Abstract
Since amphibian declines were first proposed as a global phenomenon over a quarter century ago, the conservation community has made little progress in halting or reversing these trends. The early search for a “smoking gun” was replaced with the expectation that declines are caused by multiple drivers. While field observations and experiments have identified factors leading to increased local extinction risk, evidence for effects of these drivers is lacking at large spatial scales. Here, we use observations of 389 time-series of 83 species and complexes from 61 study areas across North America to test the effects of 4 of the major hypothesized drivers of declines. While we find that local amphibian populations are being lost from metapopulations at an average rate of 3.79% per year, these declines are not related to any particular threat at the continental scale; likewise the effect of each stressor is variable at regional scales. This result - that exposure to threats varies spatially, and populations vary in their response - provides little generality in the development of conservation strategies. Greater emphasis on local solutions to this globally shared phenomenon is needed.
Similar content being viewed by others
Introduction
The challenge in understanding the drivers of population dynamics, including species declines, is a classic example of the problem of pattern and scale in ecology1. On one hand, observations of ecological processes made at local scales often inadequately describe patterns at regional or continental scales2. In turn, conservation strategies derived from broad scale assessments are not always relevant to local conservation efforts. For over 20 years amphibian ecologists have generally followed the ‘declining population’ paradigm3, conducting research to identify the complex causes underlying declines4,5,6,7,8, and have called for the collection of additional data at more locations to further document and evaluate drivers of declines5,6,7,9. The implicit expectation is that observations from an increasing number of locations in space and time will help to identify universal laws underlying an observed phenomenon, from which more informed predictions and management decisions can be generated10,11. Information is treated equally in this passive approach to improving management, even though some insights are more valuable than others (i.e., those which can be taken advantage of to increase population size or distribution10). This has been a general criticism of conservation-oriented research and monitoring programs12. Since amphibian declines were first proposed as a global phenomenon over a quarter century ago, the collective efforts of thousands of scientists have helped quantify the severity of such declines and identified a handful of consensus causes5,8,13. These approaches have helped prioritize areas for conservation14,15, and while there have been a handful of local successes, the global decline was not halted16. In fact, declines have continued across North America since at least the 1960s17, with surprisingly few conservation success stories. This should not be unexpected – if the distribution of threats, population response, or causes of population declines vary among locations, then understanding causes of amphibian declines at large spatial scales may not reveal which threats are driving local populations18.
Existing large-scale analyses of amphibian declines have relied on expert opinion and range maps extrapolated from species’ detections to evaluate causes of endangerment7,8,13. For example, IUCN species assessments assume that threat categories reflect changes in population abundances or occurrence, though other criteria (including range size or population threat information) can result in the same level of species’ endangerment. A central finding has been that while dominant threats vary among large geographic areas, a significant proportion of declines have no identified causes, especially in North America8,13. Accordingly, a handful of threats including climate change, disease risk, and land use have been used to forecast continental-scale amphibian extinction risk7,8,19. However, these analyses and accompanying predictions are based on limited empirical data on amphibian metapopulations, and thus are largely untested. We use a continental scale, long-term data set of amphibian habitat occupancy from many species and populations to empirically test the generality of proposed drivers of decline. While previous assessments8,13 show that the distributions of drivers are not globally consistent, we test whether they are consistent among metapopulations, which is the appropriate scale for conservation action20. Specifically, we test whether populations are exposed to multiple threats, whether the intensity of exposure and population response to threats varies geographically, and whether, despite this variation, continental-scale patterns in declines can be identified.
