Abstract
Bisulfite plays crucial roles in diverse physiological processes. Therefore, the efficient detection of bisulfite is very important. In this study, we report a colorimetric and ratiometric fluorescent probe (CPT) with a large Stokes shift (162 nm) for bisulfite (HSO3−) based FRET mechanism. The probe can quantitatively detect HSO3− with low detection limit (45 nM) and high specificity over other common anions and biothiols. A nucleophilic addition reaction was proposed for the sensing mechanism, which was confirmed by HRMS spectra. The test strips of the probe were made and used easily. Moreover, probe CPT was used to ratiometric fluorescent imaging of exogenous and endogenous HSO3− in living cells.
Similar content being viewed by others
Introduction
Bisulfite (HSO3−), widely used as food preservative because of its antimicrobial, bacteriostasis and antioxidant property1, is found that its certain concentration level is responsible for respiratory diseases2,3. Therefore, more analytical methods should be developed for trace HSO3−. So far, many analytical methods including electrochemistry, chromatography, absorption and fluorescence spectroscopy have been developed to detect HSO3− quantitatively4,5,6,7,8. Among these, fluorescence probes are widely applied because of their high selectivity, low detection limit and suitability for real-time monitoring.
Ratiometric fluorescent probes allowing the measurement of emission intensities at two different wavelengths could overcome the limitations of intensity-based probes and provide a self-calibration correction9,10,11,12,13. One well established method for developing ratiometric probes is based Förster Resonance Energy Transfer (FRET)-an excited-state energy interaction between two fluorophores, wherein the emission profile of one fluorophore (the donor) shows a significant overlap with the excitation profile of the other fluorophore (the acceptor). The ratio emission signal is modulated by the FRET process. On the other hand, a few probes with long emission wavelength based on 2-dicyanomethylene-3-cyano-4,5,5-trimethyl-2,5-dihydrofuran (TCF) have been reported because its excellent optical properties14,15,16,17,18,19,20,21,22.
Our group has introduced a new strategy to construct ratiometric fluorescent probes based on FRET23. The FRET process induced to prohibit the donor fluorescence and enhance the acceptor fluorescence. Upon reacting with the analytes, the FRET process will be interrupted to restore the donor fluorescence. As a continuation of previous work, we report a FRET-based fluorescent probe composed of coumarin-piperazine-TCF conjugate platform for the colorimetric and ratiometric detection of HSO3−.
Results and Discussion
UV-vis absorption and fluorescence property of probe CPT
We investigated firstly the effect of water content on fluorescence spectra of the probe. The reactivity of bisulfite toward the probe was sensitive over wide water content. Considering its’ best fluorescence ratio response as well as better peak shapes, we chose the solvent ratio (EtOH/H2O = 6:4) as the vitro test solvent system (Supplementary Fig. S1). The absorption maximum of free probe CPT centered at 570 nm and 410 nm, which were assigned to TCF and coumarin moiety, respectively (Fig. 1A). When various analytes (CH3CO2−, CO32−, F−, Cl−, Br−, I−, HCO3−, NO2−, S2O32−, NO3−, SO42−, S2−, SO32−, SCN−, H2PO3−, HPO32−, Cys and GSH) were added into the solution of probe CPT, no significant absorption peaks change occurred for solutions with these analytes except for HSO3− (Fig. 1B). In the case of biothiols (GSH, Cys), although their thiol groups are nucleophilic, they possess higher pKa values (Cys 8.30, GSH 9.20). Besides, the electron-poor C = C group was often used as a reaction site to discriminate sulfite from other anion species through different activities in nucleophilic addition reaction. Upon addition of HSO3−, the maximum absorption peak at 330 nm appeared, and the maximum absorption peak at 570 nm disappeared, accompanying with the color change of solution from purple to colorless, suggesting that the conjugation system of the probe was interrupted due to the nucleophilic attack of HSO3−. So, probe CPT could serve as a “naked-eye” probe for HSO3−. Encouraged by the results, we applied probe CPT in test strips detection (Supplementary Fig. S2).
