Abstract
Not much is known about the mechanism of endophyte-mediated induction of secondary metabolite production in Catharanthus roseus. In the present study two fungal endophytes, Curvularia sp. CATDLF5 and Choanephora infundibulifera CATDLF6 were isolated from the leaves of the plant that were found to enhance vindoline content by 229–403%. The isolated endophytes did not affect the primary metabolism of the plant as the maximum quantum efficiency of PSII, net CO2 assimilation, plant biomass and starch content of endophyte-inoculated plants was similar to endophyte-free control plants. Expression of terpenoid indole alkaloid (TIA) pathway genes, geraniol 10-hydroxylase (G10H), tryptophan decarboxylase (TDC), strictosidine synthase (STR), 16-hydoxytabersonine-O-methyltransferase (16OMT), desacetoxyvindoline-4-hydroxylase (D4H), deacetylvindoline-4-O-acetyltransferase (DAT) were upregulated in endophyte-inoculated plants. Endophyte inoculation upregulated the expression of the gene for transcriptional activator octadecanoid-responsive Catharanthus AP2-domain protein (ORCA3) and downregulated the expression of Cys2/His2-type zinc finger protein family transcriptional repressors (ZCTs). The gene for the vacuolar class III peroxidase (PRX1), responsible for coupling vindoline and catharanthine, was upregulated in endophyte-inoculated plants. These endophytes may enhance vindoline production by modulating the expression of key structural and regulatory genes of vindoline biosynthesis without affecting the primary metabolism of the host plant.
Similar content being viewed by others
Introduction
Endophytes are microbes that are found to be present in almost all plants studied1. They reside inside the host plant without causing any disease symptoms or harm2,3. Most of the endophytes enter the plant through root hairs or the stomata in leaves and then disseminate systemically throughout the plant. Endophytes may be present intercellularly or intracellularly and colonize roots, aerial parts, conduction vessels and seeds4. Endophytes may promote host plant growth, improve nutrient supply and protect plants from both biotic and abiotic stresses5,6,7,8. Endophytic microbes are the potential source of therapeutically important bioactive natural products9. Few endophytic fungi produce secondary metabolites similar to their host plant e.g. vinblastine and vincristine10, taxol11, azadirachtin12, podophyllotoxin13, deoxypodophyllotoxin14, camptothecin15 and hypericin16. A major limitation in bioactive secondary metabolite production in present fermentation practices through the use of fungal endophytes is the instability of the expression of genes involved in the biosynthesis of the desired metabolite(s). During repeated subculturing under axenic monoculture conditions, production of the secondary metabolite may reduce substantially17. Therefore, the use of endophytes to enhance secondary metabolite production in planta could be a better approach compared to their independent culture in vitro.
There are many reports of endophyte-induced biosynthesis of secondary metabolites in host plants, but limited information is available about the mechanism involved. An endophytic actinobacterium Pseudonocardia strain YIM 63111 isolated from Artemisia annua induced artemisinin production in Artemisia plant by upregulating the expression of cytochrome P450 monooxygenase and cytochrome P450 oxidoreductase genes involved in artemisinin biosynthesis18. Elicitors from the endophytic fungus Trichoderma atroviride stimulate biosynthesis of tanshinone in the host plant by increasing the expression of genes related to tanshinone biosynthesis19. Catharanthus roseus is one of the most studied medicinal plants and is used as a model species for the study of plant secondary metabolism and plant-microbe interactions20,21,22,23,24. It is the sole source of antitumor bisindole alkaloids [belonging to the class terpenoids indole alkaloids (TIAs)] vinblastine and vincristine, which are extensively used in cancer chemotherapy. Due to a very low production of vinblastine and vincristine in planta and their large demand, they are exorbitantly priced. This great commercial importance has led to major efforts to increase the in planta content of these metabolites. Vinblastine and vincristine are produced by the condensation of monomeric TIAs, vindoline and catharanthine. Due to complicated structures of these alkaloids, their chemical synthesis in large scale is not economically feasible25,26. Diverse approaches such as transgenic generation, tissue culture practices, phytohormone treatments are being attempted to achieve enhanced production of important TIAs. Most of the genes that encode enzymes for TIA biosynthesis and regulatory components such as transcriptional activators and repressors have been identified20,27,28,29,30,31 (Fig. 1). Transgenic and tissue culture approaches have their own limitations. It is difficult to increase the in planta content of bisindole alkaloids due to their cytotoxicity; a better approach may be to produce monomers and semisynthetically fuse them to produce bisindoles20. Use of endophytes to enhance secondary metabolite production in the host plant could be a sustainable approach. Earlier, we have shown that bacterial endophytes enhance the in planta content of key TIAs as well as plant growth and biomass32. In the present study, efforts were made to identify and characterize fungal endophytes capable of enhancing the vindoline content in C. roseus and to study the possible mechanism involved. Towards this end, fungal endophytes were isolated from alkaloid-rich genotype (cv. Dhawal) of C. roseus20 and their effect was checked on another C. roseus genotype (cv. Prabal) that produces a comparatively lower amount of TIAs. The idea was to explore the role, if any, of endophytes isolated from an alkaloid-rich genotype in improving the alkaloid content of low-alkaloid genotypes.
Schematic representation of the terpenoid indole alkaloid (TIA) biosynthetic pathway.
Enzyme abbreviations: DXS, 1-deoxy-D-xylulose-5-phosphate synthase; IPP, isopentenyl pyrophosphate; DMAPP, dimethylallyl pyrophosphate; IDI1, isopentenyl diphosphate isomerase 1; CPR, cytochrome P450 reductase; G10H, geraniol 10-hydroxylase; UGT8, UDP-sugar glucosyltransferase 8, 7-DLH, 7-deoxyloganic acid hydroxylase; LAMT, loganic acid O-methyltransferase; SLS, secologanin synthase; AS, anthranilate synthase; TDC, tryptophan decarboxylase; STR, strictosidine synthase; SGD, strictosidine glucosidase; T16H, tabersonine 16-hydroxylase; 16OMT, 16-hydoxytabersonine-O-methyltransferase; NMT, N-methyltransferase; D4H, desacetoxyvindoline-4-hydroxylase; DAT, deacetylvindoline-4-O-acetyltransferase; and PRX, vacuolar class III peroxidase.
