Abstract
The growing problem of antibiotic-resistant microorganisms results in an urgent need for substitutes to conventional antibiotics with novel modes of action and effective activities. Antimicrobial peptides (AMPs), produced by a wide variety of living organisms acting as a defense mechanism against invading pathogenic microbes, are considered to be such promising alternatives. AMPs display a broad spectrum of antimicrobial activity and a low propensity for developing resistance. Therefore, a thorough understanding of AMPs is essential to exploit them as antimicrobial drugs. Considering this, we developed a comprehensive user-friendly data repository of antimicrobial peptides (DRAMP), which holds 17349 antimicrobial sequences, including 4571 general AMPs, 12704 patented sequences and 74 peptides in drug development. Entries in the database have detailed annotations, especially detailed antimicrobial activity data (shown as target organism with MIC value) and structure information. Annotations also include accession numbers crosslinking to Pubmed, Swiss-prot and Protein Data Bank (PDB). The website of the database comes with easy-to-operate browsing as well as searching with sorting and filtering functionalities. Several useful sequence analysis tools are provided, including similarity search, sequence alignment and conserved domain search (CD-Search). DRAMP should be a useful resource for the development of novel antimicrobial peptide drugs.
Similar content being viewed by others
Introduction
Inappropriate and irrational use of antibiotics has resulted in the emergence of multi-drug resistant microorganisms, spurring an urgent need to develop new generations of antibiotics with novel modes of action and effective activities. Antimicrobial peptides (AMPs), both natural and synthetic which possess mechanisms of antimicrobial activity that are different from those of conventional antibiotics can provide a therapeutic alternative to fight antibiotic-resistant microorganisms1. The natural AMPs are isolated and characterized from practically all-living organisms, ranging from prokaryotes to humans2. In general, AMPs are small (<10 kDa), cationic and amphipathic molecules with a substantial proportion (≥30%) of hydrophobic residues3, thus capable of interacting with microbial membranes through non-specific interactions with the membrane lipids. AMPs display a broad spectrum of antimicrobial activity, being effective against not only gram-positive and gram-negative bacteria, fungi, viruses, protozoa but also insects and some kinds of cancer4. Much information suggests that AMPs have major effects on infection and inflammation in mammals by influencing diverse cellular processes5. AMPs may act via a range of mechanisms which include, but are not limited to, bacterial membrane disruption6, the formation of membrane-spanning pores7, the inhibition of cell wall biosynthesis8, and translocation across the cytoplasmic membrane to act on intracellular targets9. Several antimicrobial peptides or analogues in recent years have been in advanced clinical development for localized or systemic infections10,11. For example, MX-226 (omiganan pentahydrochloride 1% gel; Migenix), a bovine indolicidin-based peptide, was developed for the prevention of contamination of central venous catheters. In a completed Phase III study, MX-226 demonstrated a significant therapeutic effect on local catheter site infections12. Despite those achievements, the therapeutic use of antimicrobial peptides is very limited. Deep understanding of mechanisms of action and the structure-activity relationship of AMPs is necessary to develop new approaches to AMP drugs with improved activity and reduced toxicity. DRAMP is an antimicrobial peptide database created with the objective of providing a useful resource for sequence- and structure-activity studies on AMPs. It currently harbors 17349 entries from extensive literature search and integrates a number of analytical tools to assist researches on AMPs.
Construction and Content
Data collection
Antimicrobial peptides in DRAMP were collected from Pubmed, Swiss-prot and Lens13 by using keywords such as ‘antimicrobial peptide’, ‘antibacterial peptide’, ‘antifungal peptide’, ‘antiviral peptide’, ‘antitumor peptide’, ‘anticancer peptide’, ‘antiparasitic peptides’ or ‘insecticidal peptide’. The hits were registered into the database if: i) their antimicrobial activities have been demonstrated; ii) the amino acid sequences of peptides have been elucidated; iii) precursor and signal regions have been removed to remain mature sequences; iv) they contain less than 100 amino acid residues. The sequences were divided into General dataset and Patent dataset based on their reference literature. Each entry of General dataset contains following major fields: general field including peptide sequence, length, name, Swiss-prot ID, family, gene, source; activity field containing biological activity, target organisms with MIC values, binding target; structure field including structure type, description, PDB ID; physicochemical field such as Boman index, mass and half-life; literature field including Pubmed ID, journal, author, title; comment field describing detailed biological function of peptides. Patent dataset were annotated with sequence, length, name, source, activity, Patent ID, patent type, publication date, also publication as, title, abstract.