Results
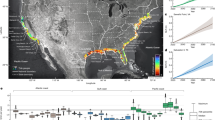
By relating spatially varying intensities of each threat (Fig. 1) to trends in the habitat occupancy of amphibian populations, we identified the relative contribution of each threat to decline in the number of populations across the continent. We provide strong evidence that the average rate of decline in amphibian metapopulations is 3.79% per year (95% CI {−2.49, −5.04}; Table 1), consistent with previous assessments13,17,21, and that similar rates of decline occur across all five regions we examined. This rate of decline means that on average, the number of occupied sites for an amphibian species will decrease by 50% within 19 years. However, we find not only that the number and relative intensity of threats varies geographically (Fig. 2), but also that population responses to threats vary when sub-basins were grouped into regions (Fig. 3). Because of this, our empirical data show that no single cause of decline can be identified. This results from substantial variation in the distribution and intensity of threats among sub-basins (Fig. 2a), which resulted in spatial variation in the number and combination of threat values to which amphibians in each sub-basin were exposed (Fig. 2b). High intensity of threats in sub-basins were most frequently land-use and drought (Fig. 2b) while high intensities of disease risk or pesticide application occurred in less than half of the sub-basins. Many sub-basins were exposed to multiple threats (Fig. 2c). Although the average annual population trend across all populations was negative (Table 1), no single threat was consistent in explaining the observed trends (Fig. 3a). Likewise, though we found that amphibian responses to these threats varied spatially, we did not find strong region-specific drivers of population trends (Fig. 3b–f). These results show that there is not a single consistent response to threats across the continental scale, and indicate that declines are discrete phenomena with spatially varying etiology.
Values are summarized and normalized at the HUC sub-basin scale, using ArcGIS (ver. 10.2. Redlands, CA: Environmental Systems Research Institute). (a) Human influence index. (b) Suitability for Batrachochytrium dendrobatidis. (c) Average annual pesticide application. (a–c) Red indicates higher than average threat intensity while green is lower than average. Locations of amphibian community data indicated with circles; symbols indicate region groupings (see Table 1; circles = Northeast, diamonds = Southeast, plus = Midwest, triangles = West coast, squares = Rocky Mountains). (d) The mean difference in the 30-year normal from the 2001–11 annual average precipitation; blue indicates wetter sub-basins with above average difference in precipitation while red indicates drier sub-basins.
Normalized intensities for the 4 threats [HII = Human influence index; Bd = Batrachochytrium dendrobatidis suitability; Pest = annual average pesticide application; Prec = the difference in the 30-year normal from the annual average precipitation (2001–2011); droughts are represented as positive values to correspond with an increase in the threat] considered in our analysis (a) average exposure for each threat by sub-basin (sub-basins are sorted by number of threats with above-average values, and intensity of the climate threat; sub-basin identifiers omitted for clarity), (b) the frequency of above-average values of each of the four threats for all sub-basins and for those with time-series data for species, (c) the number of above-average threats for all sub-basins and species in our time-series, and (d) exposure to each threat for 54 anuran species and 2 anuran species complexes (A01–A56) and 29 caudate (C01–C29) species [corresponding species names in Table S3].
The amphibian assemblages represented in our data differed among sub-basins, thus the exposure of each species to the number (Fig. 2b) and identity (Fig. 2c) of threats differs. Most species (72%) were exposed to higher-than-average intensities of two threats, with 13% exposed to three, and 13% exposed to a single threat. Two species (Anaxyrus americanus and Pseudacris maculata) were exposed to higher-than-average intensity levels of all four threats. On average, species were exposed most frequently to drought and Human Influence Index (HII) threats (72 and 83% of values, respectively, were greater than average). Most anurans were exposed to a combination of drought and human influence, while salamanders were most commonly exposed to a combination of human influence and Bd, or human influence and drought (Fig. 2d). Our findings suggest that in some populations, and for some species, a single factor may predominate, while in others a combination of threats drives population declines.
Discussion
Our analysis uses data from across the United States to empirically test the relationship between change in the number of amphibian populations and hypothesized threats. We did not find support for a consistent relationship between rates of amphibian declines and distribution of stressors at the continental level. Instead our results suggest that declines result from locally driven processes and the influence of multiple interacting stressors that in turn lead to emergent declines at the continental scale. Our use of occupancy models provides statistically unbiased information that populations of amphibians are in decline across the United States. This result is more alarming than previous work identifying declining trends in abundance in North American amphibian populations17 because we show that populations themselves go locally extinct. The absence of a single cause of these declines, even at smaller regional scales, suggests that even regional conservation action plans may be ineffective in combatting threats to amphibian populations.
