −159.11 to −120.86; P < 0.00001), shorter hospital stay length (MD: −2.10, 95% CI: −3.47 to −0.73; P = 0.003), lower intraoperative transfusion requirements (OR: 0.52, 95% CI: 0.28 to 0.99, P = 0.05), shorter time to flatus passage (MD: −0.97, 95% CI = −1.06 to −0.88, P < 0.00001) and shorter time to resume a normal diet (MD: −1.71.95% CI = −3.31 to −0.12, P = 0.04). There were no significant differences in surgery-related complications, oncologic clearance, disease-free survival and overall survival between the two groups. However, RRCS was associated with a longer operative time. RRCS is safe and effective.
Similar content being viewed by others
Introduction
Minimally invasive surgery is widely applied in many surgical fields. Robotic-assisted surgery is an advanced minimally invasive technique, has been applied in many branches of surgery (gynecologic1, urologic2 and gastrointestinal3), seems to achieve promising results and has gained worldwide attention.
Robotic-assisted colorectal surgery was first reported in 20024. Since then, a variety of reports regarding robotic-assisted rectal surgery have been published5,6,7,8,9. Robotic surgery is considered a good choice in the treatment of rectal cancer because this technique can overcome difficulties associated with the anatomy of the pelvis and has certain advantages10,11,12, including 3D imaging, dexterity and ambidextrous capability, lack of tremors, motion scaling and a short learning curve13,14. Some reports have already indicated that robotic colorectal surgery has some benefits over conventional laparoscopic surgery based on observational comparative studies or randomized controlled trials15,16. However, the feasibility and safety of robotic-assisted rectal surgery compared with open rectal surgery in treating rectal cancer are not well elucidated. We conducted this meta-analysis to assess the safety and efficacy of robotic surgery versus open surgery in treating rectal cancer.
Methods
Search strategy
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement17. A comprehensive literature search was carried out by reviewers using the following electronic databases: PubMed, EMBASE, Web of Knowledge and the Cochrane Library. The search was conducted in July 2014 and the language was restricted to English. The following search terms were used: robot or robotic or robot-assisted or da vinci or davinci, open, rectal or rectum or colorectal and cancer or tumor or carcinoma.
The study inclusion criteria were as follows: (1) the study was a comparative study that compared the safety and efficiency between robotic rectal cancer surgery (RRCS) and open rectal cancer surgery (ORCS); (2) the study included quantitative outcome data (e.g., operative time, length of hospital stay, complications, pathological parameters and survival outcomes); (3) if the same institution and/or authors reported several studies, only the study with the greatest patient population or the highest quality study was included in the analysis; and (4) the study was published in English. We excluded editorials, comments, meeting abstracts, review articles and non-relevant topic studies.
Data extraction
The primary relevant data from all the included studies were extracted by two reviewers (GXL, ZZ). The extracted data included the following: the basic characteristics of the study, including first author, year and country of publication; the publication journal name; the basic patient characteristics, including age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) score, case number and tumor stage; short-term outcomes, including intraoperative data, postoperative data and oncologic clearance; and long-term outcomes, including disease-free survival and overall survival. All available data were extracted using standard data extraction by one reviewer and were checked by another reviewer.
Quality assessment
Two reviewers (GL, YL) independently evaluated the quality of each included study using the modified Newcastle-Ottawa scale (available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp), which is widely used for cohort study assessment. The quality assessment consisted of three major categories: patient selection, comparability of the RRCS and ORCS groups and outcome assessment18. The details of this quality assessment are provided in Table S1. Any disagreement was resolved via discussion among the author group.