Results
Isolation of fungal endophytes that enhance the vindoline biosynthetic potential of C. roseus
A total of seven fungal endophytes were isolated from C. roseus genotype Dhawal and their potential to enhance vindoline content was examined in both genotypes Dhawal and Prabal. It was found that out of the seven fungal endophytes, CATDLF5 and CATDLF6, identified as Curvularia sp. and Choanephora infundibulifera by ITS sequencing, respectively (Fig. 2) were found to improve the vindoline content in genotype Prabal but could not improve the same in genotype Dhawal in the preliminary glass house trials (Supplementary Fig. S1). Therefore, to evaluate the possible mechanism involved in fungal endophyte-mediated enhancement of vindoline biosynthesis, further study was performed on genotype Prabal. The isolated endophytes were used to inoculate endophyte-free seedlings of C. roseus genotype Prabal. After 90 d of growth, the presence of inoculated endophytes in the leaves of endophyte-inoculated plants was examined and vindoline content was measured. High-performance liquid chromatography (HPLC) analysis of leaves of CATDLF5- and CATDLF6-inoculated plants was performed and vindoline content was compared with two types of controls–(i) endophyte-free control [C] and (ii) natural control [NC] plants containing the naturally present endophytes (these plants were not made endophyte-free by the treatment of fungicide and bactericide). The purpose to include the natural control was to get an idea of the effect of the natural endophytic populations occurring in the plants vis-à-vis the endophyte-free control plants. Leaves of CATDLF5- and CATDLF6-inoculated plants had 403% and 229% higher vindoline content as compared to that in endophyte-free control plants, respectively (Fig. 2; Supplementary Fig. S2). It was observed that endophyte-free control and natural control plants did not differ significantly in their vindoline content. To rule out the possibility of CATDLF5 and CATDLF6 endophytes producing vindoline themselves, they were examined for the presence of vindoline in their culture media (independent of host) by ethyl acetate extraction followed by HPLC. The selected endophytes were not able to produce vindoline in the culture medium (data not shown).
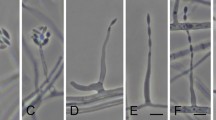
Fungal endophytes isolated from Catharanthus roseus and their effect on vindoline content in plant leaves.
Upper panel: Fungal endophytes isolated from the surface sterilized leaves of C. roseus (cv. Dhawal) plants. Surface sterilized leaves were cut into small pieces and kept on potato dextrose agar plate, whereby endophytes were found to originate from the margin of the pieces (inset picture). Lower panel: Endophyte-free C. roseus (cv. Prabal) plants (generated from seeds treated with bactericide and fungicide) were used to study the effect of treatment with endophytes (CATDLF5 and CATDLF6 isolated from cv. Dhawal) on leaf vindoline content. Two types of controls were included in the study-(i) the endophyte-free control [C] plants that originated from C. roseus seeds treated with bactericides and fungicides and were thus devoid of any naturally occurring endophyte and (ii) the natural control [NC] plants that originated from C. roseus seeds that were not treated with any bacteriocide and fungicide and contained all the naturally occurring endophytes present in the plants. Fungal endophyte inoculums (1 × 108 spore/conidia mL−1) prepared in phosphate buffer saline (PBS) were used to treat roots of experimental plants. The roots of both the controls–endophyte-free (C) and natural (NC) plants were treated with PBS. Third leaves of 90 d-old plants were sampled for vindoline content (% dry weight basis). As biological replicates, three plants per treatment were analyzed. For each biological replicate, three technical replicates were run on the HPLC and the mean of the three technical replicates represented the particular biological replicate. Statistical analysis was carried out for the data obtained for the three biological replicates (n = 3). Asterisks indicate significant differences as compared to the endophyte-free control (C) (Duncan’s multiple range test *P < 0.05).
Effect of CATDLF5 and CATDLF6 inoculation on photosynthetic efficiency of C. roseus
To examine the effect of endophytes on primary metabolism of the plant, photosynthetic efficiency of plants inoculated with CATDLF5 and CATDLF6 was measured and compared with endophyte-free control and natural control plants. Chlorophyll content, chlorophyll fluorescence, net CO2 assimilation, stomatal conductance and transpiration rate were measured.
Chlorophyll and carotenoid content were not affected by endophyte inoculation (Table 1). Natural control plants had ~12% higher total chlorophyll content as compared to endophyte-free control plants. Chlorophyll content in CATDLF5- and CATDLF6-treated plants were similar to endophytes-free controls. Chlorophyll fluorescence, which is believed as the signature of photosynthesis of a plant33 and represents functional status of photosynthetic apparatus and electron transport chain was not affected by endophyte inoculation. Fv/Fm of natural control plants was higher than that of endophyte-free control plants as well as CATDLF5- and CATDLF6-inoculated plants. The Fv/Fm indicates the quantum efficiency of photosynthesis. The Fv is the variable fluorescence, which represents Fm-Fo. The Fo indicates the initial minimal fluorescence emitted from a dark-adapted leaf sample and the Fm is the maximal fluorescence measured during the first saturation pulse after dark adaptation. Fv/Fm of CATDLF5- and CATDLF6-inoculated plants and endophyte-free control plants was ~0.73 however it was ~0.83 in natural control plants (Table 1).
Net CO2 assimilation, stomatal conductance and transpiration rate
Natural control plants had 23% higher net CO2 assimilation than that of endophyte-free control plants (Table 1). CATDLF5 and CATDLF6 inoculation did not affect the net CO2 assimilation in C. roseus plants. Stomatal conductance and transpiration rate were 11.3% and 23.7% higher in natural control plants as compared to endophyte-free control plants, respectively (Table 1). CATDLF5- and CATDLF6-inoculated plants had almost similar stomatal conductance and transpiration rate as compared to endophyte-free control plants.
Starch and biomass production
As photosynthetic efficiency of natural control plants was higher, accumulation of the primary photosynthetic end product starch and biomass of C. roseus plant was found to be higher in natural control plants compared to endophyte-free control plants. Natural control plants had 27% and 56% higher starch and biomass production, respectively as compared to that of endophyte-free control plants (Table 1). CATDLF5- and CATDLF6-inoculated plants had an amount of starch and biomass similar to endophyte-free control plants.
Growth parameters
To evaluate the effect of endophyte inoculation on the growth of C. roseus plant, the number of leaves, siliques, branches and plant height were measured. Natural control plants showed better plant growth than that of endophyte-free control plants and CATDLF5- and CATDLF6-inoculated plants. Number of leaves, siliques, branches and plant height was higher in natural control plants (Table 1). CATDLF5 and CATDLF6 inoculation did not affect the growth of the C. roseus plants.
Expression of vindoline biosynthetic pathway genes
To understand the role of CATDLF5 and CATDLF6 in modulation of TIA biosynthesis in the host plant, the expression of different genes involved in TIA biosynthetic pathway was measured by quantitative real time PCR (qRT-PCR) in the third leaves of 90 d-old C. roseus plants inoculated individually with CATDLF5 and CATDLF6 endophytes as well as endophyte-free control plants and natural control plants. Endophyte-free control plants were used as the calibrator. Transcript abundance of CPR, DXS, G10H, UGT8, LAMT, SLS, AS, TDC, STR, SGD, T16H, 16OMT, D4H and DAT was quantified (Figs 3 and 4). CATDLF5- and CATDLF6-inoculated and natural control plants had 2–4 fold higher TDC expression than that of endophyte-free control plants. Expression of LAMT and AS was higher in CATDLF5-treated and natural control plants. CATDLF6 did not affect the expression of LAMT and AS. Expression of CPR, DXS and SLS in the plants were not affected by the presence of endophytes. UGT8 was downregulated after endophyte treatment. G10H expression was enhanced post-endophyte treatment. Expression of STR, which catalyzes the condensation of secologanin and tryptamine to produce strictosidine was higher in natural control and CATDLF5- and CATDLF6-inoculated plants. In the case of SGD, which codes the protein that catalyzes the first step in the post-strictosidine pathway, the transcript abundance was found to be higher in the natural control plants but lower in the CATDLF5- and CATDLF6-inoculated plants as compared to the endophyte-free control plants. Transcript abundance of other genes for enzymes involved in steps downstream to strictosidine biosynthesis like 16OMT, D4H and DAT was found to be higher in CATDLF5- and CATDLF6-inoculated plants as well as the natural control plants as compared to the endophyte-free control plants. Interestingly, there was no significant change in the transcript abundance of T16H in the endophyte-inoculated plants and the two types of control (natural and endophyte-free) plants.