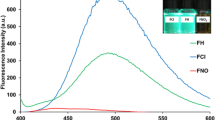
To examine the ability of probe CPT to sense HSO3−, the fluorescence titration was conducted. The probe (2.5 μM) alone displayed two obvious fluorescence bands at 470 nm and 632 nm (fluorescence quantum yield Φ = 0.39, rhodamine B as standard), which attributed to coumarin and TCF unit, respectively (Supplementary Fig. S3). Upon addition of HSO3− incrementally from 0 to 5 equiv., fluorescence emission band at 470 nm (Φ = 0.072, quinine sulfate as standard) increased gradually while the fluorescence at 632 nm decreased (Fig. 2A), which implies the acceptor moiety was destroyed by the reaction of the probe with HSO3−. The maximum emission peak underwent a 162 nm blue shift (from 632 nm to 470 nm) with an isoemission point at 589 nm, accompanying the fluorescence change from red to blue. These distinct responses confirmed that the FRET process was regulated by the reaction of the probe with HSO3−. The ratios of emission intensity at 470 and 632 nm (I470/I632) exhibited a drastic change from 2.841 to 546.986, a 192-fold enhancement with increasing concentrations of HSO3−. On the other hand, the probe showed no fluorescence intensity change toward other analytes (Fig. 2B). Further, we explored the interference of these analytes. The results demonstrated that probe CPT had high selectivity toward HSO3− and had almost no interference from other analytes (Supplementary Fig. S4).
Moreover, we also conducted the absorption titration experiments (Supplementary Fig. S5). Upon addition of HSO3− to the solution of probe CPT, new absorption bands at 330 nm enhanced consistently with the increasing concentrations (0–3 equiv.). Meanwhile, absorption bands at 410 nm were almost no change, however, absorption bands at 570 nm decreased. The results implied the reaction of TCF moiety with HSO3−, which was consistent with that from fluorescence titration. Ratiometric signaling of the fluorescence output of the probe (2.5 μM) at two different wavelengths indicated that the ratios reached a plateau when 15 μM of HSO3− was added (Supplementary Fig. S6). In addition, there was a linear correlation between the fluorescence intensity ratios and the concentrations of HSO3− from 0 to 7 μM, and the limit of detection (LOD) was calculated to be 45 nM, which is superior to most reported probes (Supplementary Table S1).
As an important factor for evaluating the probe in practical sensing, response time toward HSO3− was tested (Supplementary Fig. S7). Clearly, the ratios of emission intensity reached maximum at around 1 h, indicating the reaction accomplished. In the study, we utilized 10 equivalents (relatively lower) of HSO3−, causing relatively rapid response (Supplementary Table S1). To obtain information concerning the pH effects, lg(I470/I632) changes of probe CPT (2.5 μM) was investigated at different pH (Supplementary Fig. S8). The results indicated that the probe was stable and can function well over a wide range of relatively alkaline pH (7.0–10.0). As expected, there was hardly any response to HSO3− in acidic condition, which could be demonstrated by the facts that HSO3− cannot exist under stronger acid conditions.
Recognition mechanism
The supposed ratiometric fluorescence mechanism is shown in Fig. 3. For the probe alone, FRET between the coumarin and the TCF moiety resulted in red emission from the TCF acceptor with the excitation of coumarin moiety. Upon the addition of HSO3−, the energy transfer from coumarin donor to TCF acceptor was interrupted because of the breakage of C = C bond by the addition reaction with HSO3−. The reaction product of the probe with HSO3− displayed coumarin emission.
To confirm the FRET mechanism, we synthesized the donor and the acceptor (Supplementary). The addition of HSO3− to the donor caused no fluorescence change at 470 nm, while the addition of HSO3− to the acceptor quenched the fluorescence at 640 nm (Supplementary Fig. S9). These results confirmed that the conjugated structure of the acceptor was interrupted. The MS spectra of the reaction product between the acceptor and HSO3− also supported the deduction (calcd for C22H24N5O4S: 454.52, found 454.50) (Supplementary Fig. S10). Moreover, the overlap between the absorption spectra of the acceptor and the emission spectra of the donor showed that the FRET process occurred though the intramolecular energy transfer efficiency is not so effectively (44.5%) (Supplementary Fig. S11). Pleasantly, a large emission shift (162 nm) with two-well resolved emission bands (632/470 nm) before and after interaction with HSO3− generated, which could ensure accuracy in determining their intensities and ratios24. Finally, to further clarify the proposed mechanism, HRMS of the reaction product from probe CPT and HSO3− was carried out. The results confirmed that the treatment of probe CPT with HSO3− could afford nucleophilic addition product, accompanying a new peak appeared at 721.2068 (Supplementary Fig. S12).