While there is value in empirically testing hypothesized factors underlying amphibian declines, the continuing pace of population extirpations and declines in abundance suggests a need for a new approach. Discussions at the 1st World Congress of Herpetology in Canterbury, UK, a quarter century ago, presaged the documentation of serious and global declines in amphibian populations. However, there is no longer a lack of research on potential causes of decline, as was the case in 1989. Rather, broad recognition of the multitude and complexity of factors driving amphibian declines9 emphasizes the need to increase the development and application of local science-based strategies for conservation of amphibian populations. In fact, understanding the causes of a decline may not necessarily lead to better management; for example, identifying populations which are at risk for Bd-induced decline has not led directly to better management, as few proposed solutions have been effective in the field22. More generally, understanding the causes of declines does not always mean that the best management action can be identified or implemented. While declines of amphibians and loss of biodiversity are globally consistent problems, most conservation actions must be implemented locally, at the scale of a population or metapopulations, to be most effective. Because we find that amphibian populations are exposed to multiple drivers and because the degree of exposure varies spatially, a single recipe for conservation is impractical. While some local conservation actions have proven successful23 thus far, solutions to threats at large scales are elusive24. Assuming few technological constraints (which may be addressed through research), a focus on local drivers could improve conservation. Similar to the principle of subsidiarity in solving water management problems25, amphibian conservation can beneficially consider global context, but focus on local actions.
Developing feasible strategies to increase population sizes, maintain population distributions, and support population resilience to environmental change requires two seemingly paradoxical capacities: a better understanding of which threats are most important locally, and a willingness to test conservation actions despite uncertainty in the identification of threats and the response of populations to management. We may use scientific approaches that allow us to learn about the causes of the declines while we implement conservation on the ground. This approach to developing management policies (e.g., under an adaptive management framework26), where we reduce uncertainties about the effect of specific management strategies on the target populations through active management, has proven successful (e.g., harvest management27). Under this approach to conservation, the investment in research is related directly to the potential improvement for populations under management26,28. Some uncertainty will always remain regardless of research, but the ability to take conservation actions given uncertainty is needed, especially where populations are facing catastrophic declines29. A shift in the approach to amphibian conservation towards action under uncertainty could improve efficiency in optimizing management in response to local population declines. This would include explicit identification of uncertainties which most matter to choosing among management options, greater transparency in management decision making, and increased sharing of successes and failures in a formal framework for evidence-based conservation decision making. This approach to learning – about what threatens local populations and what strategies will work to maintain populations – may be best implemented when resource managers incorporate more quantitative rigor into the development and application of management strategies, and when researchers make science more understandable, and immediately relevant, to managers.
Experimental Procedures
We obtained unbiased estimation of trends in amphibian occurrence probabilities (i.e., changes in the number of populations that occur when local extinctions exceed (re-)colonizations within a metapopulation) from 61 study areas across North America (Fig. 1; Table S1), incorporating data from 389 time-series of 83 species of amphibians (Table S2) using a hierarchical occupancy model30. Study areas were mainly Federal and State protected areas, and we had no a priori expectation that amphibian populations or resource conditions were in decline or deteriorating when surveys were initiated. Data came from field surveys of multiple habitat units (e.g., wetlands, stream segments, forest patches) within each protected area and included habitats where amphibian species were expected to have a chance of occurring. Site selection was unconditional on initial amphibian presence, and sites at each study area were selected under a probabilistic design (e.g., simple random sampling, stratified random sampling); this means that though site selection differed among study areas, statistical inference is appropriate at the scale of each study area. Survey methods differed among habitats and species, but were generally chosen to maximize the probability of detecting the target species and sometimes multiple standard methods for detecting amphibians were used. Multiple species may have been detected during a single survey occasion. Surveys were repeated on multiple occasions within a time period when each species had the possibility of occupying a site (i.e., sites were assumed to be demographically ‘closed’ during the duration of surveys). These detection-nondetection data were analyzed in our occupancy model, and used to estimate detection probabilities, thereby accounting for unequal effort and other sources of observation error so that inference is on the (true) state of a population (i.e., present or absent) and is unconditional on whether or not a species was detected at a site during sampling occasions31.