Effect of endophytes (CATDLF5 and CATDLF6) on expression of genes involved in secologanin and tryptamine biosynthesis.
Transcript abundance of (a) DXS, (b) CPR, (c) G10H, (d) UGT8, (e) LAMT, (f) SLS, (g) AS, (h) TDC was analyzed. NC- the natural control plants that originated from C. roseus seeds that were not treated with any bacteriocide and fungicide and contained all the naturally occurring endophytes present in the plants. C- the endophyte-free control plants that originated from C. roseus seeds treated with bactericides and fungicides and were thus devoid of any naturally occurring endophyte. The endophyte-free control (C) was used as the calibrator. For normalization, C. roseus actin gene was used as the endogenous gene. Data are means ± SD (n = 3 biological replicates) and Y-axis represents relative quantity (RQ). Asterisks indicate significant differences as compared to the endophyte-free control (C) (Duncan’s multiple range test *P < 0.05).
Effect of endophytes (CATDLF5 and CATDLF6) on expression of genes involved in vindoline biosynthesis.
Transcript abundance of (a) STR, (b) SGD, (c) T16H, (d) 16OMT, (e) D4H, (f) DAT was analyzed. NC- the natural control plants that originated from C. roseus seeds that were not treated with any bacteriocide and fungicide and contained all the naturally occurring endophytes present in the plants. C- the endophyte-free control plants that originated from C. roseus seeds treated with bactericides and fungicides and were thus devoid of any naturally occurring endophyte. The endophyte-free control (C) was used as the calibrator. For normalization, C. roseus actin gene was used as the endogenous gene. Data are means ± SD (n = 3 biological replicates) and Y-axis represents relative quantity (RQ). Asterisks indicate significant differences as compared to the endophyte-free control (C) (Duncan’s multiple range test *P < 0.05).
PRX1 expression and H2O2 measurement
The expression of class III peroxidase (PRX1), which utilizes H2O2 to oxidize the coupling of vindoline and catharanthine to yield bisindole alkaloids was higher in CATDLF5 and CATDLF6-inoculated and natural control plants as compared to that in endophyte-free control plants (Fig. 5a). H2O2 production was lower in natural control plants as well as in CATDLF5- and CATDLF6-inoculated plants as compared to endophyte-free control plants (Fig. 5b).
Effect of endophytes (CATDLF5 and CATDLF6) on PRX1 expression and hydrogen peroxide concentration.
(a) Transcript abundance of PRX1, (b) Hydrogen peroxide concentration in the plant leaf. NC- the natural control plants that originated from C. roseus seeds that were not treated with any bacteriocide and fungicide and contained all the naturally occurring endophytes present in the plants. C- the endophyte-free control plants that originated from C. roseus seeds treated with bactericides and fungicides and were thus devoid of any naturally occurring endophyte. In the qRT-PCR analysis, the endophyte-free control (C) was used as the calibrator and for normalization, C. roseus actin gene was used as the endogenous gene. In (a) Y-axis represents relative quantity (RQ). Data are means ± SD (n = 3 biological replicates). Values with different letters are significantly different (Duncan’s multiple range test *P < 0.05).
Regulatory components of TIA biosynthesis
Since TIA biosynthesis is regulated by transcriptional activators (ORCA2, ORCA3, BPF1 and MYC2) (Fig. 6) and repressors (ZCT1, ZCT2, ZCT3, GBF1 and GBF2) (Fig. 7), their transcript abundance was quantified to understand the mechanism of endophyte-mediated increment in vindoline biosynthesis. ORCA2 expression was not affected in CATDLF5- and CATDLF6-inoculated plants. When compared to endophyte-free plants, CATDLF5 inoculation enhanced the expression of ORCA3, BPF1 and MYC2, but CATDLF6 inoculation could enhance MYC2 expression only. Also, CATDLF6 downregulated BPF1 expression. In natural control plants, ORCA3 expression was significantly (~5 fold) higher than that in endophyte-free control plants whereas there was no significant difference in expression of ORCA2, BPF1 and MYC2 in both types of control (natural and endophyte-free) plants. CATDLF5 and CATDLF6 inoculation downregulated the expression of ZCT1, ZCT2, ZCT3 and GBF2 as compared to the endophyte-free control plants. Unexpectedly, GBF1 expression was higher in CATDLF5- and CATDLF6-inoculated plants as compared to that in the endophyte-free control plants. Natural control plants showed increased ZCT1, ZCT2, GBF1 and GBF2 expression and decreased ZCT3 expression as compared to that in the endophyte-free control plants. Transcript abundance of MPK3, which has a possible role in the stress-induced biosynthesis of TIAs, was unaffected by CATDLF5 and CATDLF6 inoculation (Fig. 7).
Effect of endophytes (CATDLF5 and CATDLF6) on expression of transcriptional activators.
Transcript abundance of (a) ORCA2, (b) ORCA3, (c) BPF1, (d) MYC2 was analyzed. NC- the natural control plants that originated from C. roseus seeds that were not treated with any bacteriocide and fungicide and contained all the naturally occurring endophytes present in the plants. C- the endophyte-free control plants that originated from C. roseus seeds treated with bactericides and fungicides and were thus devoid of any naturally occurring endophyte. The endophyte-free control (C) was used as the calibrator. For normalization, C. roseus actin gene was used as the endogenous gene. Data are means ± SD (n = 3 biological replicates) and Y-axis represents relative quantity (RQ). Asterisks indicate significant differences as compared to the endophyte-free control (C) (Duncan’s multiple range test *P < 0.05).
Effect of endophytes (CATDLF5 and CATDLF6) on expression of transcriptional repressors and mitogen activated protein kinase 3.
Transcript abundance of (a) ZCT1, (b) ZCT2, (c) ZCT3, (d) GBF1, (e) GBF2, (f) MPK3 was analyzed. NC- the natural control plants that originated from C. roseus seeds that were not treated with any bacteriocide and fungicide and contained all the naturally occurring endophytes present in the plants. C- the endophyte-free control plants that originated from C. roseus seeds treated with bactericides and fungicides and were thus devoid of any naturally occurring endophyte. The endophyte-free control (C) was used as the calibrator. For normalization actin gene was used as the endogenous gene. Data are means ± SD (n = 3 biological replicates) and Y-axis represents relative quantity (RQ). Asterisks indicate significant differences as compared to the endophyte-free control (C) (Duncan’s multiple range test *P < 0.05).
