Abstract
Endoscopic ultrasound-guided fine needle core biopsy (EUS-FNB) has been used as an effective method of diagnosing pancreatic malignant lesions. It has the advantage of providing well preserved tissue for histologic grading and subsequent molecular biological analysis. In order to estimate the diagnostic accuracy of EUS-FNB for pancreatic malignant lesions, studies assessing EUS-FNB to diagnose solid pancreatic masses were selected via Medline. Sixteen articles published between 2005 and 2015, covering 828 patients, met the inclusion criteria. The summary estimates for EUS-FNB differentiating malignant from benign solid pancreatic masses were: sensitivity 0.84 (95% confidence interval (CI), 0.82–0.87); specificity 0.98 (95% CI, 0.93–1.00); positive likelihood ratio 8.0 (95% CI 4.5–14.4); negative likelihood ratio 0.17 (95% CI 0.10–0.26); and DOR 64 (95% CI 30.4–134.8). The area under the sROC curve was 0.96. Subgroup analysis did not identify other factors that could substantially affect the diagnostic accuracy, such as the study design, location of study, number of centers, location of lesion, whether or not a cytopathologist was present and so on. EUS-FNB is a reliable diagnostic tool for solid pancreatic masses and should be especially considered for pathology where histologic morphology is preferred for diagnosis.
Similar content being viewed by others
Introduction
Pancreatic cancer is a devastating disease with a poor prognosis, which is partially due to delayed diagnosis because of the late onset of symptoms1. Despite the many advancements that have been made in medical therapy in the past decade, there are still limited treatment modalities for advanced disease. Many epidemiologic surveys have shown that the 5-year survival rate is below 5%2,3. A significant proportion of patients could extend their survival time by surgery if their tumors were diagnosed at an early stage4. So early detection and accurate staging are crucial for the right treatment choice.
Tissue acquisition is of great importance to confirm diagnosis and guide treatment in pancreatic solid mass. The endoscopic ultrasound (EUS)-guided minimally invasive tissue acquisition techniques have become the standard of choice to sample pancreatic tissue that could only be biopsied through open techniques in the past5. The EUS method can detect lesions that are not seen by other imaging modalities and fine needle aspiration (FNA) is reported to be able to give a definitive cytological diagnosis4. A recent meta-analysis reported that the sensitivity and specificity of EUS-guided FNA (EUS-FNA) for pancreatic neoplasms were 85% and 98%, respectively6. The complication rate of EUS-FNA is approximately 1%–2%7. Having become widely accepted as safe and effective, EUS-FNA is considered a minimally invasive method of diagnosing pancreatic cancer8. Despite the widespread usage of EUS-FNA, one limitation related to this technique is that it often only provides a cytologic specimen with scant cellularity and lack of histologic architecture, which restrains us from making a complete tissue analysis for diagnosis and grade differentiation, especially for sarcomas or lymphomas9. As we know, in the era of molecular profiling and personalized oncologic therapies, a complete histologic sample for evaluation of molecular marker expression has become of paramount importance. Another limitation of EUS-FNA is the unclear number of passes required to achieve an adequate sample without an onsite cytopathologist8.
A fine needle biopsy (FNB) specimen containing core tissue may theoretically overcome the limitations associated with EUS-FNA and have a greater diagnostic accuracy, because it can provide well preserved cellular architecture for histological evaluation. To meet these expectations, many endeavors have been made to devise needles that could be compatible with an echoendoscope for obtaining a tissue core. Finally, different EUS-compatible core biopsy needles including a 19 gauge (G) Trucut biopsy needle, 19/22/25 G ProCore needle and nitinol-based flexible 19G needle have been developed and put into clinical practice based on different design mechanisms10,11,12,13. These needles make procurement of larger amounts of tissue available with preserved architecture for histological analysis. Many papers have reported the diagnostic accuracy of EUS-FNB for the differentiation of solid pancreatic masses, with a sensitivity and specificity ranging from 61–100% and 90–100%, respectively14. The objective of this meta-analysis was to evaluate the accuracy of EUS-FNB for the diagnosis of malignant solid pancreatic masses based on previous published literature.