Statistical analysis
This meta-analysis was performed using the Review Manager software (version 5.2, provided by the Cochrane Collaboration) and Stata software (version 11.0). Dichotomous variables were analyzed using odds ratios (ORs) with 95% confidence intervals (95% CIs). Survival outcomes were estimated using hazards ratios (HRs) and standard errors. Continuous variables were analyzed using mean differences (MDs) and 95% CIs. A fixed-effects model or a random-effects model was applied according to heterogeneity, which was evaluated by the I2 measure of inconsistency. Heterogeneity was present when the I2 statistic was greater than 50% and a random-effects model was adopted. However, when the I2 statistic value was less than 50%, a fixed-effects model was used. Publication bias was evaluated by a funnel plot of postoperative complications and Egg’s and Begg’s tests. Sensitivity analysis was performed by excluding the low-quality studies. A P value less than 0.05 was considered to be significant.
Results
Literature search
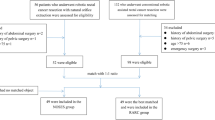
The PubMed, EMBASE, Web of Science and Cochrane Library search identified a total of 277 studies. After excluding duplicates using Endnote software, 180 abstracts were carefully reviewed by two independent reviewers using a standard study selection form. After this process, the reviewers identified 10 studies for a comprehensive review. Three studies were excluded according to the inclusion criteria19,20,21, leaving 7 studies that were included in our analysis22,23,24,25,26,27,28. The study selection process is shown in Fig. 1. A total of 1074 patients with 498 (9.00%) cases of RRCS and 576 cases of ORCS were analyzed. Information on the studies and the participants is shown in Table 1. The quality assessment score of each included study is also provided in Table 1 and the details of each included study assessment are provided in Table S2.
Meta-analysis
Intraoperative data
Operative times
The operative time was reported in all of the included studies22,23,24,25,26,27,28. The pooled data revealed that the operative time was significantly longer in the RRCS group compared with the ORCS group (MD = 55.76; 95% CI = 29.31–82.22; P < 0.0001) and the heterogeneity was high (P < 0.00001, I2 = 91%) (Fig. 2A).
Estimated blood losses
Five of the studies assessed intraoperative estimated blood loss (EBL)22,23,24,25,26. The EBL was significantly lower, by 139.98 ml, in the RRCS group compared with the ORCS group (MD: −139.98, 95% CI: −159.11 to −120.86; P < 0.00001). There was no significant heterogeneity (I2 = 33%, P = 0.20) (Fig. 2B).
Intraoperative transfusion
Intraoperative transfusion was mentioned in three studies26,27,28. Our results revealed that intraoperative transfusion requirements were reduced in the RRCS group compared with the ORCS group (OR: 0.52, 95% CI: 0.28–0.99, P = 0.05) and the analysis revealed significant heterogeneity (P = 0.28, I2 = 21%) (Fig. 2C).
Postoperative data
Overall postoperative complications
All of the included studies mentioned this index22,23,24,25,26,27,28 and the overall postoperative complication rates were similar between studies. The pooled data showed that the total postoperative complications of the two groups were not significantly different (OR = 1.00, 95% CI: 0.75 −1.32, P = 0.97) and there was no evidence of heterogeneity (P = 0.56, I2 = 0) (Fig. 3A).
Meta-analysis of postoperative complications associated with robotic-assisted rectal cancer surgery versus open rectal cancer surgery.
(A) overall postoperative complications, (B) postoperative mortality, (C) anastomotic leakage, (D) wound infection, (E) pelvic abscess, (F) ileus, (G) bleeding, (H) urinary retention.
Postoperative mortality
Two studies mentioned postoperative mortality24,28. The pooled data analysis indicated that the mortality rate was not different between the two techniques (OR: 0.87, 95% CI: 0.11–6.86, P = 0.90) and there was no heterogeneity (P = 0.70, I2 = 0) (Fig. 3B).
Anastomotic leakage
Seven studies reported anastomotic leakage events22,23,24,25,26,27,28. The anastomotic leakage rate was 6.63% in the RRCS group and 4.51% in the ORCS group. The pooled data analysis revealed that this rate was not significantly different between the two groups (OR = 1.54, 95% CI = 0.90–2.66, P = 0.12) and there was no evidence of heterogeneity (P = 0.93, I2 = 0) (Fig. 3C).