Discussion
Fungal endophytes have been isolated from C. roseus plants and their host and tissue specificity was suggested34. A fungal endophyte isolated from C. roseus plant was able to produce vinblastine and vincristine in culture medium10. Previously, we reported two bacterial endophytes V1 and V3 identified as Staphylococcus sciuri and Micrococcus sp., respectively isolated from C. roseus plants that had increased ~38–68% vindoline content and also enhanced the biomass and plant growth32. However, in the present work, we identified two fungal endophytes CATDLF5 (Curvularia sp.) and CATDLF6 (Choanephora infundibulifera) from alkaloid-rich genotype Dhawal that were able to increase vindoline content by 229–403% in low alkaloid genotype Prabal. We tried to reveal the role of the endophytes in enhancing the biosynthesis of key TIA (vindoline) in the host plant. The endophytes were isolated from field-grown C. roseus plants and then their ability to enhance vindoline content in C. roseus plants was examined by inoculating them into endophyte-free plants. To have a comprehensive picture, we used two types of control (endophyte-free and natural) plants in the present study. However, the presence of cryptic (non-culturable) endophytes in the endophyte-free plants that were generated for the study could not be completely ruled out. Nevertheless, the effect of any non-culturable microbe present both in the endophyte-free control as well as endophyte (CATDLF5 and CATDLF6)-inoculated plants will not matter as the only variable will be the presence of the additionally inoculated endophyte (CATDLF5 or CATDLF6) when comparing the endophyte-free control with the endophyte-treated plants. Natural control plants, which have all the natural complement of cryptic and non-cryptic endophytes were also used and compared with endophyte-free control plants as well as CATDLF5- and CATDLF6-inoculated plants.
Although the endophytes CATDLF5 and CATDLF6 were able to substantially enhance the vindoline content in the C. roseus plant, they did not affect the photosynthetic efficiency of the host plant, which was measured in terms of chlorophyll content and fluorescence, net CO2 assimilation, stomatal conductance and transpiration rate. The chlorophyll fluorescence was used as a non-invasive tool to monitor plant performance35. Fv/Fm measurement confirmed that natural control plants had more efficient and functional photosynthetic machinery as evident from higher Fv/Fm value in them as compared to that of endophyte-free control plants indicating the possible role of some endophytes in plant physiological processes. Also, higher chlorophyll content in natural control plants may be a reason for higher Fv/Fm value. CATDLF5 and CATDLF6 did not affect the efficiency of photosynthetic apparatus and the plants inoculated with them had a Fv/Fm value (~0.75) similar to that of endophyte-free control plants. As Fv/Fm value was higher in natural control plants their net CO2 assimilation, transpiration rate and stomatal conductance was also higher than that of endophyte-free control plants. The final end product of photosynthetic metabolism is starch and its accumulation depends on the photosynthetic efficiency of plants36. Natural control plants showed higher starch accumulation. Better foliage growth and enhanced biomass of the natural control plants compared to endophyte-free control plants suggests the presence of endophytes that can promote photosynthesis and growth of the host plant. Previously we have reported a bacterial endophyte V3, a Micrococcus sp., isolated from C. roseus that was able to promote plant growth and biomass32. Endophytic fungi that promote photosynthetic efficiency, growth and yield of the plant have also been reported for Mentha piperita37. A root fungal endophyte Piriformospora indica was able to promote plant growth and yield38. In future, such endophytes promoting plant growth could be considered for forming a consortium with endophytes enhancing secondary metabolites.
Induction of vindoline biosynthesis by selected endophytes could be the result of upregulation of key structural genes of the TIA biosynthetic pathway such as G10H, LAMT, AS, TDC, STR, 16OMT, D4H and DAT. However, it seems that each endophyte differentially regulates the expression of a different set of genes involved in TIA biosynthesis. Both the endophytes also upregulated the expression of vacuolar localized C. roseus class III peroxidase (PRX1), which dimerizes the monomeric TIA precursors (vindoline and catharanthine) to form bisindole alkaloids39. PRX1 is also known to be involved in scavenging of H2O2 and its homeostasis in plant cell40. As suggested previously, PRX1-secondary metabolites together serve as a central sink and buffer of H2O2 levels in plant cells and fine-regulation of this system is needed for efficient TIA production and plant homeostasis40. However, there may be further complexities involved as well. Higher PRX1 expression in CATDLF5- and CATDLF6-inoculated plants as compared to natural control plants could not result into lower H2O2 accumulation, whereas higher PRX1 expression in natural control plants compared to endophyte-free control plants could reduce the accumulation of H2O2 but could not increase the vindoline content as in the case of CATDLF5- and CATDLF6-inoculated plants. Although vindoline is a substrate for PRX1, there seems to be no direct relationship between PRX1 expression and vindoline content in the present study. In natural control plants, lower H2O2 production indicates the presence of some endophytes in host plant that can scavenge H2O2 or having antioxidative properties. Endophytes promoting the antioxidative potential of host plants have been isolated from Datura stramonium41 and Solanum nigrum42.
Biosynthesis of TIA is regulated by transcriptional regulators. Three Cys2/His2-type zinc finger protein family transcriptional factors ZCT1, ZCT2 and ZCT3, have been identified to repress the activity of TDC and STR promoters43. ZCTs also repress the activating activity of ORCAs, APETALA2/ethylene response factor domain transcription factors43. Transcriptional activities of TDC, STR and D4H promoters are also regulated by ORCA2 and ORCA344,45. Downregulation of ZCT expression (in CATDLF5- and CATDLF6-inoculated plants) and upregulation of ORCA3 expression (in CATDLF5-inoculated plants only) may be the reason for higher TDC and STR transcript abundance. Previously it was found that transgenic plants overexpressing ORCA3 had induced expression of TDC, STR, AS and D4H46. An earlier study28 revealed that ORCA expression was activated by the basic helix-loop-helix transcription factor CrMYC2. The selected endophytes regulated the expression of STR by differentially modulating the expression of two G-box-binding factors GBF1 and GBF2 that act as transcriptional repressors of STR promoters47. Expression of mitogen-activated protein kinase MPK3 was not affected by selected endophytes, although stresses such as wounding, UV-treatment and methyl jasmonate application induce MPK3 expression48. This confirmed that the selected endophytes induced in planta vindoline biosynthesis without creating any major type of stress response in the host plant.
Results of the present study suggest that the presence of natural endophytes in the plant probably plays an important role in promoting the growth of the host plant by increasing photosynthetic pigment synthesis, photosynthetic rate, stomatal conductance, transpiration rate, which results in increased starch accumulation and biomass. The absence of these natural endophytes (in endophyte-free plants) resulted in reduced growth parameters. Our study strongly suggests that the presence of some endophytes is able to promote the biosynthetic potential of the plant for some key secondary metabolites. Two such endophytes (CATDLF5 and CATDLF6) could enhance vindoline content by modulating the expression of genes involved in TIA biosynthesis and its regulatory components. For future applications, we suggest formulation and testing of microbial consortia comprised of endophytes like bacterial V3 (that enhance plant growth rate, biomass and vindoline content) and fungal CATDLF5/CATDLF6 (that enhance vindoline content appreciably but have no effect on plant growth/biomass). Such consortia may provide a win-win situation by providing high biomass coupled with high vindoline content, which in turn might play a key role in reducing the price of the costly bisindole alkaloids.