Clinical peptides were extracted from literature as separate clinical dataset. The dataset contains 74 antimicrobial peptide entries which have been developed by companies as drug candidates into preclinical or clinical trial stage. Data in this dataset were organized as sequence, name, description, activity, medical use, stage of development, comments, company and reference. It is worthy to note that some clinical peptides whose amino acid sequences are absent were also included in this dataset as we don’t want to lose any clinical information. The architecture of the datasets in DRAMP is shown in Fig. 1.
“IdGeneralAMPs”, “IdClinicalAMPs” and “IdPatentAMPs” are storage structures of the General, Clinical and Patent dataset respectively. The General and Patent datasets fit into two subsets: natural peptides and synthetic peptides. Plant AMPs and bacteriocins belonging to natural AMPs are further stored as separate datasets. About half general peptides in DRAMP are available of MIC values along with target organisms and 253 general entries possess known structures.
Database construction and maintenance
DRAMP was built on Linux platform (32-bits operating system) with Apache web server (version 2.2.22) and MySQL server (v 5.5.29) as the back-end. HTML, PHP and JavaScript was applied to develop the front-end web interfaces. The maintenance of DRAMP contains regular data update, backup, recovery and web optimization.
Utility
The main web page of DRAMP contains the following interfaces: Search, Browse, Tools, Statistics, Download and Links. A brief description of the interfaces is given below and the screenshots are given in Fig. 2.
(i)Search page: simple search and advanced search capabilities are constructed in search page though a quick search has been integrated in Home page. Simple search allows users to search the database in a specific field, such as sequence, name or reference, from the drop down menu. Advanced search (Fig. 2 (a)) is a more comprehensive search allowing a combination of keywords like sequence, length, source, peptide name, gene, structure, structure method, biology activity, target organism, binding target, cell toxicity, post-translational modification (PTM) and database ID.
(ii)Browse page: users can browse the database and download sequences that they are interested in. As shown in Fig. 2(b), data can be browsed in General dataset, Patent dataset and Clinical dataset. General data may be further viewed in natural or synthetic sources or in plant AMPs or in bacteriocins. Besides, sequences can be browsed in different activities, viz. antimicrobial, antibacterial, antifungal, antiviral, anticancer, antitumor, antiprotozoal and insecticidal.
(iii)Detailed information page (Fig. 2 (d)): clicking on DRAMP ID of an entry in query or browse results (Fig. 2(c)) can get detailed information page. This page presents all annotations for the entry which are divided into general information, activity information, structure information, physicochemical information, comments information and literature information. In structure part, a helical wheel diagram is shown and if a sequence has the known structure, it can be directly viewed. Besides, amino acid distribution histogram and hydropathy plot are drawn by a sequence’s physicochemical information.
(iv)Tools page: tools integrated in DRAMP include similarity search, alignment search, conserved domain search and physicochemical properties calculator, as Fig. 2(e) shown. Similarity search contains three methods, namely Blast14, Fasta15 and Ssearch15. Blast search can be operated against the local database, or Swiss-prot, or PDB. Alignment search includes global, local and multiple alignment using methods of StretcherP16, MatcherP16 and Clustal Omega17 respectively. Conserved domain search (CD-Search)18 allows users to detect structural and functional domains in protein sequences. ProtParam program19 and Chou & Fasman algorithm20 are integrated for physiochemical properties prediction and protein secondary structure prediction. Users can use the tools by either pasting their peptide sequences in FASTA format or uploading a text file with sequences of their interest.
(v)Statistics page: data statistics of DRAMP are presented in this page, including percentage of amino acids occurrence in AMPs, distribution of peptides length, top 8 AMPs for antimicrobial activity against common microbes in DRAMP.