Results
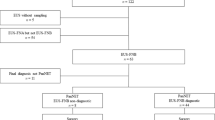
The Medline search, with preset search strategy, yielded 1491 records. Following an initial review of titles, 1362 records were excluded immediately. Seventy records were further excluded by abstract review for a variety of reasons and the remaining 59 were left for full-text review. Manual review of the references of retrieved articles and the related-article function in PubMed identified 15 additional records concerning EUS-FNB in pancreatic mass lesions, of which most were excluded due to conference abstracts while three papers were included for full-text review 10,15,16. A further 56 records were excluded because they did not fit the criteria stated above, leaving 18 papers published between 2006 and 2015 that were selected in the meta-analysis. Finally, one paper was excluded as both the true negative and the false positive were zero12 and another one was excluded for the final diagnosis of some cases were not given17. Therefore, 16 studies were included in the final analysis10,13,16,18,19,20,21,22,23,24,25,26,27,28,29,30. Of the 16 papers, 12 were prospective studies, whereas the remaining four were retrospective studies. The median patient numbers included in the selected study was 50 (range 12–113). The detailed flow chart is shown in Fig. 1. The characteristics of included studies as well as QUADAS scores are described in Table 1.
All procedures of the studies were performed with standardized protocol by experienced investigators using either an Olympus (Olympus Medical Systems, Tokyo, Japan) or Pentax (Pentax Medical Company, Montvale, NJ, USA) convex or linear-array echoendoscope. The EUS-FNB procedure was performed with a 19-gauge, 22-gauge or 25-gauge Echotip ProCore biopsy needle (Wilson–Cook Medical, Winston-Salem, NC, USA), a flexible 19-gauge needle (Boston Scientific Corporation, Marlborough, MA, USA), or a 19 gauge QuickCore Trucut needles (Wilson–Cook Medical). Thirteen studies reported the inadequate or technial failures cases and atypical or suspicious cases separately. One study reported the inadequate or technial failures cases and atypical or suspicious cases in total. Two studies have such cases, but the exact numbers were unextractable (Table 2). Inadequate specimens were included in the assessment of sensitivity and specificity in the assessment of diagnostic performance. No serious complications were reported during any of the procedures. The pathological types of malignant pancreatic lesions included adenocarcinoma, solid-pseudopapillary tumor, malignant neuroendocrine tumor, acinar cell carcinoma, metastatic cancer, lymphoma and sarcoma. The benign solid pancreatic masses were mainly pancreatic inflammatory mass.
Diagnostic accuracy
The forest plot of sensitivity and specificity of EUS-FNB for the diagnosis of malignant solid pancreatic mass is shown in Fig. 2. The sensitivity ranged from 0.43–1.00, whereas specificity was uniformly high and ranged from 0.90–1.00. The pooled sensitivity (random-effect model) and specificity (fixed-effect model) were 0.84 (95% CI, 0.82–0.87) and 0.98 (95% CI, 0.93–1.00), respectively. Significant heterogeneity was found in sensitivity (I2 = 87%), whereas it was not found in specificity (I2 = 0.0%). It was noted that the pooled PLR was 8.0 (95% CI 4.5–14.4), NLR was 0.17 (95% CI 0.10–0.26, Table 2) and DOR was 64 (95% CI 30.4–134.8, Table 2). The I2 values of PLR, NLR and DOR were 0.0%, 81.9% and 0.0%, respectively, indicating that there was no significant heterogeneity among studies regarding PLR and DOR, but not NLR.
Forest plot of sensitivity and specificity estimates for EUS-FNB in the diagnosis of malignant pancreatic lesions.
The point estimates of sensitivity and specificity from each study are shown as solid circles. Error bars indicate 95% CIs. EUS-FNB, endoscopic ultrasound-guided fine needle core biopsy; CI, confidence interval.