Methods
Plant material and growth conditions
Seeds of two C. roseus genotypes (cv. Prabal and cv. Dhawal) were obtained from the National Gene Bank for Medicinal and Aromatic Plants at CSIR-CIMAP, Lucknow and grown in pots (17 cm height ×22 cm top diameter ×12 cm bottom diameter and 3.7 L volume) filled with 3.5 kg of autoclaved soil and vermicompost mixture (2:1 v/v). The plants were watered with sterile water. Plants were grown in a greenhouse in natural photoperiod and light intensity at 25 °C ± 2 °C.
Isolation of endophytes
Endophytes were isolated from healthy green leaves of field grown C. roseus (cv. Dhawal) plants. For surface sterilization leaves were washed in running tap water and then dipped in 1% sodium hypochlorite for 10 min and rinsed 4 times in 0.02 M sterile potassium phosphate buffer pH 7.0. Sterility check was performed by taking 100 μL of an aliquot from the final wash and transferring to 5 mL nutrient broth and keeping in an incubator shaker (200 rpm at 28 °C) for 7 days. Samples were discarded if the growth was observed in the sterility check samples. Surface sterilized leaves were cut into small pieces by using sterilized surgical blade and kept on potato dextrose agar (PDA) (200 g L−1 potato infusion, 20 g L−1 dextrose, 15 g L−1 agar; pH 5.6) plate. Sterilized leaves were also macerated in a sterile pestle and mortar with sterile distilled water. The extract (100 μL) was taken and serial dilutions were made. Each dilution of the sample was plated on PDA plate. The plates were incubated at 28 °C for 7 days. Emerged fungal colonies were separated under sterilized conditions and grown on PDA plates for further use. Isolated fungal endophytes were identified by sequencing the internal transcribed spacer (ITS; 18S rDNA) region.
Fungal ITS fragment amplification and sequencing
Genomic DNA from endophytes was isolated by using CTAB method49. Primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) used for PCR amplification of the ITS were taken from a previous study50. Each PCR reaction mixture (25 μL) contained 1 μL genomic DNA (100 ng), 1 μL each of 10 μM forward and reverse primers, 0.5 μL 10 mM deoxyribonucleotide triphosphate mixture, 2.5 μL 10X PCR buffer (100 mM Tris-HCl, pH 8.3; 500 mM KCl; 15 mM MgCl2; 0.01% gelatin), 0.2 μL Taq DNA polymerase (5 U μL−1) (Sigma-Aldrich Inc. MO, USA) and 18.8 μL autoclaved MilliQ water. The thermocycling conditions consisted of an initial denaturation step of 94 °C for 5 min, followed by 30 cycles of 94 °C for 45 s, 57.4 °C for 45 s and 72 °C for 2 min and a final extension at 72 °C for 5 min in an Eppendorf Master cycler gradient PCR system. PCR products were visualized on 0.8% agarose gels and the products were excised and purified with Nucleo-pore PCR Clean-up Gel Extraction kit (Genetix Biotech Asia Pvt. Ltd., India) following the manufacturer’s instructions. DNA sequencing was performed with a 3130XL genetic analyzer (Applied Biosystems, USA). Nucleotide sequence similarities were determined by using NCBI BLAST search (http://blast.ncbi.nlm.nih.gov/blast/Blast.cgi). Partial sequence data for the ITS fragment have been deposited in the GenBank (NCBI) under accession numbers KT001517 (CATDLF5) and KT001518 (CATDLF6).
Treatment of C. roseus plants with endophytes
Endophyte-free C. roseus (cv. Prabal) plants were used to study the effects of treatment with endophytes isolated from cv. Dhawal. Endophyte-free plants were generated according to an earlier report51. Seeds were rinsed thoroughly with water and incubated in fungicide Bavistin (containing carbendazim 50% W.P., BASF India Limited) and bactericide K-Cycline (containing tetracycline hydrochloride 10% w/w and streptomycin sulphate 90% w/w, Karnataka Antibiotics & Pharmaceuticals Ltd. Bangalore, India) solution at room temperature with shaking at 100 rpm. Carbendazim, tetracycline hydrochloride and streptomycin sulphate were chosen because no phytotoxic effects were observed on the treated seedlings. After 24 h of treatment, seeds were washed five times with autoclaved water and an aliquot of seeds was crushed using a sterile pestle and mortar with sterile distilled water. Triturate was plated on nutrient agar and potato dextrose agar and plates were incubated at 28 °C for 10 days. When no microbial growth was found in the incubated plates, the remaining seeds were treated as endophyte-free and were used to grow endophyte-free nursery stock of C. roseus in pots (7 cm height ×30 cm top diameter ×20 cm bottom diameter and 3.0 L volume) filled with 3.0 kg of autoclaved soil and vermicompost mixture (2:1 v/v) and watered with sterile water. Pots were maintained in a greenhouse (natural photoperiod and light intensity at 25 °C ± 2 °C) for one month and sample seedlings were re-checked for their endophyte-free status. Thirty days-old endophyte-free seedlings were uprooted carefully to minimize damage to their roots and used for inoculation with the selected/target endophytes. For inoculation, the roots of the seedlings were dipped in individual endophyte spore suspension (1 × 108 spore/conidia mL−1) prepared in phosphate buffered saline (PBS) (8 g L−1 sodium chloride, 0.2 g L−1 potassium chloride, 1.44 g L−1 disodium hydrogen phosphate, 0.24 g L−1 potassium dihydrogen phosphate; pH 7.4) for 3 h. The plants were re-planted in autoclaved soil and vermicompost mixture (2:1 v/v) filled pots (17 cm height ×22 cm top diameter ×12 cm bottom diameter and 3.7 L volume) and grown under greenhouse conditions. Plants were watered with sterile water as and when required.
For all experimental analyses, endophyte-treated plants were compared with two types of controls-(i) the endophyte-free control [C] plants that originated from C. roseus seeds treated with bactericides and fungicides and were thus devoid of any naturally occurring endophyte and (ii) the natural control [NC] plants that originated from C. roseus seeds that were not treated with any bacteriocide and fungicide and contained all the naturally occurring endophytes present in the plants. The purpose to include the natural control was to get an idea of the effect of the natural endophytic populations occurring in the plants vis-à-vis the endophyte-free control plants. The roots of both the controls–endophyte-free (C) and natural (NC) plants were dipped in PBS for 3 h before re-planting in autoclaved soil and vermicompost mixture (2:1 v/v) filled pots. To ensure the presence of a sufficient number of inoculated endophytes in the soil, the inoculation (10 mL pot−1 of the individual endophyte suspension containing 1 × 108 spore/conidia mL−1) was repeated at 15-days after the first inoculation. Before carrying out further experimental analyses, the presence of endophytes in the inoculated C. roseus plants was confirmed by incubation of small pieces of surface- sterilized leaves on PDA plate. Inoculated endophytes were found to emerge from the margin of pieces cut from the leaves of endophyte-inoculated plants.
To minimize variations related to plant developmental stage and other variables, sampling for all analyses were carried out at the same (intermediate-neither too young nor too old/senescent) stage (90 d) and position of leaves (third leaf from top).