Wound infection
Four studies reported the incidence of wound infection23,24,26,28. The combined data revealed that this parameter was not different between the two groups (OR = 0.37, 95% CI: 0.05–2.50, P = 0.31) (Fig. 3D).
Pelvic abscess
Four studies described the number of pelvic abscess events23,24,26,28. The pooled analysis indicated that there was no significant difference in this variable between the two approaches (OR = 1.11, 95% CI: 0.47–2.61, P = 0.80) and there was no heterogeneity (P = 0.55, I2 = 0) (Fig. 3E).
Ileus
Ileus events were reported in four studies22,26,27,28. The incidence of ileus in the RRCS group was 3.81%, compared with 3.26% in the ORCS group. The results showed no significant difference between the two groups (OR = 1.11, 95% CI: 0.47–2.61, P = 0.80) and there was no heterogeneity among the studies (Fig. 3F).
Bleeding
Three studies described bleeding22,26,28. The pooled data analysis revealed no significant difference in bleeding between the two techniques (OR: 2.05, 95% CI: 0.52–8.13, P = 0.31) and no significant heterogeneity existed among the studies (Fig. 3G).
Urinary retention
Three studies reported urinary retention22,24,28. The combined data indicated that urinary retention in the two groups was not significantly different (OR: 0.52, 95% CI: 0.10 to 2.77, P = 0.44) and there was no significant heterogeneity (Fig. 3H).
Length of hospital stay
All of the studies22,23,24,25,26,27,28 described length of hospital stay (LOS). The pooled data of the included studies showed that the LOS was significantly reduced in the RRCS group compared with the ORCS group (MD: −2.10, 95% CI: −3.47 to −0.73; P = 0.003). However, the heterogeneity was high (P < 0.00001, I2 = 92%) (Fig. 4A).
Pain score
Pain scores were reported in two studies27,28. The pooled data of the two studies showed no difference between the two approaches with respect to pain scores (MD: −0.61, 95% CI: −1.78 to 0.57, P = 0.31) and there was high heterogeneity (P = 0.003, I2 = 92%) (Fig. 4B).
Flatus passage
Six studies reported the time to flatus passage22,23,25,26,27,28. The combined data indicated that RRCS significantly reduced the time to first flatus passage by 0.97 days compared with the ORCS group (MD: −0.97, 95% CI = −1.06 to −0.88, P < 0.00001), without significant heterogeneity among the studies (P = 0.79, I2 = 0) (Fig. 4C).
Time to diet resumption
The time to normal diet resumption included the time to normal diet resumption and the time to resumption of a soft diet22,23,25,26,28. The combined analysis showed that the RRCS group had a significantly shorter time to diet resumption (MD: −1.71. 95% CI = −3.31 to −0.12, P = 0.04). However, there was high heterogeneity among the studies (P < 0.00001, I2 = 97%) (Fig. 4D).
Meta-analysis of the pathological details
Kang et al.26 and Park et al.28 described proximal margin indices and the combined data indicated no differences in this parameter (MD: 2.23, 95% CI: −1.19 to 5.65; P = 0.20, I2 = 88) (Fig. 5A). Six studies reported distal margins22,23,25,26,27,28 and there was no difference between the two groups in this parameter (MD: 0.17, 95% CI: −0.14 to 0.48; P = 0.27) (Fig. 5B), but there was significant heterogeneity (P = 0.0003, I2 = 79). Two studies mentioned circumferential resection margins22,28 and the pooled data revealed no significant differences (MD: −0.22, 95% CI: −1.82 to 1.38, P = 0.79) (Fig. 5C).