(vi) Download page: install packages of useful tools such as Blast are collected in this page for download.
(vii) Links page: links of AMP-related databases are collected here for linking to these resources.
Statistics and findings
The current version of DRAMP holds 17349 entries in total, which include 4571 general AMPs, 12704 patented sequences and 74 clinical peptides. About half general peptides and 22 clinical peptides are available of MIC values along with target organisms and the top 8 antimicrobial activity are listed in Table 1. 253 general peptides in DRAMP have known structures with PDB ID. To advance our understanding of features of antimicrobial peptides as a basis for peptide design, statistics were carried out in DRAMP. It is found that 90.7% of natural AMPs have a positive net charge with an average value of 3.45 while almost all synthetic AMPs possess positive charges with an average value of 4.78, by analyzing 4001 natural and 570 synthetic sequences in general dataset. 60% natural sequences range from 10 to 50, as can be seen in Fig. 3, while a majority of synthetic peptides have a length of less than 25 amino acids. Figure 4 illustrates the contents of hydrophobic residues in natural and synthetic AMPs. Most of natural peptides possess hydrophobic content in the 30−45% range while most of synthetic peptides range from 45% to 55%. Figure 5 summarizes the basic amino acids distribution. As shown, glycine, cysteine and lysine make up the predominant composition in natural peptides, which is coincident to the statistical result given by APD221 in its website. In contrast, synthetic peptides have a lower ratio of glycine but a higher ratio of arginine. A summary of activities distribution of natural peptides is made in Fig. 6. A specific peptide here may have different activities and thus can be counted twice or more. Most peptides in DRAMP are shown to antibacterial activity (56.8%), followed by antifungal (28.4%). These findings may be useful in developing natural peptide templates or designing novel peptides with improved activities.
Discussion
Comparison with other databases
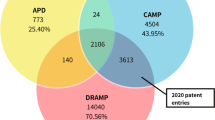
At present, there exist a few databases relating to AMPs, but most of them are specialized to certain categories of AMPs. Some have focused on AMPs produced by plants (PhytAMP)22, bacteria (Bactibase)23, shrimp (Penbase)24 and milk (MilkAMP)25, while others have focused on certain properties of AMPs like hemolytic activity (Hemolytik)26, activity and structure (DBAASP)27, antibacterial activity (YADAMP)28, anuran defense peptides (DADP)29, antiviral activity (AVPdb)30 and anti-HIV activity (HIPdb)31. Those databases have played an important role in certain categories of AMPs but have limits on sequences collection. To the best of our knowledge, there exsit four databases dealing with AMPs from diverse origins and they are APD221, DAMPD32, CAMP33 and LAMP34. APD2 is the most popular public collections of AMPs, harboring 2625 mature sequences. DAMPD is a replacement of the ANTIMIC35 database and has extended its entries to 1232, including both precursor and mature sequences. CAMP holds 8164 entries (experimentally validated (2774) and predicted (5390)) and has integrated an antimicrobial activity prediction function based on machine learning algorithms. LAMP is a cross-linking database providing hyperlinks to other databases. Compared to these databases, DRAMP holds diverse annotations of AMPs including sequence information, structure information, physicochemical information, patent information, clinical information, reference information and especially antimicrobial activity information (shown as target organisms with MIC values). Moreover, multiple tools are available on the database for sequence analysis. DRAMP is expected to be a useful resource for rational design of novel antimicrobial peptides.
Future directions
The physicochemical properties and activity relationship extracted from the collection of AMPs has proved to be a powerful resource for the design of novel antimicrobial peptides. The Nebraska Medical Center has carried out the design of anti-MRSA peptides based on peptide information in the APD database36. SVM-based activity prediction tools have been implemented in CAMP and C-PAmP37. We will conduct more data mining and design work based on our collection of AMPs in the future. For example, novel peptides are being developed based on 121 experimentally validated anti-MRSA peptides38.