Analysis of vindoline content
Extraction of C. roseus leaves for alkaloid analysis was done according to an earlier report52. It is known that vindoline content depends upon plant developmental stage20. Hence, sampling was carried out in plants of the same stage (90-d old) and from the same position of leaves (third leaf) in each case to minimize unintended variations. Leaf powder (1 g) was extracted thrice with 30 mL of 90% ethanol for 12 h at room temperature. The alcohol extract was filtered through Whatman No. 1 filter paper. The filtrates were concentrated in vacuo (Rotavapor R-210, Büchi, Switzerland) to 10 mL, diluted with water (10 mL), acidified with 3% HCl (10 mL) and washed with hexane three times (3 × 30 mL). The aqueous portion was separated and basified with liquid ammonia to pH 8.5 and extracted three times with chloroform (3 × 30 mL). The chloroform extract was washed with water, dried over anhydrous sodium sulphate, concentrated under vacuum and redissolved in 1 mL methanol. Vindoline content in the methanol extract of leaf tissue was measured by using HPLC as described previously53. In brief, separation was achieved on C18 Symmetry® Waters (250 × 4.6 mm, 5μm) using the mobile phase consisting 70:30 (v/v) mixture of 100 mM ammonium acetate (pH 7.3) (A): acetonitrile (B), at a flow rate of 1 mL min−1 for 5 min. The mobile phase was changed in a linear gradient to 36A:64B over 10 min and maintained for 15 min. The flow rate was increased to 1.4 mL min−1 over 5 min. The A:B ratio was then increased to 20:80 in 5 min and maintained for 15 min. The flow rate and mobile phase ratio were then returned to 1 mL min−1 and 70:30 and the column was allowed to re-equilibriate. The analysis was performed with Empower Pro software (Waters, Milford, MA, USA). Pure vindoline (Sigma-Aldrich, St. Louis, MO, USA) was used as a standard. The selectivity and specificity of the vindoline determination was established through-(i) studying peak purity plots using a PDA detector, (ii) UV-Vis spectra matching, (iii) spiking with reference compound of known concentration and (iv) LC-MS specificity (Supplementary Figs S3 and S4).
Photosynthetic pigments, chlorophyll fluorescence and photosynthesis measurement
Total chlorophyll, chlorophyll a, chlorophyll b and carotenoid content was measured in the plants. Leaf tissue was kept overnight in 100% methanol at 4 °C and quantified as described previously54.
Chlorophyll fluorescence and photosynthesis in terms of net CO2 assimilation, stomatal conductance and transpiration rate were measured in the attached plant leaf using portable photosynthesis system (CIRAS-3 PP Systems, USA) attached with chlorophyll fluorescence module (CFM-3). CO2 concentration in sample chamber was maintained at 400 ppm (ambient CO2) and chamber air temperature was maintained at 25 °C (ambient temperature). Leaf was incubated in dark for 15 min before chlorophyll fluorescence measurement. For CO2 assimilation, stomatal conductance and transpiration rate measurement, the leaf was pre-exposed for 15 min at 400 μmol photons m−2 s−1 light, 400 ppm CO2 and 25 °C temperature.
Starch estimation
Starch content was estimated in the leaves by the acid digestion method as described previously55. Leaf discs of equal diameter were homogenized in liquid nitrogen. These homogenized samples were washed with acetone and hot 80% ethanol to remove interfering substances until the extract became colorless. The starch was extracted and solubilized by 35% perchloric acid. This starch solution was used for the colorimetric assay by using anthrone reagent (1.146 g of anthrone powder in 500 mL of concentrated sulfuric acid and 200 mL of water). Samples were placed in a boiling-water bath for 12 min and immediately placed in ice. Absorbance was recorded at 625 nm on a spectrophotometer.
Hydrogen peroxide estimation
Hydrogen peroxide was determined in the plant leaves as described previously56 with slight modifications. Leaf tissues (200 mg) were homogenized in 1 mL of 0.1% (w/v) trichloroacetic acid solution on ice. The homogenate was centrifuged at 12,000 g for 15 min at 4 °C and 0.4 mL of the supernatant was then added to 0.4 mL of 10 mM potassium phosphate buffer, pH 7.0 and 0.8 mL of 1 M potassium iodide and mixed. The absorbance was measured at 390 nm by using a spectrophotometer. The content of H2O2 was calculated by comparison with a H2O2 calibration curve.
Measurement of plant biomass, plant height, number of leaves, branches and siliques
For biomass measurement, aerial parts of the plants were harvested and dried at 70 °C for 5 d and their dry weights were measured. Plant height, number of leaves, siliques and branches were also measured.
qRT-PCR analysis
Total RNA was extracted from the leaves using TRI-reagent (Sigma-Aldrich, St. Louis, USA) according to the manufacturer’s instructions. RNA quantification was performed by using NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific). To remove genomic DNA contamination, DNase I, RNase-free enzyme (Thermo Scientific) was used. First-strand cDNA synthesis was done from 5 μg of total RNA using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific) with oligo(dT)18 primer according to the manufacturer’s protocol. Transcript abundance of structural and regulatory genes involved in vindoline biosynthesis was analyzed. Primers used for the qRT-PCR were designed using Primer Express Software v3.0.1 (Applied Biosystems) (Supplementary Table S1)48,57,58. PCR mixtures included 5 μL Power SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK), 300 nM each of the forward and reverse primers and 1 μL of 10 times diluted cDNA as a template in a reaction volume of 10 μL. PCR conditions were an initial denaturation of 10 min at 95 °C, followed by 40 cycles of denaturation at 95 °C for 15 sec and annealing/extension at 60 °C for 1 min each. Fluorescent signal intensities were recorded on an Applied Biosystems StepOnePlusTM Real-Time PCR System and analyzed using StepOne software (Applied Biosystems). Specificity of qRT-PCR was examined by subjecting all amplicons to a melting curve analysis using the dissociation method (Applied Biosystems). The threshold cycle (Ct) for each gene was normalized against the Ct for actin of C. roseus, which was used as the endogenous gene. The endophyte-free control (C) was used as the calibrator and the relative quantification 2−∆∆Ct method was used59,60.
Statistical analysis
For each treatment (endophytes and controls) three individual plants were analyzed for qRT-PCR, vindoline content and hydrogen peroxide measurement whereas for analysis of physiological and growth parameters six individual plants were analyzed as biological replicates. For each biological replicate, three technical replicates were run to keep a check on analytical errors and the mean of the three technical replicates represented the particular biological replicate. Technical replicates were carried out for qRT-PCR, vindoline content and hydrogen peroxide measurement but not for physiological and growth parameters analyses. Statistical analyses were carried out for the data obtained for the three biological replicates by applying ANOVA, suitable for a completely randomized design (CRD), using the ASSISTAT Version 7.6 beta (2012) software. Comparisons among means were carried out using Duncan’s multiple range tests at a significance level of P ≤ 0.05.
Additional Information
How to cite this article: Pandey, S. S. et al. Fungal endophytes of Catharanthus roseus enhance vindoline content by modulating structural and regulatory genes related to terpenoid indole alkaloid biosynthesis. Sci. Rep. 6, 26583; doi: 10.1038/srep26583 (2016).