Statistical model
Using Markov Chain Monte Carlo methods30,32, we estimated trends of amphibian populations for each metapopulation (i.e., each combination of species and study area), and the effects of four of the most widely cited threats [land-use, contaminants, climate change and disease risk5,6,8,13], as there is evidence that these threats have contributed to the extinction of local populations [e.g., (26–29)]. Trends were estimated for each metapopulation, and represent the balance of local extinctions and colonizations over each time-series; a negative trend occurs when extinctions exceed colonizations. We defined 5 regions as geographic clusters of protected areas.
For all studies, multiple observations were collected within a year, which allowed us to estimate unbiased trends across years. We denote year by y and time-series by k, so that ψyk is the probability a site in the kth time-series in the yth year is occupied by a given species and pyk is the probability of observing a given species during a visit to a site, given that site is occupied by the species. We denote the number of visits to the ith site in a given year for a given time series as Niyk and the number of times the species was observed at the site during those visits as Yiyk. The true, but unobserved, state of a site is denoted by ziyk, where z = 1 if a site is occupied and z = 0 if not.
Observations Yiyk are modeled with a binomial distribution as:


Note the binomial probability is conditional on the true occupancy state of the site, which is a Bernoulli trial with probability of occurrence for the specific combination of year and time-series. To accommodate variation of detection probabilities among different years and time-series, we assumed a random distribution for the detection parameters:

We estimated occupancy trends as a derived parameter within the MCMC algorithm, calculating the average rate of change in number of occupied sites (λ) in each time-series as a function of the estimated trend parameters in the logit-linear model as

where ψi and ψf are occupancy at the start and end of each time series.
Our interest was in understanding the factors associated with annual variation in occupancy probabilities. We expected that relationships would not be evident among threats and continental-scale population trends if declines are driven by different factors among populations, but that relationships may be detectable at regional scales if these threats influence populations more consistently at a smaller spatial scale.
We fit a generalized linear mixed model, where the model included two random components, αk, a random-effect for time-series intercept, and δyk, a random-error component to account for sub-sampling of sites within each combination of year and time-series, where:

Distributions for random-effects were specified as:


A fixed model, βX, was used to specify the effect of year (continuous), threat, and interaction between threat and year. Covariates for threats were scaled and centered to have a mean of 0 and standard deviation of 1 and year was centered to have a mean of 0 at the level of the time-series. To account for model uncertainty in the inclusion of parameters and minimize potential effects of correlation among threat covariates, we fit parameters using a Bayesian LASSO (least absolute shrinkage and selection operator) approach33,34, by specifying the β parameters to come from a double-exponential distribution, where:

and τ, the regularization parameter was estimated from the data used to fit the model (example code to implement a LASSO provided in Supporting Online Material). To allow for a single value of τ to be estimated, we specified year covariates and interactions between year and threats to have a mean of 0 and standard deviation of 1 when fitting the model. We then back-transformed estimates so the estimated effect of year represented the average annual change in occupancy across all time-series and the estimated interaction effects represented the average annual effect of a 1-standard deviation increase in the value of a threat. The Markov Chain Monte Carlo-estimated posterior probabilities of the parameters (i.e., the standardized partial regression coefficients) can be interpreted as the relative contribution of each threat to the observed population trends.
Priors were chosen as follows: logit−1(μα) and logit−1(μp) were uniform (0,1) on the real scale; σp, σα, and σδ were uniform (0, 3); and τ was uniform (0, 20). Models were fit using JAGS run via R (v.2.15.2; R Core Development Team 2012). We used a burn-in sample of 5000 iterations and then estimated the posterior distribution of parameters based on the next 25000 samples. Three chains were run and model convergence was assessed based on Gelman-Rubin statistics35.
Threats
Disease risk was defined as the suitability for occurrence of the fungal pathogen B. dendrobatidis (Bd)36, as it is classified as one of the “enigmatic” causes of amphibian decline13,37. We used spatially referenced predictions of Bd suitability for the United States, which were generated under the environmental niche model described in36 (provided by D. Olsen, US Forest Service). In a binomial regression model, the detection of Bd was found to be different among major ecosystems of the US, generally increasing with number of species, and increasing with decreasing minimum temperature (details of model found in36). Predicted suitability under this model is qualitatively similar to the global model38 in8. There are no comparable data on other emerging amphibian pathogens such as Ranavirus or Batrachochytium salamandrivorans.