All the included studies reported the number of retrieved lymph nodes22,23,24,25,26,27,28. The combined data indicated that the two groups did not differ significantly in this parameter (MD: 1.49, 95% CI: −0.82 to 3.79; P = 0.21) (Fig. 5D) and there was significant heterogeneity (P < 0.00001, I2 = 79). Moreover, the pooled data showed no significant differences in the number of retrieved positive lymph nodes between the two groups (MD: 0.07, 95% CI: −0.29 to 0.44, P = 0.70) (Fig. 5E).
Long-term outcomes
Disease-free survival (DFS)
Two studies reported DFS outcomes25,28. Kang et al. reported that the 2-year DFS was 83.5% in the RRCS group and 79.7% in the ORCS group. Ghezzi et al.25 reported that the 5-year DFS was 73.2% and 69.5% in the RRCS and ORCS groups, respectively. The combined data indicated no differences in DFS between the two arms (HR: 0.84, 95% CI: 0.53–1.35, P = 0.47) and no heterogeneity (P = 0.87, I2 = 0) (Fig. 6).
Overall survival
Ghezzi et al.25 reported that the 5-year overall survival rate was higher in the RRCS group than the ORCS group, but this difference was not significant (85.0% vs. 76.1%). Future studies should be conducted to assess this index.
Publication bias
A funnel plot analysis of all studies was performed in the meta-analysis of overall postoperative complications between RRCS and ORCS. Visually, all of the studies were within the limits of the 95% CIs (Fig. 7). Moreover, the statistical test indicated no evidence of publication bias (Egg’s test P = 0.174, Begg’s test P = 0.764).
Sensitivity analysis
A sensitivity analysis was performed by excluding the studies with a low quality score (Score ≤ 6)27,28. The results were not influenced, with the exception of the time to flatus passage. The results are listed in Table 2.
Discussion
Several meta-analyses have evaluated the efficacy and safety of robotic-assisted surgery versus open surgery in gastric cancer29,30, pancreatic disease31, renal disease32 and bladder cancer33. All of these studies have indicated that robotic-assisted surgery is safe and effective. However, no meta-analysis has been conducted to evaluate robotic-assisted surgery compared with open surgery for rectal cancer.
This meta-analysis assessed the efficacy and safety of RRCS versus ORCS. This meta-analysis indicated that RRCS may provide certain benefits over ORCS. Compared with ORCS, RRCS is associated with a lower EBL (MD: −139.98, 95% CI: −159.11 to −120.86; P < 0.00001), shorter LOS (MD: −2.10, 95% CI: −3.47 to −0.73; P = 0.003), less intraoperative transfusion requirements (OR: 0.52, 95% CI: 0.28 to 0.99, P = 0.05), shorter time to flatus passage (MD: −0.97, 95% CI = −1.06 to −0.88, P < 0.00001) and shorter time to diet resumption (MD: −1.71.95% CI = −3.31 to −0.12, P = 0.04). There were no significant differences in overall postoperative complications, anastomotic leakage, pain scores, wound infection, ileus, pelvic abscess, bleeding, urinary retention, postoperative mortality, proximal margin, distal margin, circumferential resection margin, number of lymph nodes retrieved, number of positive lymph nodes retrieved, DFS and overall survival between the two groups. However, the disadvantage of RRCS is that it was associated with a longer operative time (MD: −139.98, 95% CI: −159.11 to −120.86; P < 0.00001).
The combined data indicated that compared with the ORCS group, the EBL was significantly lower in the RRCS group. Due to a lower EBL, it is possible to suggest that RRCS may significantly reduce the probability of transfusion. Indeed, our meta-analysis suggested that the transfusion rate was significantly lower in the RRCS group compared with the ORCS group. Thus, reduced transfusion rates may prevent the recurrence of cancer34. Patients with cancer who receive more intraoperative blood transfusions are at greater risk for cancer recurrence and the volume of transfused blood at surgery is an independent risk factor for cancer recurrence35. In addition, more EBL may indicate an unfavorable prognosis of patients with cancer36,37.