References
Kusari, S., Hertweck, C. & Spiteller, M. Chemical ecology of endophytic fungi: origins of secondary metabolites. Chem. Biol. 19, 792–798 (2012).
Ryan, R. P., Germaine, K., Franks, A., Ryan, D. J. & Dowling, D. N. Bacterial endophytes: recent developments and applications. FEMS Microbiol. Lett. 278, 1–9 (2008).
Lodewyckx, C. et al. Endophytic bacteria and their potential applications. Crit. Rev. Plant Sci. 21, 583–606 (2002).
Bernardi-Wenzel, J. B. et al. Evaluation of foliar fungal endophyte diversity and colonization of medicinal plant Luehea divaricata (Martius et Zuccarini). Biol. Res. 43, 375–384 (2010).
Knoth, J. L., Kim, S. H., Ettl, G. J. & Doty, S. L. Biological nitrogen fixation and biomass accumulation within poplar clones as a result of inoculations with diazotrophic endophyte consortia. New Phytol. 201, 599–609 (2014).
Rodriguez, R. & Redman, R. More than 400 million years of evolution and some plants still can’t make it on their own: Plant stress tolerance via fungal symbiosis. J. Exp. Bot. 59, 1109–1114 (2008).
Schulz, B. & Boyle, C. The endophytic continuum. Mycol. Res. 109, 661–686 (2005).
Rodriguez, R. J., Redman, R. S. & Henson, J. M. In The Fungal Community: its Organization and Role in the Ecosystem 3rd edn, (eds Dighton, J. et al. ) Ch. 34, 683–695 (CRC Press, Boca Raton, 2005).
Mousa, W. K. & Raizada, M. N. The diversity of anti-microbial secondary metabolites produced by fungal endophytes: an interdisciplinary perspective. Front. Microbiol. 4, 65 (2013).
Kumar, A., Patil, D., Rajamohanan, P. R. & Ahmad, A. Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS One 8, e71805 (2013).
Soliman, S. S. M., Tsao, R. & Raizada, M. N. Chemical inhibitors suggest endophytic fungal paclitaxel is derived from both mevalonate and non-mevalonate-like pathways. J. Nat. Prod. 74, 2497–2504 (2011).
Kusari, S., Verma, V. C., Lamshoeft, M. & Spiteller, M. An endophytic fungus from Azadirachta indica A. Juss. that produces azadirachtin. World J. Microbiol. Biotechnol. 28, 1287–1294 (2012).
Eyberger, A. L., Dondapati, R. & Porter, J. R. Endophyte fungal isolates from Podophyllum peltatum produce podophyllotoxin. J. Nat. Prod. 69, 1121–1124 (2006).
Kusari, S., Lamshöft, M. & Spiteller, M. Aspergillus fumigatus Fresenius, an endophytic fungus from Juniperus communis L. Horstmann as a novel source of the anticancer pro-drug deoxypodophyllotoxin. J. Appl. Microbiol. 107, 1019–1030 (2009).
Puri, S. C., Verma, V., Amna, T., Qazi, G. N. & Spiteller, M. An endophytic fungus from Nothapodytes foetida that produces camptothecin. J. Nat. Prod. 68, 1717–1719 (2005).
Kusari, S., Lamshöft, M., Zühlke, S. & Spiteller, M. An endophytic fungus from Hypericum perforatum that produces hypericin. J. Nat. Prod. 71, 159–162 (2008).
Kusari, S., Singh, S. & Jayabaskaran, C. Biotechnological potential of plant-associated endophytic fungi: hope versus hype. Trends Biotechnol. 32, 297–303 (2014).
Li, J. et al. An endophytic Pseudonocardia species induces the production of artemisinin in Artemisia annua. PloS one 7, e51410 (2012).
Ming, Q. et al. Elicitors from the endophytic fungus Trichoderma atroviride promote Salvia miltiorrhiza hairy root growth and tanshinone biosynthesis. J. Exp. Bot. 64, 5687–5694 (2013).
Shukla, A. K. & Khanuja, S. P. S. In OMICS Applications in Crop Science, (ed Barh, D. ) Ch. 10, 325–384 (CRC Press, Boca Raton, 2013).
Torres, A. R. et al. Colonization of Madagascar periwinkle (Catharanthus roseus), by endophytes encoding gfp marker. Arch. Microbiol. 195, 483–489 (2013).
Ferreira Filho, A. S. et al. Endophytic Methylobacterium extorquens expresses a heterologous β-1, 4-endoglucanase A (EglA) in Catharanthus roseus seedlings, a model host plant for Xylella fastidiosa. World J. Microbiol. Biotechnol. 28, 1475–1481 (2012).
Lacava, P. T., Araújo, W. L. & Azevedo, J. L. Evaluation of endophytic colonization of Citrus sinensis and Catharanthus roseus seedlings by endophytic bacteria. J. Microbiol. 45, 11–14 (2007).
Monteiro, P. B. et al. Catharanthus roseus, an experimental host plant for the citrus strain of Xylella fastidiosa. Plant Dis. 85, 246–251 (2001).
van Der Heijden, R., Jacobs, D. I., Snoeijer, W., Hallard, D. & Verpoorte, R. The Catharanthus alkaloids: pharmacognosy and biotechnology. Curr. Med. Chem. 11, 607–628 (2004).
O’Keefe, B. R., Mahady, G. B., Gills, J. J., Beecher, C. W. & Schilling, A. B. Stable vindoline production in transformed cell cultures of Catharanthus roseus. J. Nat. Prod. 60, 261–264 (1997).
Suttipanta, N. et al. The transcription factor CrWRKY1 positively regulates the terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Plant Physiol. 157, 2081–2093 (2011).
Zhang, H. et al. The basic helix-loop-helix transcription factor CrMYC2 controls the jasmonate-responsive expression of the ORCA genes that regulate alkaloid biosynthesis in Catharanthus roseus. Plant J. 67, 61–71 (2011).
Zhou, M. L. et al. Molecular regulation of terpenoid indole alkaloids pathway in the medicinal plant, Catharanthus roseus. J. Med. Plants Res. 4, 2760–2772 (2010).
Liu, D. H. et al. Terpenoid indole alkaloids biosynthesis and metabolic engineering in Catharanthus roseus. J. Integr. Plant Biol. 49, 961–974 (2007).
Memelink, J. & Gantet, P. Transcription factors involved in terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Phytochem. Rev. 6, 353–362 (2007).
Tiwari, R. et al. Bacterial endophyte-mediated enhancement of in planta content of key terpenoid indole alkaloids and growth parameters of Catharanthus roseus. Ind. Crop. Prod. 43, 306–310 (2013).
Papageorgiou, G. C. & Govindjee. (Ed.). In Chlorophyll a fluorescence: a signature of photosynthesis, Vol. 19 (Springer, 2004).
Kharwar, R. N., Verma, V. C., Strobel, G. & Ezra, D. The endophytic fungal complex of Catharanthus roseus (L.) G. Don. Curr. Sci. 95, 228–233 (2008).
Adams III, W. W. & Demmig-Adams, B. In Chlorophyll a fluorescence: a signature of photosynthesis, Vol. 19 (eds Papageorgiou, G. C. & Govindjee ) Ch. 22, 583–604 (Springer, 2004).