Conclusion
DRAMP is an open-access, manually curated database aiming at making a comprehensive repository of AMPs. DRAMP mainly holds three datasets, general, patented and clinical dataset. Activity information (MIC values) and structure information are manually collected from the literature. Clinical entries with pharmaceutical information are registered in clinical dataset. User-friendly interfaces have been established to facilitate peptides searching, browsing and alignment. DRAMP should help promote our understanding of antimicrobial peptides and should provide a valuable resource for the development of novel drugs.
Availability and requirements
DRAMP can be accessed freely at http://dramp.cpu-bioinfor.org.
Additional Information
How to cite this article: Fan, L. et al. DRAMP: a comprehensive data repository of antimicrobial peptides. Sci. Rep. 6, 24482; doi: 10.1038/srep24482 (2016).
References
Cruz, J., Ortiz, C., Guzman, F., Fernandez-Lafuente, R. & Torres, R. Antimicrobial peptides: promising compounds against pathogenic microorganisms. Curr. Med. Chem. 21, 2299–2321 (2014).
Li, Y., Xiang, Q., Zhang, Q., Huang, Y. & Su, Z. Overview on the recent study of antimicrobial peptides: Origins, functions, relative mechanisms and application. Peptides 37, 207–215 (2012).
Giuliani, A., Pirri, G. & Nicoletto, S. F. Antimicrobial peptides: an overview of a promising class of therapeutics. Cent. Eur. J. Biol. 2, 1–33 (2007).
Gaspar, D., Veiga, A. S. & Castanho, M. A. From antimicrobial to anticancer peptides. A review. Front Microbiol. 4, 294 (2013).
Lai, Y. & Gallo, L. R. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 30, 131–141 (2009).
Fjell, C. D., Hiss, J. A., Hancock, R. E. & Schneider, G. Designing antimicrobial peptides: form follows function. Nat. Rev. Drug Discov. 11, 37–51 (2011).
Brogden, K. A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3, 238–250 (2005).
Hasper, H. E. et al. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 313, 1636–1637 (2006).
Otvos, L. Jr. Antibacterial peptides and proteins with multiple cellular targets. J. Pept. Sci. 11, 697–706 (2005).
Harrison, P. L., Abdel-Rahman, M. A., Miller, K. & Strong, P. N. Antimicrobial peptides from scorpion venoms. Toxicon. 88, 115–137 (2014).
Andres, E. Cationic antimicrobial peptides in clinical development, with special focus on thanatin and heliomicin. Eur. J. Clin. Microbiol. 31, 881–888 (2012).
Marr, A. K., Gooderham, W. J. & Hancock, R. E. Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr. Opin. Pharmacol. 6, 468–472 (2006).
Jefferson, O. A., Köllhofer, D., Ehrich, T. H. & Jefferson, R. A. Transparency Tools in Gene Patenting for informing policy and practice. Nat. Biotechnol. 31, 1086–1093 (2013).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Pearson, W. R. & Lipman, D. J. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85, 2444–2448 (1988).
Rice, P., Longden, I. & Bleasby, A. EMBOSS: The European Molecular Biology Open Software Suite. Trends Genet. 16, 276–277 (2000).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Bauer, A. M. & Bryant, S. H. CD-Search: protein domain annotations on the fly. Nucleic Acids Res. 32, W327–W331 (2004).
Gasteiger, E. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook (ed. John, M. W. ) 571–607 (Humana Press, 2005).
Kumar, T. A. CFSSP: Chou & Fasman Secondary Structure Prediction Server. Wide Spectrum 1, 15–19 (2013).
Wang, G., Li, X. & Wang, Z. APD2: the updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 37, D933–D937 (2009).
Hammami, R., Ben, H. J., Vergoten, G. & Fliss, I. PhytAMP: a database dedicated to antimicrobial plant peptides. Nucleic Acids Res. 37, D963–D968 (2009).
Hammami, R., Zouhir, A., Le L. C., Ben, H. J. & Fliss, I. BACTIBASE second release: a database and tool platform for bacteriocin characterization. BMC Microbiol. 10, 22 (2010).
Gueguen, Y. et al. PenBase, the shrimp antimicrobial peptide penaeidin database: Sequence-based classification and recommended nomenclature. Dev. Comp. Immunol. 30, 283–288 (2006).