Another advantage of RRCS is its associated shorter LOS. The shorter LOS may be explained by the following rationale: RRCS is a minimally invasive surgery and it may provide faster wound recovery and reduce postoperative pain. RRCS has a shorter time to flatus passage and a faster recovery to normal diet resumption.
The overall complication rate was similar between the two techniques (7% in the RRCS group and 8% in the ORCS group). This finding also indicated that RRCS is as safe and feasible as ORCS. The most frequently occurring events included anastomotic leakage, wound infection, ileus and pelvic abscess. Our analysis indicated that the two groups had no differences in those regards.
Anastomotic leakage is a major surgical complication of gastrointestinal surgery29 and it represents one of the most dreaded complications of colorectal cancer. The mortality rate is high in patients with colorectal anastomotic leakage that required re-operation and accounts for almost 40% of the deaths after colorectal cancer surgery29. The anastomotic leakage rate was 6.49% (31/478) in the RRCS group and 4.5% (25/556) in the ORCS group, which was not a significant difference. The rate (6.49%) was consistent with previous reports for robotic surgery (1.8–12.1%)5,38,39 and was similar to the rate of 7% that was reported in open rectal cancer surgery based on a multicenter randomized controlled trial40.
The present study revealed no difference in oncologic clearance (proximal margin, circumferential resection margin, distal margin and number of retrieved lymph nodes). Resection margins and number of retrieved lymph nodes have been regarded as quality indicators in rectal cancer surgery41 because the distal margin, circumferential resection margin and number of retrieved lymph nodes were important parameters for the evaluation of prognosis in rectal cancer42,43,44,45. Several studies have indicated that RRCS can achieve good-quality performance.
The long-term follow-up was reported in two studies. The results indicated that there was no difference in DFS or overall survival between the two groups. Hara et al. reported that the 5-year overall survival and DFS rates were 88.6% and 76.6%, respectively, for patients diagnosed with stage III rectal cancer who had undergone robotic surgery7. The 3-year overall survival rate was 97%46.
Robotic rectal cancer surgery can achieve promising survival rates. Due to the limited number of studies, more studies are mandatory to establish the value of robotic surgery for rectal cancer in the future.
We should note the disadvantage of RRCS, namely, it requires a longer operative time compared with ORCS. This result is mainly attributed to the docking and preparation times associated with RRCS. A previous study reported that the median operative time for RRCS ranged from 240 to 310 min46. With increasing experience, the operative time would be reduced in robotic surgery29. It was reported that a surgical team requires approximately 30 cases to become comfortable and proficient with RRCS28.
The following limitations of this meta-analysis should be considered. First, the included studies were not randomized controlled trials; some studies were prospective studies and some were retrospective studies. Thus, the studies may have been biased and the results should be interpreted with caution. Second, as a novel technique, the cost-effectiveness of RRCS should be considered. Of the included studies, only that of Berten et al.23 reported the total cost, which was 11214€ for RRCS and 9858€ for ORCS; the combined data for this index were not available. However, a variety of studies have indicated that the cost of robotic colorectal surgery is higher than the cost of laparoscopic colorectal surgery8,47,48,49. The high capital and running costs of robotic systems have precluded their widespread use in many countries50. Third, the studies included patients with different basic characteristics and treatments and these differences may have affected some of the results. Moreover, the surgeries in the included cases were carried out by different surgeons and the different experiences and techniques of the surgeons may have affected some of the results34. Fourth, only two studies reported the survival outcomes after long-term follow-up; more studies are needed to assess the survival outcome as well as the recurrence events. Fifth, the studies included in this meta-analysis were limited to those published in the English language because the authors of the present study were not literate in other languages. Thus, studies published in English may have more frequently supported our hypotheses and studies reported in other languages may have more frequently refuted our hypotheses.