The sROC curve of EUS-FNB diagnosing solid pancreatic lesions is shown in Fig. 3. The area under the sROC curve (AUC) is a method to assess the discriminating capability of a test. It represents an analytical summary of test performance. A higher AUC value means a better discriminating ability, a value of 1.0 means that the test has almost a perfect discrimination. The AUC value of EUS-FNB diagnosed malignant pancreatic lesions was 0.96 (SE = 0.013), indicating that the overall diagnostic accuracy of EUS-FNB was quite high. Index Q is another method to assess the diagnostic performance of an sROC curve. The index Q corresponds to the intersection point of the sROC curve with a diagonal line from the left upper corner to the right lower corner, in which sensitivity and specificity have a highest equal value. An index Q close to 1 means that the test has almost a perfect discrimination. The present Index Q value was 0.90, also suggesting a relatively high diagnostic accuracy.
Subgroup analyses and meta-regression
Subgroup analysis based on factors potentially affecting the diagnostic accuracy is shown in Table 3. It was of note that needle type greatly influenced the diagnostic accuracy. The 19-gauge flexible needle had the highest sensitivity (97%) while the Trucut needle had the lowest sensitivity (62%). There was little difference among specificities. Although differences in diagnostic accuracy were observed and heterogeneity was reduced, the subgroups analysis could not adequately explain the heterogeneity with regard to sensitivity and NLR (all of the I2 were still above 50%).
The DOR is a single indicator of test accuracy that combines the data from sensitivity and specificity into a single entity31. The DOR equals the value of the PLR divided by the NLR. A higher value indicates a better discriminatory capability of a test31. To assess the effect of study characteristics, such as study design, single center versus multicenter and so on, the DOR, meta-regression analysis was performed. Consistent with the subgroup analysis, although the DOR appeared improved in some subgroups, as shown in Table 3, none of the relative DOR (RDOR) reached statistical significance, demonstrating that the study characteristics, including study design, single center versus multicenter and so on, did not significantly affect the diagnostic accuracy.
Publication bias
Egger’s test and Begg’s funnel plot was used to analyze potential publication bias of the meta-analysis32. The Egger’s test showed a value of 2.20 (95% CI −2.04 ∼ 6.44, P = 0.285) on a per-patient analysis and the Begg’s test showed a P value of 0.39 for the included studies (Fig. 4). These results indicated that there was no potential for publication bias.
Discussion
The EUS-guided FNA has displaced surgical biopsy and became the standard practice of diagnosing pancreatic solid mass with the advantages of technical ease, lower cost and decreased morbidity33. As the treatment modality is transiting to a more personalized approach, there is now an increased demand for additional tissue and histologic sections in addition to cytopathology from FNA specimens for the purpose of improved diagnostic accuracy and molecular characterization of tumors8. It is reasonable that larger-caliber or needles more specifically designed for obtaining core tissue would facilitate subsequent molecular biological analysis and histologic grading. However, unlike surgical resection specimens, EUS-FNB takes samples only from a very limited area of the suspected lesion, which enhances the chance of sampling and histologic interpretation errors due to tumor heterogeneity9. In fact, two studies have found sufficient tissue yielded high reproducibility and interobserver agreement while limited EUS-guided materials yielded significant discordance between the histologic and cytologic evaluations34,35. The actual benefit of EUS-FNB for pancreatic lesions still needs evaluation.
This meta-analysis has shown that EUS-FNB as a diagnostic tool for malignant pancreatic masses has a pooled sensitivity of 84% and a higher pooled specificity of 99%. We report an index Q value from the sROC of 0.9 and an AUC of 0.96, demonstrating a high degree of overall diagnostic accuracy. The DOR is used as an overall measure of the diagnostic accuracy of a diagnostic test. It is calculated as the odds of positivity among diseased persons, divided by the odds of positivity among non-diseased individuals31. The DOR value ranges from 0 to infinity and a DOR value of 1.0 suggests that a test has no discriminability between patients with the disease and those without it31. In our study, we report a pooled DOR of 64.0, which also confirms a high level of overall diagnostic accuracy.