Cell imaging of probe CPT
CPT was of low cytotoxicity (Supplementary Fig. S13) and had excellent photostability (Supplementary Fig. S14). Encouraged by the aforementioned results, we further investigated the applicability of CPT for exogenous and endogenous HSO3− detection in Hela cells. Staining Hela cells with CPT gave strong fluorescence in red channel and weak fluorescence in the blue channel. Further incubated with NaHSO3 the cells displayed a distinct fluorescence increase in blue channel, accompanied by the dramatic fluorescence drop in red channel (Supplementary Fig. S15). These results indicated that CPT can provide ratiometric detection for HSO3− in cells.
Further, we investigated the response of CPT toward endogenous HSO3− in cells. Endogenous HSO3− in cells can be produced from thiosulfate via thiosulfate sulphurtransferase (TST), which is abundant in mammalian liver cells25. Therefore, HepG2 cells (human liver cancer cells) and L-02 cells (human normal liver cells) were chosen to investigate the capability of this probe for the detection of endogenous bisulfite. A clear fluorescence change was observed only when HepG2 cells were incubated with CPT (5 μM) for 1 h followed by incubating with 500 μM GSH and 250 μM Na2S2O3 for 0.5 h (Fig. 4). These results demonstrated that the probe was capable of detecting endogenous bisulfite in HepG2 cells. However, no significant fluorescence change was observed in L-02 cells incubated with probe CPT (5 μM) and GSH/Na2S2O3 (Supplementary S16). The comparison between these results demonstrated that CPT could potentially be applied to differentiate liver cancer cells and normal liver cells.
(A) The first row (vertically): HepG2 cells were incubated with CPT (5 μM) for 40 min; The second row: HepG2 cells were incubated with 500 μM GSH and 250 μM Na2S2O3 for 1 h, and then with CPT (5 μM) for 40 min; The third row: HepG2 cells were incubated with 10 mM TNBS (2,4,6-trinitrobenzenesulphonate, known as a TST inhibitor) for 0.5 h, then with 500 μM GSH and 250 μM Na2S2O3 for another 1 h, followed by CPT (5 μM) for 40 min; The fourth row: HepG2 cells were incubated with 500 μM GSH for 1 h, then with CPT (5 μM) for another 40 min. (B) From left to right: the relative ratio of blue/red fluorescence intensity of row 1, 2, 3 and 4 in (A). The ratio images were all obtained as Fblue/Fred. Images were acquired from 405–555 nm for blue fluorescence, and from 560–700 nm for red fluorescence. λex = 405 nm.
Conclusions
In summary, we have developed a new colorimetric and ratiometric fluorescent probe based on FRET between coumarin and TCF fluorophor with a large Stokes shift (162 nm) for detecting HSO3−. The probe exhibits a clear HSO3− induced change in the intensity ratio of the two emission bands of coumarin and TCF, with high selectivity and sensitivity. The probe has a detection limit as low as 45 nM, which is superior to most reported probes. Moreover, the probe was used to ratiometric fluorescent imaging of endogenous HSO3− in living cells.
Methods
Apparatus and chemicals
Melting points were measured on an XD-4 digital micro-melting point apparatus. 1H NMR and 13C NMR spectra were recorded on a Bruker Avance 300 MHz spectrometer. HRMS spectra were obtained on a Q-TOF6510 spectrograph (Agilent). UV-vis spectra were measured by using a Hitachi U-4100 spectrophotometer. Twice-distilled water was used throughout all experiments. All the pH measurements were made with a PHS-3C pH meter. Thin-layer chromatography (TLC) was conducted on silica gel 60 F254 plates (Merck KGaA) and column chromatography was conducted over silica gel (mesh 200–300). All of fluorescence spectra were obtained by the excitation at 390 nm, slit 8/9 nm. All the samples were investigated in EtOH-H2O solution (6:4 v/v, 10 mM PBS, pH 8.0). Quartz cuvettes with a 1 cm path length and 3-mL volume were involved in fluorescence and UV -vis spectra measurements. All reagents were purchased from J&K, Aladdin and Sinopharm Chemical Reagent Co. and used without further purification.