In conclusion, this meta-analysis suggests that RRCS is safe and effective. RRCS was associated with reduced EBL, less intraoperative transfusion requirements, a shorter time to flatus passage, a shorter time to resumption of a normal diet and reduced LOS. There were no significant differences in complication rates, oncologic clearance and survival outcomes between the two groups. However, RRCS was associated with a longer operative time compared with ORCS. Future well-designed, larger, randomized controlled studies should be performed to assess the clinical and financial benefits and oncologic outcomes of RRCS to establish its role in the minimally invasive management of rectal cancer.
Additional Information
How to cite this article: Liao, G. et al. Robotic-assisted surgery versus open surgery in the treatment of rectal cancer: the current evidence. Sci. Rep. 6, 26981; doi: 10.1038/srep26981 (2016).
References
Desai, P. H., Lin, J. F. & Slomovitz, B. M. Milestones to optimal adoption of robotic technology in gynecology. Obstet gynecol 123, 13–20 (2014).
Autorino, R., Zargar, H. & Kaouk, J. H., Robotic-assisted laparoscopic surgery: recent advances in urology. Fertil Steril 102, 939–949 (2014).
Maeso, S. et al. Efficacy of the Da Vinci surgical system in abdominal surgery compared with that of laparoscopy: a systematic review and meta-analysis. Ann Surg 252, 254–262 (2010).
Weber, P. A., Merola, S., Wasielewski, A. & Ballantyne, G. H. Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis Colon Rectum 45, 1689–1694, 1695–1696 (2002).
Baik, S. H. et al. Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol 16, 1480–1487 (2009).
Collinson, F. J. et al. An international, multicentre, prospective, randomised, controlled, unblinded, parallel-group trial of robotic-assisted versus standard laparoscopic surgery for the curative treatment of rectal cancer. Int J Colorectal Dis 27, 233–241 (2012).
Hara, M. et al. Robotic-assisted surgery for rectal adenocarcinoma: short-term and midterm outcomes from 200 consecutive cases at a single institution. Dis Colon Rectum 57, 570–577 (2014).
Delaney, C. P., Lynch, A. C., Senagore, A. J. & Fazio, V. W. Comparison of robotically performed and traditional laparoscopic colorectal surgery. Dis Colon Rectum 46, 1633–1639 (2003).
Kariv, Y. & Delaney, C. P. Robotics in colorectal surgery. Minerva Chir 60, 401–416 (2005).
Pigazzi, A., Ellenhorn, J. D., Ballantyne, G. H. & Paz, I. B. Robotic-assisted laparoscopic low anterior resection with total mesorectal excision for rectal cancer. Surg Endosc 20, 1521–1525 (2006).
Baek, S. J. et al. Robotic surgery for rectal cancer can overcome difficulties associated with pelvic anatomy. Surg Endosc 29, 1419–1424 (2015).
Baik, S. H. et al. Robotic tumor-specific mesorectal excision of rectal cancer: short-term outcome of a pilot randomized trial. Surg Endosc 22, 1601–1608 (2008).
Park, J. S., Choi, G. S., Lim, K. H., Jang, Y. S. & Jun, S. H. Robotic-assisted versus laparoscopic surgery for low rectal cancer: case-matched analysis of short-term outcomes. Ann Surg Oncol 17, 3195–3202 (2010).
Liao, G. X. et al. Meta-analysis of outcomes compared between robotic and laparoscopic gastrectomy for gastric cancer. Asian Pac J Cancer Prev 14, 4871–4875 (2013).
Yang, Y. et al. Robot-assisted versus conventional laparoscopic surgery for colorectal disease, focusing on rectal cancer: a meta-analysis. Ann Surg Oncol 19, 3727–3736 (2012).
Memon, S., Heriot, A. G., Murphy, D. G., Bressel, M. & Lynch, A. C. Robotic versus laparoscopic proctectomy for rectal cancer: a meta-analysis. Ann Surg Oncol 19, 2095–2101 (2012).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS MED 6, e1000097 (2009).
Arezzo, A. et al. Laparoscopy for rectal cancer is oncologically adequate: a systematic review and meta-analysis of the literature. Surg Endosc 29, 334–348 (2015).