The threat from climate change relates to an increased risk of extinction in response to changes in precipitation, mainly resulting in increased frequency and intensity of droughts39. This threat from climate change was calculated as the mean difference in mean annual precipitation from (2001–2011) and the 30-year normal (1981–2010; available from: http://www.prism.oregonstate.edu/, accessed 13 March 2014), which was a proxy for water availability. To obtain the difference in annual average precipitation during the period of our surveys (2001–11), we subtracted the 10-year average annual precipitation for each sub-basin over the period of record (monthly data obtained from PRISM for 2001–11; http://www.prism.oregonstate.edu/recent/; accessed 13 March 2014) from the 30-year (1981–2010) normal precipitation (http://www.prism.oregonstate.edu/normals/; accessed 13 March 2014).
The threat from land use change was evaluated because it is the single most important threat to biodiversity in general40, and both human influence (Human Influence Index; HII41) and the application of pesticides42,43 are components of this threat. The human influence index (HII), developed by the Wildlife Conservation Society and the Center for International Earth Science Information Network41, is an index of direct human disturbance which incorporates human population density, land transformation, accessibility, and electrical power infrastructure to represent direct human influence on the landscape. We summarized the 1-km2-pixel HII raster map provided by41 as the mean HII within each sub-basin.
The threat from contaminants was described by the estimated annual pesticide use values for compounds known or hypothesized to be important to amphibians (Table in44 and updated by K. Smalling, USGS pers. comm.). We used the ‘EPest-high’ application estimation method from42,43 for each county from 2001–11, which treats non-reporting of application as missing values which are estimated from nearby data. Values were summarized at the sub-basin scale by weighting the average application by the area of the county falling within each sub-basin, and thus reflect the average pesticide application per unit area.
For each threat, we calculated the average, normalized intensity (the value of each threat) within each sub-basin (i.e., United States Geological Survey (USGS) HUC4-scale sub-basins; http://pubs.usgs.gov/wsp/wsp2294/html/pdf.html) for use in our occupancy models. We investigated the correlations among the normalized threats for all 204 sub-basins in the continental United States using the Band Collection Statistics tool in Spatial Analyst in ArcGIS 10.2. Not all sub-basins had amphibian population data; correlations among threats for the subset of sub-basins (n = 55) with amphibian species data were determined independently. All correlations were <0.47, except a positive correlation between Bd and climate for the 55 sub-basins with amphibian data (ρ = 0.648) results from the inclusion of precipitation in the suitability model of36.
Additional Information
How to cite this article: Grant, E. H. C. et al. Quantitative evidence for the effects of multiple drivers on continental-scale amphibian declines. Sci. Rep. 6, 25625; doi: 10.1038/srep25625 (2016).
References
Levin, S. A. The problem of pattern and scale in ecology. Ecology 73, 1943–1967 (1992).
Melbourne, B. A. & Chesson, P. The scale transition: scaling up population dynamics with field data. Ecology 87, 1478–88 (2006).
Caughley, G. Directions in conservation biology. J. Anim. Ecol. 63, 215–244 (1994).
Alford, R. A. & Richards, S. J. Global amphibian declines: a problem in applied ecology. Annu. Rev. Ecol. Syst. 30, 133–165 (1999).
Collins, J. P. & Storfer, A. Global amphibian declines : sorting the hypotheses. Divers. Distrib. 9, 89–98 (2003).
Beebee, T. J. & Griffiths, R. A. The amphibian decline crisis: A watershed for conservation biology? Biol. Conserv. 125, 271–285 (2005).
Sodhi, N. S. et al. Measuring the meltdown: drivers of global amphibian extinction and decline. PLoS One 3, e1636 (2008).
Hof, C., Araújo, M. B., Jetz, W. & Rahbek, C. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature 480, 516–9 (2011).