Cell culture and cell imaging of the probe in HepG2 cells and L-02 cells
Hela cells were cultured in a 6-well plate in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum in an atmosphere of 5% CO2 and 95% air at 37 °C.
HepG2 cells or L-02 cells were cultured in a 6-well plate in DMEM supplemented with 10% fetal bovine serum in an atmosphere of 5% CO2 and 95% air at 37 °C. The probe CPT was dissolved in DMSO to get the stock solution (10 mM) and diluted to 5 μM before use. HepG2 cells or L-02 cells were incubated with a mixture of 500 μM GSH and 250 μM Na2S2O3 for 1 h, and then treated with 5 μM CPT for 40 min. For control experiments, CPT loaded HepG2 cells were pretreated with 10 mM TNBS for 0.5 h, and then treated with a mixture of 500 μM GSH and 250 μM Na2S2O3 for another 1 h. Simultaneously, CPT loaded HepG2 cells were treated with 500 μM GSH only for 40 min. Subsequently, excited at 405 nm, the cells were imaged under a confocal microscope (LSM 700) and the images were collected at emission channels of 405–555 nm (blue channel) and 560–700 nm (red channel), respectively.
Statistical analysis
Data are presented as mean ± SEM and analysis involved the use of GraphPad Prism 5. Images were processed by Adobe Photoshop CS5 (Adobe, San Jose, USA). P < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Wu, W.-L. et al. An effective colorimetric and ratiometric fluorescent probe based FRET with a large Stokes shift for bisulfite. Sci. Rep. 6, 25315; doi: 10.1038/srep25315 (2016).
References
De Azevedo, L. C. et al. Evaluation of the formation and stability of hydroxyalkylsulfonic acids in wines. J. Agric. Food Chem. 55, 8670–8680 (2007).
Sang, N. et al. SO2 inhalation contributes to the development and progression of ischemic stroke in the brain. Toxicol. Sci. 114, 226–236 (2010).
Claudia, R. C. & Francisco, J. C. Application of flow injection analysis for determining sulphites in food and beverages: a review. Food Chem. 112, 487–493 (2009).
Mohr, G. J. A chromoreactand for the selective detection of HSO3- based on the reversible bisulfite addition reaction in polymer membranes. Chem. Commun. 2646–2647 (2002).
Isaac, A., Wain, A. J., Compton, R. G., Livingstone, C. & Davis, J. A novel electroreduction strategy for the determination of sulfite. Analyst 130, 1343–1344 (2005).
Yu, L. H., Kim, J. M. & Schemid, R. D. Determination of sulphite in wine through flow-injection analysis based on the suppression of luminol chemiluminescence. Anal. Chim. Acta 26, 317–323 (1992).
Hassan, S. S., Hamza, M. S. & Mohamed, A. H. A novel spectrophotometric method for batch and flow injection determination of sulfite in beverages. Anal Chim Acta 570, 232–239 (2006).
Yang, Y. T. et al. A novel coumarin-based fluorescent probe for selective detection of bissulfite anions in water and sugar samples. Sens. Actuators B 166–167, 665–670 (2012).
Huang, K. et al. Ratiometric and colorimetric detection of hydrogen sulfide with high selectivity and sensitivity using a novel FRET-based fluorescence probe. Dyes Pigments 118, 88–94 (2015).
Wu, M. Y., Li, K., Li, C. Y., Hou, J. T. & Yu, X. Q. A water-soluble near-infrared probe for colorimetric and ratiometric sensing of SO2 derivatives in living cells. Chem. Commun. 50, 183–185 (2014).
Kaur, A., Haghighatbin, M. A., Hogan, C. F. & New, E. J. A FRET-based ratiometric redox probe for detecting oxidative stress by confocal microscopy, film and flow cytometry. Chem. Commun. 51, 10510–10513 (2015).