Kim, J. C., Kwak, J. Y., Yoon, Y. S., Park, I. J. & Kim, C. W. A comparison of the technical and oncologic validity between robot-assisted and conventional open abdominoperineal resection. Int J Colorectal Dis 29, 961–969 (2014).
Kim, J. C. et al. Open versus robot-assisted sphincter-saving operations in rectal cancer patients: techniques and comparison of outcomes between groups of 100 matched patients. Int J Med Robot 8, 468–475 (2012).
Biffi, R. et al. Operative blood loss and use of blood products after full robotic and conventional low anterior resection with total mesorectal excision for treatment of rectal cancer. J Robot Surg 5, 101–107 (2011).
Barnajian, M., Pettet, D. R., Kazi, E., Foppa, C. & Bergamaschi, R. Quality of total mesorectal excision and depth of circumferential resection margin in rectal cancer: A matched comparison of the first 20 robotic cases. Colorectal Dis 16, 603–609 (2014).
Bertani, E. et al. Assessing appropriateness for elective colorectal cancer surgery: clinical, oncological and quality-of-life short-term outcomes employing different treatment approaches. Int J Colorectal Dis 26, 1317–1327 (2011).
DeSouza, A. L. et al. A comparison of open and robotic total mesorectal excision for rectal adenocarcinoma. Dis Colon Rectum 54, 275–282 (2011).
Ghezzi, T. L. et al. Robotic versus open total mesorectal excision for rectal cancer: Comparative study of short and long-term outcomes. Eur J Surg Oncol 40, 1072–1079 (2014).
Kang, J. et al. The impact of robotic surgery for mid and low rectal cancer: a case-matched analysis of a 3-arm comparison–open, laparoscopic and robotic surgery. Ann Surg 257, 95–101 (2013).
Kim, J. C. et al. Completely abdominal intersphincteric resection for lower rectal cancer: feasibility and comparison of robot-assisted and open surgery. Surg Endosc 28, 2734–2744 (2014).
Park, J. S., Choi, G. S., Lim, K. H., Jang, Y. S. & Jun, S. H. S052: a comparison of robot-assisted, laparoscopic and open surgery in the treatment of rectal cancer. Surg Endosc 25, 240–248 (2011).
Liao, G. et al. Robotic versus open gastrectomy for gastric cancer: a meta-analysis. Plos one 8, e81946 (2013).
Zong, L., Seto, Y., Aikou, S. & Takahashi, T. Efficacy Evaluation of Subtotal and Total Gastrectomies in Robotic Surgery for Gastric Cancer Compared with that in Open and Laparoscopic Resections: A Meta-Analysis. PLos one 9, e103312 (2014).
Zhang, J., Wu, W. M., You, L. & Zhao, Y. P. Robotic versus open pancreatectomy: a systematic review and meta-analysis. Ann Surg Oncol 20, 1774–1780 (2013).
Wu, Z. et al. Robotic versus open partial nephrectomy: a systematic review and meta-analysis. PLos One 9, e94878 (2014).
Tang, K. et al. Robotic vs. open radical cystectomy in bladder cancer: A systematic review and meta-analysis. Eur J Surg Oncol 40, 1399–1411 (2014).
Liao, G. et al. Robotic-assisted versus laparoscopic colorectal surgery: a meta-analysis of four randomized controlled trials. World J Surg Oncol 12, 122 (2014).
Amato, A. & Pescatori, M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev D5033 (2006).
Morner, M. E., Gunnarsson, U., Jestin, P. & Svanfeldt, M. The importance of blood loss during colon cancer surgery for long-term survival: an epidemiological study based on a population based register. Ann Surg 255, 1126–1128 (2012).
Basilico, V. et al. Anastomotic leakage following colorectal resection for cancer: how to define, manage and treat it. Minerva Chir 69, 245–252 (2014).