Li, W. D., Duan, W., Fan, P. G., Yan, S. T. & Li, S. H. Photosynthesis in response to sink-source activity and in relation to end products and activities of metabolic enzymes in peach trees. Tree Physiol. 27, 1307–1318 (2007).
Mucciarelli, M., Scannerini, S., Bertea, C. & Maffei, M. In vitro and in vivo peppermint (Mentha piperita) growth promotion by nonmycorrhizal fungal colonization. New Phytol. 158, 579–591 (2003).
Varma, A. et al. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl. Environ. Microbiol. 65, 2741–2744 (1999).
Costa, M. M. et al. Molecular cloning and characterization of a vacuolar class III peroxidase involved in the metabolism of anticancer alkaloids in Catharanthus roseus. Plant Physiol. 146, 403–417 (2008).
Ferreres, F. et al. Identification of phenolic compounds in isolated vacuoles of the medicinal plant Catharanthus roseus and their interaction with vacuolar class III peroxidase: an H2O2 affair? J. Exp. Bot. 62, 2841–2854 (2011).
Christhudas, I. N., Kumar, P. P. & Agastian, P. In Vitro α-Glucosidase inhibition and antioxidative potential of an endophyte species (Streptomyces sp. Loyola UGC) isolated from Datura stramonium L. Curr. Microbiol. 67, 69–76 (2013).
Wan, Y. et al. Effect of endophyte-infection on growth parameters and Cd-induced phytotoxicity of Cd-hyperaccumulator Solanum nigrum L. Chemosphere 89, 743–750 (2012).
Pauw, B. et al. Zinc finger proteins act as transcriptional repressors of alkaloid biosynthesis genes in Catharanthus roseus. J. Biol. Chem. 279, 52940–52948 (2004).
Li, C. Y. et al. The ORCA2 transcription factor plays a key role in regulation of the terpenoid indole alkaloid pathway. BMC Plant Biol. 13, 155 (2013).
van der Fits, L. & Memelink, J. ORCA3, a jasmonate responsive transcriptional regulator of plant primary and secondary metabolism. Science 289, 295–297 (2000).
Pan, Q. et al. Overexpression of ORCA3 and G10H in Catharanthus roseus plants regulated alkaloid biosynthesis and metabolism revealed by NMR-metabolomics. PloS one 7, e43038 (2012).
Sibéril, Y. et al. Catharanthus roseus G-box binding factors 1 and 2 act as repressors of strictosidine synthase gene expression in cell cultures. Plant Mol. Biol. 45, 477–488 (2001).
Raina, S. K. et al. CrMPK3, a mitogen activated protein kinase from Catharanthus roseus and its possible role in stress induced biosynthesis of monoterpenoid indole alkaloids. BMC Plant Biol. 12, 134 (2012).
Sambrook, J., Fritsch, E. F. & Maniatis, T. In Molecular cloning: a laboratory manual 2nd edn (Cold Spring Harbor Laboratory Press, 1989).
White, T. J., Bruns, T., Lee, S. & Taylor, J. In PCR protocols A guide to methods and applications (eds Innis, M. A. et al. ) Ch. 38, 315–322 (Academic Press, 1990).
Spiering, M. J., Greer, D. H. & Schmid, J. Effects of the fungal endophyte, Neotyphodium lolii, on net photosynthesis and growth rates of perennial ryegrass (Lolium perenne) are independent of in planta endophyte concentration. Ann. Bot. 98, 379–387 (2006).
Singh, D. V., Maithy, A., Verma, R. K., Gupta, M. M. & Kumar, S. Simultaneous determination of Catharanthus alkaloids using reversed phase high performance liquid chromatography. J. Liq. Chrom. & Rel. Technol. 23, 601–607 (2000).
Peebles, C. A. M., Hong, S. B., Gibson, S. I., Shanks, J. V. & San, K. Y. Transient effects of overexpressing anthranilate synthase α and β subunits in Catharanthus roseus hairy roots. Biotechnol. Prog. 21, 1572–1576 (2005).
Lichtenthaler, H. K. & Buschmann, C. In Current protocols in food analytical chemistry (eds Wrolstad, R. E. et al. ) F4.3.1–F4.3.8 (New York: John Wiley & Sons, 2001).
Biswal, A. K. et al. Light Intensity-dependent modulation of chlorophyll b biosynthesis and photosynthesis by overexpression of Chlorophyllide a Oxygenase in tobacco. Plant Physiol. 159, 433–449 (2012).
Pérez-Ruiz, J. M. et al. Rice NTRC is a high-efficiency redox system for chloroplast protection against oxidative damage. Plant Cell 18, 2356–2368 (2006).
Asada, K. et al. A 7-Deoxyloganetic acid Glucosyltransferase contributes a key step in secologanin biosynthesis in Madagascar Periwinkle. Plant Cell 25, 4123–4134 (2013).
Besseau, S. et al. A pair of Tabersonine 16-hydroxylases initiates the synthesis of vindoline in an organ-dependent manner in Catharanthus roseus. Plant Physiol. 163, 1792–1803 (2013).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108 (2008).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2∆∆Ct method. Methods 25, 402–408 (2001).
Acknowledgements
The Council of Scientific and Industrial Research (CSIR), India, financially supported this study through grants NWP BSC0117 (XII Five Year Plan Network Project). The authors are grateful to Director, CSIR-CIMAP, for his encouragement in providing laboratory facilities. SSP and SS gratefully acknowledge CSIR for financial assistance in the form of fellowships in NWP BSC0117 project. SS also thanks Indian Council of Medical Research (ICMR) for her Senior Research Fellowship.
Author information
Authors and Affiliations
Contributions
A.K. and S.S.P. conceived and designed the experiments. S.S.P., S.S., N.K.S. and K.S. performed the experiments. A.K., C.S.V., N.K.S., K.S., A.K.S. and S.S.P. analyzed the data. A.K., C.S.V., A.K.S. and S.S.P. wrote the paper. All authors read and approved the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Pandey, S., Singh, S., Babu, C. et al. Fungal endophytes of Catharanthus roseus enhance vindoline content by modulating structural and regulatory genes related to terpenoid indole alkaloid biosynthesis. Sci Rep 6, 26583 (2016). https://doi.org/10.1038/srep26583
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep26583
This article is cited by
-
Elicitation with sodium nitroprusside and Trichoderma improves vincristine and vinblastine yield in Catharanthus roseus cell suspension culture by modulating terpenoid indole alkaloid pathway genes
Plant Cell, Tissue and Organ Culture (PCTOC) (2024)
-
Plants and endophytes interaction: a “secret wedlock” for sustainable biosynthesis of pharmaceutically important secondary metabolites
Microbial Cell Factories (2023)
-
Co-occurrence of two ascomycete endophytes as the specialized metabolite production partners in Rheum spiciforme Royle
Symbiosis (2023)
-
Endophytic fungi mediates production of bioactive secondary metabolites via modulation of genes involved in key metabolic pathways and their contribution in different biotechnological sector
3 Biotech (2023)
-
Vinca alkaloids as a potential cancer therapeutics: recent update and future challenges
3 Biotech (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.