We also used both PLR and NLR as our measures of diagnostic accuracy for they can be more easily interpreted and applied to clinical practice36. A PLR value of 8.0 indicates that patients with malignant pancreatic mass have about a eight-fold higher chance of being EUS-FNB test-positive compared with those without malignant pancreatic mass. By comparison, the NLR was found to be 0.17, meaning that if the EUS-FNB test result was negative, the likelihood that this patient has malignant pancreatic mass is approximately 17%. So, on the basis of the currently available data, the PLR was high enough to be used as a valuable tool for rule-in diagnosis while the NLR was not sufficiently low enough to be used as a rule-out diagnosis.
As we have shown above, there was a significant heterogeneity with regard to sensitivity and NLR among the studies included. On subgroup analysis, based on several potential predefined sources of heterogeneity, we noted that differences in diagnostic accuracy occurred in different subgroups. However, heterogeneity was not adequately explained by analysis of these subgroups for all of them did not reach statistical significance (all I2 > 50%). Then, we used the meta-regression analysis to evaluate the effect of study characteristics, such as study design, study centers, etc., on RDOR. Consistently, with all of the factors, meta-regression was not statistically significant. We also analyzed potential publication bias according to the recommended guidelines. There was no significant publication bias found. We believed the variability in study population, research procedures and measurements may lead to heterogeneity in this study.
It had been reported that the complication rate for EUS-FNA was as low as 1% to 2%, with complications more usually occur when EUS-FNA was performed on cystic lesions than on solid lesions37. We found the EUS-FNB had a comparable complication rate with EUS-FNA. Of the studies included in this meta-analysis, the reported complications ranged from 0 to 7.5%. Only one study had greater than 5% complications. Examples of complications include self-limiting pancreatitis, infection, bleeding, abdominal pain requiring analgesics, aspiration pneumonia and cholangitis due to biliary obstruction. No deaths or late complications related to EUS-FNB were reported.
Appropriate adjustment of the tip of the scope, avoiding puncture of vessels during operation and use of antibiotics when pancreatic masses contain cystic components can help reduce FNB-related complications38. Theoretically, the size of the needle and the number of passes made may also influence the overall risk of complications. At present, no study has definitely evaluated whether the size of the needle can influence the risk of adverse events. Based on the very low overall rate of EUS-FNB complications, it is reasonable to recommend that a larger sample size needle should be used when necessary. However, it is also recommended that the diagnosis be made with the minimal number of passes to avoid unnecessary risks.
A few limitations of this meta-analysis should be mentioned. First, letters to the editors, conference abstracts and non-English-language studies were excluded from this analysis and may have led to publication bias, although that was not statistically significant in this meta-analysis. However, we reviewed these letters and abstracts and found that the overall results were similar to the results in the included articles, which may reduce the potential of publication bias. Second, classifying the suspicious/atypical results on EUS-FNB as true positive may have led to overestimates of the diagnostic accuracy while classifying a few undiagnosed benign cases due to technique failure as false negative may have led us to underestimate the diagnostic accuracy. The rationale that we classified the suspicious/atypical as true positive was based on the conception that such a result could alert patient’s awareness of monitoring and subsequent imaging, which was of great usefulness for a better prognosis. Another important issue needed to address was that some studies had an unacceptably low sensitivity that couldn’t be easily explained by different populations. We believed that such low sensitivities mainly came from the high number of false negative due to the technical failure, especially the EUS-TNB needles were prone to fail to sample some lesions in the head of pancreas for the earlier studies. Also, the diagnostic accuracy was heavily reliant upon the experience of the endoscopists, studies performed at less experienced institutions tended to yielding lower sensitivities. However, the studies included in this analysis did not reflect the levels of experience at these institutions. Besides, malignant pancreatic mass is not always diagnosed by histologic analysis. It was diagnosed in some rare cases based just on the clinical course. With one study in our analysis the minimal clinical follow-up of just 5 months might lead to biased results.
In conclusion, EUS-FNB is a reliable and accurate diagnostic test for malignant pancreatic lesions, especially for the suspicion of pathology where histologic morphology was preferred for diagnosis. With improvements in technology, it could become the standard of choice. However, it should be interpreted in combination with clinical data and other conventional tests because the negative predictive value of this test is not high enough.