Blaustein, A. R. et al. The complexity of amphibian population declines: understanding the role of cofactors in driving amphibian losses. Ann. N. Y. Acad. Sci. 1223, 108–19 (2011).
Williams, B. K., Szaro, R. & Shapiro, C. Adaptive Management: The U.S. Department of the Interior Technical Guide. (2009). U.S. Department of Interior.
Nie, M. A. & Schultz, C. A. Decision-making triggers in adaptive management. Conserv. Biol. 26, 1137–44 (2012).
Nichols, J. D. & Williams, B. K. Monitoring for conservation. Trends Ecol. Evol. 21, 668–73 (2006).
Stuart, S. N. et al. Status and trends of amphibian declines and extinctions worldwide. Science (80-.). 306, 1783–1786 (2004).
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. & Kent, J. Biodiversity hotspots for conservation priorities. Nature 403, 853–8 (2000).
Botkin, D. B. et al. Forecasting the effects of global warming on biodiversity. Bioscience 57, 227–236 (2007).
Hoffmann, M. et al. The impact of conservation on the status of the world’s vertebrates. Science (80-.). 330, 1503–1509 (2010).
Houlahan, J. E., Findlay, C. S., Schmidt, B. R., Meyer, A. H. & Kuzmin, S. L. Quantitative evidence for global amphibian population declines. Nature 404, 752–5 (2000).
Wiens, J. A. & Bachelet, D. Matching the multiple scales of conservation with the multiple scales of climate change. Conserv. Biol. 24, 51–62 (2010).
Ficetola, G. F., Rondinini, C., Bonardi, A., Baisero, D. & Padoa-Schioppa, E. Habitat availability for amphibians and extinction threat: a global analysis. Divers. Distrib. 21, 302–311 (2014).
Marsh, D. M. & Trenham, P. C. Metapopulation dynamics and amphibian conservation. Conserv. Biol. 15, 40–49 (2001).
Adams, M. J. et al. Trends in amphibian occupancy in the United States. PLoS One 8, e64347 (2013).
Woodhams, D. C. et al. Mitigating amphibian disease: strategies to maintain wild populations and control chytridiomycosis. Front. Zool. 8, 8 (2011).
Smith, R. K. & Sutherland, W. J. Amphibian conservation: global evidence for the effects of interventions. Exeter, Pelagic. (Pelagic Publishing, 2014).
Mendelson III, J. R. et al. Confronting amphibian declines and extinctions. Science (80-.). 313, 48 (2006).
Hering, B. J. G., Sedlak, D. L., Tortajada, C. & Biswas, A. K. Local perspectives on water. Science (80-.). 349, 479–480 (2015).
McCarthy, M. A. & Possingham, H. P. Active adaptive management for conservation. Conserv. Biol. 21, 956–963 (2007).
Nichols, J. D., Runge, M. C., Johnson, F. A. & Williams, B. K. Adaptive harvest management of North American waterfowl populations: a brief history and future prospects. J. Ornithol. 148, 343–349 (2007).
Williams, B. K., Eaton, M. J. & Breininger, D. R. Adaptive resource management and the value of information. Ecol. Modell. 222, 3429–3436 (2011).
Martin, T. G. et al. Acting fast helps avoid extinction. Conserv. Lett. 5, 274–280 (2012).
Royle, J. A. & Dorazio, R. M. Hierarchical models of animal abundance and occurrence. J. Agric. Biol. Environ. Stat. 11, 249–263 (2006).
Mackenzie, D. I. et al. Estimating site occupancy rates when detection probabilities are less than one. Ecology 83, 2248–2255 (2002).
Miller, D. A. & Grant, E. H. C. Estimating occupancy dynamics for large-scale monitoring networks: amphibian breeding occupancy across protected areas in the northeast. U.S. Ecol. Evol. 5, 4735–4746 (2015).
Park, T. & Casella, G. The Bayesian Lasso. J. Am. Stat. Assoc. 103, 681–686 (2008).
Hooten, M. B. & Hobbs, N. T. A guide to Bayesian model selection for ecologists. Ecol. Monogr. 83, 3–28 (2015).