Dai, X. et al. An effective colorimetric and ratiometric fluorescent probe for bisulfite in aqueous solution. Anal. Chim. Acta. 888, 138–145 (2015).
Yang, J. et al. Novel tumor-specific and mitochondria-targeted near-Infrared-emission fluorescent frobe for SO2 derivatives in living cells. ACS Sensors 1, 166–172 (2016).
Chen, T. et al. A red emission fluorescent probe for hydrogen sulfide and its application in living cells imaging. Tetrahedron Lett. 54, 2980–2982 (2013).
Liu, C. Y. et al. A highly selective colorimetric and far-red fluorescent probe for imaging bisulfite in living cells. RSC Adv. 4, 33507–33513 (2014).
Ipuy, M. et al. Fluorescent push-pull pH-responsive probes for ratiometric detection of intracellular pH. Org. Biomol. Chem. 12, 3641–3648 (2014).
Zhang, A. R. et al. A systematic study of the structure–property relationship of a series of nonlinear optical (NLO) julolidinyl-based chromophores with a thieno[3,2-b]thiophene moiety. J. Mater. Chem. C 3, 370–381 (2015).
Zhou, X. et al. A new highly selective fluorescent K+ sensor. J. Am. Chem. Soc. 133, 18530–18533 (2011).
Yuan, L., Lin, W., Zheng, K., He, L. & Huang, W. Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem. Soc. Rev. 42, 622–661 (2013).
Feng, L. et al. A highly selective long-wavelength fluorescent probe for the detection of human carboxylesterase 2 and its biomedical applications. Chem. Commun. 50, 14519–14522 (2014).
Zhu, B. et al. A highly selective ratiometric visual and red-emitting fluorescent dual-channel probe for imaging fluoride anions in living cells. Biosens. Bioelectron. 52, 298–303 (2014).
Park, J., Kim, H., Choi, Y. & Kim, Y. A ratiometric fluorescent probe based on a BODIPY-DCDHF conjugate for the detection of hypochlorous acid in living cells. Analyst 138, 3368–3371 (2013).
Li, D. P. et al. A mitochondria-targeted fluorescent probe for ratiometric detection of endogenous sulfur dioxide derivatives in cancer cells. Chem. Commun. 52, 2760–2763 (2016).
Yuan, L. et al. Engineering a FRET strategy to achieve a ratiometric two-photon fluorescence response with a large emission shift and its application to fluorescence imaging. Chem. Commun. 6, 2360–2365 (2015).
Chen, W. et al. Selective, highly sensitive fluorescent probe for the detection of sulfur dioxide derivatives in aqueous and biological environments. Anal. Chem. 87, 609–616 (2015).
Acknowledgements
This study was supported by the Natural Science Foundation of Shandong Province (ZR2014BM004) and Major Project of Science and Technology of Shandong Province (NO. 2015ZDJS04001 and 2015ZDJS04003).
Author information
Authors and Affiliations
Contributions
B.-X.Z. designed the study. W.-L.W. did the synthesis of this fluorescent probe and performed the probes characterization. Z.-Y.W. performed the cell assays. B.-X.Z., J.-Y.M., W.-L.W. and X.D. wrote and edited the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wu, WL., Wang, ZY., Dai, X. et al. An effective colorimetric and ratiometric fluorescent probe based FRET with a large Stokes shift for bisulfite. Sci Rep 6, 25315 (2016). https://doi.org/10.1038/srep25315
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep25315
This article is cited by
-
Fluorescence Imaging of Mitochondria with Three Different Sets of Signals Based on Fluorene Cation Fluorescent Probe
Journal of Fluorescence (2019)
-
A new fluorescent probe for colorimetric and ratiometric detection of sulfur dioxide derivatives in liver cancer cells
Scientific Reports (2017)
-
Polymer vesicle sensor through the self-assembly of hyperbranched polymeric ionic liquids for the detection of SO2 derivatives
Chinese Journal of Polymer Science (2017)
-
An HBT-Based Near-Infrared Fluorescent Probe for Colorimetric and Ratiometric Detection of Bisulfite and its Application in Living Cells
Journal of Fluorescence (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.