Methods
Search strategy and study selection
A comprehensive search of Medline (using PubMed as the search engine) was done to identify suitable studies up to May 2015. The search used a combination of terms (“biopsy” or “aspiration”) AND (“endoscopy” or “endoscopic”) AND (“pancreas” or “pancreatic”). The bibliographies of retrieved articles were searched to identify relevant studies manually. The related-articles function in PubMed was also used to further identify relevant articles. The search was not restricted to any particular language, but only articles written in English were retrieved for full evaluation. Data extraction and quality control were performed by two reviewers (YTY and LYL) for each selected study. Disagreements were resolved by making a consensus.
Studies included in the meta-analysis met the following criteria: (1) adult patients with suspected pancreatic solid mass; (2) final diagnosis was resolved by at least one of these criteria: (i) surgical diagnosis based on a resected specimen; (ii) typical histological or cytological characteristics of the EUS-FNA or EUS-FNB examination; (iii) clinical follow-up of at least five months for suspicion of benign pancreatic disease; (3) provided sufficient data to extract the diagnostic results such as true-positive, true-negative, false-positive and false-negative; (4) solid pancreatic mass was the only lesion or contained other lesions but pancreatic mass cases were analyzed separately and the number of patients with pancreatic lesions was over ten; and (5) written in English. The exclusion criteria were: (1) conference abstracts and letters to editors; (2) pediatric or animal studies; (3) assessing pancreatic cystic lesions; and (4) providing insufficient data to construct a 2 × 2 contingency table for calculating specificity and sensitivity.
Data extraction and quality assessment
The cytological or histological results in some articles were reported as inadequate, benign, atypical, suspicious, or malignant. Then, we included atypical and suspicious cytology results as positive for malignancy, whereas we included cases of inadequate or technique failure as false negative when benign cases constituted only a very small fraction of total. Further information extracted from each article included: (1) publication year; (2) country of origin; (3) prospective or retrospective; (4) number of centers; (5) length of study; (6) number of benign and malignant patients; (7) needle types; (8) mean or median of passes; (9) lesion size; (10) lesion location; and (11) whether or not a cytopathologist was on site for all cases. To evaluate the study quality and potential for bias, an assessment was conducted using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tool39. A total of 14 items were assessed for each study, with a maximum score 14. Disagreement between the two extracting authors was resolved by consensus.
Statistical analyses
Standard methods recommended for meta-analyses of diagnostic test evaluations were used36. The estimates of diagnosis accuracy including sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR) and diagnostic odds ratio (DOR) were calculated for each study. Pooled results were constructed by using both the Mantel–Haenszel method (fixed-effect model) and the DerSimonian–Laird method (random-effect model) based on whether significant heterogeneity was absent or not36. The Cochrane Q test was used to estimate heterogeneity among the studies. Heterogeneity across the studies rather than from chance was expressed as inconsistency (I2), in the form of a percentage. An I2 above 50% was considered significant heterogeneity across the studies, which meant that the random-effect model rather than fixed-effect model method was used to calculate the pooled estimates40.
Summary receiver operating characteristic (sROC) analysis was performed based on the Moses and colleagues method41, which was used to reflect the discriminating ability of a diagnostic test. The sROC curve is a plot of the true positive rate (sensitivity) as a function of the false positive rate (1-specificity)42. An sROC curve is constructed based on a linear regression model to fit these points. The constructed linear regression equation to fit these points is as follows: D = β × S + α, where D = ln[TPR/(1−TPR)] − ln[FPR/(1−FPR)] and S = ln[TPR/(1−TPR)] + ln[FPR/(1−FPR)] and α is used as the y-intercept while β is the regression coefficient of independent variable S.
To explore the heterogeneity across the studies, subgroup analyses were performed according to: origin of study; study design (retrospective versus prospective); number of centers; location of lesion; and whether or not a cytopathologist was present for all cases. The variables in the subgroup analysis were used as covariates to perform meta-regression analysis to further explore potential sources of heterogeneity. We analyzed the effects of covariates on DOR according to the Moses–Shapiro–Littenberg model with recommended methods43. The publication bias for meta-analyses was analyzed using funnel plots and the Egger test and was evaluated in the form of a funnel plot of standard error (SE) in the DOR (x) versus ln (DOR) (y).