Kim, C. W., Kim, C. H. & Baik, S. H. Outcomes of robotic-assisted colorectal surgery compared with laparoscopic and open surgery: a systematic review. J Gastrointest Surg 18, 816–830 (2014).
Hellan, M., Anderson, C., Ellenhorn, J. D., Paz, B. & Pigazzi, A. Short-term outcomes after robotic-assisted total mesorectal excision for rectal cancer. Ann Surg Oncol 14, 3168–3173 (2007).
Guillou, P. J. et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365, 1718–1726 (2005).
Bosch, S. L. & Nagtegaal, I. D. What Is “Good Quality” in Rectal Cancer Surgery? The Pathologist’s Perspective. Recent Results Cancer Res 203, 41–46 (2014).
La Torre, M. et al. The importance of lymph node retrieval and lymph node ratio following preoperative chemoradiation of rectal cancer. Colorectal Dis 15, e382–e388 (2013).
Dhadda, A. S., Bessell, E. M., Scholefield, J., Dickinson, P. & Zaitoun, A. M. Mandard tumour regression grade, perineural invasion, circumferential resection margin and post-chemoradiation nodal status strongly predict outcome in locally advanced rectal cancer treated with preoperative chemoradiotherapy. Clin Oncol (R Coll Radiol) 26, 197–202 (2014).
Madbouly, K. M., Abbas, K. S. & Hussein, A. M. Metastatic lymph node ratio in stage III rectal carcinoma is a valuable prognostic factor even with less than 12 lymph nodes retrieved: a prospective study. Am J Surg 207, 824–831 (2014).
Mezhir, J. J. et al. Whole-mount pathologic analysis of rectal cancer following neoadjuvant therapy: implications of margin status on long-term oncologic outcome. Ann Surg 256, 274–279 (2012).
Pigazzi, A. et al. Multicentric study on robotic tumor-specific mesorectal excision for the treatment of rectal cancer. Ann Surg Oncol 17, 1614–1620 (2010).
Akmal, Y., Baek, J. H., McKenzie, S., Garcia-Aguilar, J. & Pigazzi, A. Robot-assisted total mesorectal excision: is there a learning curve ? Surg Endosc 26, 2471–2476 (2012).
DeSouza, A. L. et al. Robotic assistance in right hemicolectomy: is there a role? Dis Colon Rectum 53, 1000–1006 (2010).
Halabi, W. J. et al. Robotic-assisted colorectal surgery in the United States: a nationwide analysis of trends and outcomes. World J Surg 37, 2782–2790 (2013).
Aly, E. H. Robotic colorectal surgery: summary of the current evidence. Int J Colorectal Dis 29, 1–8 (2014).
Author information
Authors and Affiliations
Contributions
Z.Z., G.X.L., Y.-B.L. and X.L. conducted the literature search, identified the studies for exclusion and inclusion, extracted data from the retrieved studies, performed the meta-analysis and drafted the manuscript. G.L.,Y.-B.L., X.L. and H.D. provided comments on the experiment design and the manuscript and read and approved the final manuscript. All authors reviewed the paper and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Liao, G., Li, YB., Zhao, Z. et al. Robotic-assisted surgery versus open surgery in the treatment of rectal cancer: the current evidence. Sci Rep 6, 26981 (2016). https://doi.org/10.1038/srep26981
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep26981
This article is cited by
-
Technical outcomes of robotic-assisted surgery versus laparoscopic surgery for rectal tumors: a single-center safety and feasibility study
Surgery Today (2023)
-
Lower need for allogeneic blood transfusion after robotic low anterior resection compared with open low anterior resection: a propensity score-matched analysis
Journal of Robotic Surgery (2023)
-
Do specific operative approaches and insurance status impact timely access to colorectal cancer care?
Surgical Endoscopy (2021)
-
Robotics Total Mesorectal Excision Up To the Minute
Indian Journal of Surgical Oncology (2020)
-
Robotic proctectomy for rectal cancer in the US: a skewed population
Surgical Endoscopy (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.