Gelman, A. & Rubin, D. B. lnference from iterative simulation using multiple sequences. Stat. Sci. 7, 457–472 (1992).
Olson, D. H. et al. Mapping the global emergence of Batrachochytrium dendrobatidis, the amphibian chytrid fungus. PLoS One 8, e56802 (2013).
Berger, L. et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl. Acad. Sci. 95, 9031–6 (1998).
Rödder, D., Kielgast, J. & Lötters, S. Future potential distribution of the emerging amphibian chytrid fungus under anthropogenic climate change. Dis. Aquat. Organ. 92, 201–7 (2010).
Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 3, 52–58 (2012).
Foley, J. A. et al. Global consequences of land use. Science. 309, 570–4 (2005).
Sanderson, E. W., Jaiteh, M., Levy, M. A., Redford, K. H., Wannebo, A. V. & Woolmer, G. 2003. The human footprint and the last of the wild. Bio Science 52, 891–904 (2002).
Thelin, G. & Stone, W. W. Estimation of annual agricultural pesticide use for counties of the conterminous United States, 1992–2009. (US Geological Survey Scientific Investigation Report 2013–5009, 2013).
Baker, N. T. & Stone, W. W. Preliminary estimates of annual agricultural pesticide use for counties of the conterminous United States, 2010–2011. (U.S. Geological Survey Open-File Report 2013–1295, 2013).
Baker, N. J., Bancroft, B. A. & Garcia, T. S. A meta-analysis of the effects of pesticides and fertilizers on survival and growth of amphibians. Sci. Total Environ. 449, 150–156 (2013).
Acknowledgements
This work was conducted as part of the Amphibian Decline Working Group supported by the John Wesley Powell Center for Analysis and Synthesis, funded by the US Geological Survey. Data deposited at the US Geological Survey’s John Wesley Powell Center for Analysis and Synthesis. The authors declare no competing financial interests. This manuscript is contribution #541 of the USGS Amphibian Research and Monitoring Initiative. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Comments from D. Hocking, P. Toschik and three anonymous reviewers improved the manuscript.
Author information
Authors and Affiliations
Contributions
E.H.C.G., D.A.W.M. and E.M. designed the study, E.H.C.G. and D.A.W.M. performed all analyses; all authors discussed the results; E.H.C.G., M.J.A., R.N.F., D.M.G., B.R.H., P.T.J.J., T.A.G.R., J.H.W., S.C.W., L.L.B., G.M.F., T.A.G., A.M.R., D.S.P., S.J.P., D.S., W.S. and E.M. contributed data and expertise on study areas from which data were collected; E.H.C.G., D.A.W.M., E.M. and B.R.S. wrote the paper, and E.H.C.G., D.A.W.M., B.R.S., E.M., S.M.A., T.C., S.S.C., M.B.J., M.E.R., M.J.A., R.N.F., D.M.G., B.R.H., P.T.J.J., T.A.G.R., J.H.W., S.C.W., L.L.B., G.M.F., T.A.G., A.M.R., D.S.P., S.J.P., W.S. and D.S. discussed the results and implications and commented on the manuscript at all stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Grant, E., Miller, D., Schmidt, B. et al. Quantitative evidence for the effects of multiple drivers on continental-scale amphibian declines. Sci Rep 6, 25625 (2016). https://doi.org/10.1038/srep25625
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep25625
This article is cited by
-
Metabolites of Xenorhabdus bacteria are potent candidates for mitigating amphibian chytridiomycosis
AMB Express (2023)
-
Simulating the response of a threatened amphibian to climate-induced reductions in breeding habitat
Landscape Ecology (2023)
-
Factors influencing persistence of a threatened amphibian in restored wetlands despite severe population decline during climate change driven weather extremes
Biodiversity and Conservation (2022)
-
Life history traits and reproductive ecology of North American chorus frogs of the genus Pseudacris (Hylidae)
Frontiers in Zoology (2021)
-
Exposure to Batrachochytrium dendrobatidis affects chemical defences in two anuran amphibians, Rana dalmatina and Bufo bufo
BMC Ecology and Evolution (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.