Théolier, J., Fliss, I., Jean, J. & Hammami, R. MilkAMP: a comprehensive database of antimicrobial peptides of dairy origin. Dairy Sci. & Technol. 94, 181–193 (2014).
Gautam, A. et al. Hemolytik: a database of experimentally determined hemolytic and non-hemolytic peptides. Nucleic Acids Res. 42, D444–D449 (2014).
Gogoladze, G. et al. DBAASP: database of antimicrobial activity and structure of peptides. FEMS Microbiol. Lett. 357, 63–68 (2014).
Piotto, S. P., Sessa, L., Concilio, S. & Iannelli, P. YADAMP: yet another database of antimicrobial peptides. Int. J. Antimicrob. Agents 39, 346–351 (2012).
Novković, M., Simunić, J., Bojović, V., Tossi, A. & Juretić, D. DADP: the database of anuran defense peptides. Bioinformatics 28, 1406–1407 (2012).
Qureshi, A., Thakur, N., Tandon, H. & Kumar, M. AVPdb: a database of experimentally validated antiviral peptides targeting medically important viruses. Nucleic Acids Res. 42, D1147–D1153 (2014).
Qureshi, A., Thakur, N. & Kumar, M. HIPdb: a database of experimentally validated HIV inhibiting peptides. Plos One 8, e54908 (2013).
Sundararajan, V. S. et al. DAMPD: a manually curated antimicrobial peptide database. Nucleic Acids Res. 40, D1108–D1112 (2012).
Waghu, F. H. et al. CAMP: Collection of sequences and structures of antimicrobial peptides. Nucleic Acids Res. 42, D1154–D1158 (2014).
Zhao, X. W., Wu, H. Y., Lu, H. R., Li, G. D. & Huang, Q. S. LAMP: A Database Linking Antimicrobial Peptides. Plos One 8, e66557 (2013).
Brahmachary, M. et al. ANTIMIC: a database of antimicrobial sequences. Nucleic Acids Res. 32, D586–D589 (2004).
Mishra, B. & Wang, G. S. Ab Initio Design of Potent Anti-MRSA Peptides Based on Database Filtering Technology. J. Am. Chem. Soc. 134, 12426–12429 (2012).
Niarchou, A., Alexandridou, A., Athanasiadis, E. & Spyrou, G. C-PAmP: Large Scale Analysis and Database Construction Containing High Scoring Computationally Predicted Antimicrobial Peptides for All the Available Plant Species. Plos One 8, e79728 (2013).
GU, J. & DAI, J. Advances in Clinical Research of New-type Anti-MRSA Antibiotics. Anti Infect Pharm. 6, 223–228 (2009).
Acknowledgements
The authors thank Dr. Jiao Chen for her critical reading of the manuscript. This research was supported by grants from the National High Technology Research and Development Program of China (863) [number 2012AA020304]; and the National Natural Science Foundation of China (grant number 31300643); and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
L.F. participated in the design of the study, performed the statistical analysis and drafted the manuscript. J.S. mainly conceived the study and collected data. M.Z. programmed the database and designed the website. J.Z. and X.L. participated in data collection and compilation. H.Z. and H.X. conceived the study and helped draft the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Fan, L., Sun, J., Zhou, M. et al. DRAMP: a comprehensive data repository of antimicrobial peptides. Sci Rep 6, 24482 (2016). https://doi.org/10.1038/srep24482
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep24482
This article is cited by
-
Rational design of stapled antimicrobial peptides
Amino Acids (2023)
-
A proline rich protein from the gingival seal around teeth exhibits antimicrobial properties against Porphyromonas gingivalis
Scientific Reports (2021)
-
A Crosstalk on Antimicrobial Peptides
International Journal of Peptide Research and Therapeutics (2021)
-
Nanoantibiotics containing membrane-active human cathelicidin LL-37 or synthetic ceragenins attached to the surface of magnetic nanoparticles as novel and innovative therapeutic tools: current status and potential future applications
Journal of Nanobiotechnology (2020)
-
Encodings and models for antimicrobial peptide classification for multi-resistant pathogens
BioData Mining (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.