The test accuracy, including sensitivity, specificity, PLR, NLR, DOR, sROC and meta-regression analyses, were performed using Meta-DiSc software (version 1.4)44. The publication bias analyses were performed using STATA software (version 12.0). Levels of significance were measured at P < 0.05.
Additional Information
How to cite this article: Yang, Y. et al. Endoscopic ultrasound-guided fine needle core biopsy for the diagnosis of pancreatic malignant lesions: a systematic review and Meta-Analysis. Sci. Rep. 6, 22978; doi: 10.1038/srep22978 (2016).
References
Jemal, A. et al. Cancer statistics, 2009. CA Cancer J Clin 59, 225–249 (2009).
Walters, S. R. & Westlake, S. Cancer survival, England, patients diagnosed 2001–2006 and followed up to 2007: one year and five year survival for 21 common cancers, by sex and age. London: Office for National Statistics ; 2009.
Howlader, N. N. et al. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations), National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/csr/1975_2009_pops09/. Accessed May 15, 2012.
Helmstaedter, L. & Riemann, J. F. Pancreatic cancer–EUS and early diagnosis. Langenbecks Arch Surg 393, 923–927 (2008).
Horwhat, J. D. et al. A randomized comparison of EUS-guided FNA versus CT or US-guided FNA for the evaluation of pancreatic mass lesions. Gastrointest Endosc 63, 966–975 (2006).
Hewitt, M. J. et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc 75, 319–331 (2012).
Adler, D. G. et al. ASGE guideline: complications of EUS. Gastrointest Endosc 61, 8–12 (2005).
Kedia, P., Gaidhane, M. & Kahaleh, M. Technical Advances in Endoscopic Ultrasound (EUS)-Guided Tissue Acquisition for Pancreatic Cancers: How Can We Get the Best Results with EUS-Guided Fine Needle Aspiration? Clin Endosc 46, 552–562 (2013).
Hebert-Magee, S. Is there a role for endoscopic ultrasound-guided fine-needle biopsy in pancreatic cancer? Endoscopy 47, 291–292 (2015).
Varadarajulu, S., Bang, J. Y. & Hebert-Magee, S. Assessment of the technical performance of the flexible 19-gauge EUS-FNA needle. Gastrointest Endosc 76, 336–343 (2012).
Larghi, A. et al. EUS-guided trucut needle biopsies in patients with solid pancreatic masses: a prospective study. Gastrointest Endosc 59, 185–190 (2004).
Itoi, T. et al. Puncture of solid pancreatic tumors guided by endoscopic ultrasonography: a pilot study series comparing Trucut and 19-gauge and 22-gauge aspiration needles. Endoscopy 37, 362–366 (2005).
Iglesias-Garcia, J. et al. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc 73, 1189–1196 (2011).
Karadsheh,Z. & Al-Haddad, M. Endoscopic ultrasound-guided fine-needle aspiration needles: which one and in what situation? Gastrointest Endosc Clin N Am 24, 57–69 (2014).
Wittmann, J., Kocjan,G., Sgouros, S. N., Deheragoda, M. & Pereira, S. P. Endoscopic ultrasound-guided tissue sampling by combined fine needle aspiration and trucut needle biopsy: a prospective study. Cytopathology 17, 27–33 (2006).
Fabbri, C. et al. Endoscopic ultrasound-guided fine-needle biopsy of small solid pancreatic lesions using a 22-gauge needle with side fenestration. Surg Endosc 29, 1586–1590 (2015).
Strand, D. S. et al. EUS-guided 22-gauge fine-needle aspiration versus core biopsy needle in the evaluation of solid pancreatic neoplasms. Diagn Cytopathol 42, 751–758 (2014).
Gines,A. et al. Prospective study of a Trucut needle for performing EUS-guided biopsy with EUS-guided FNA rescue. Gastrointest Endosc 62, 597–601 (2005).
Yun, S. S., Remotti, H., Vazquez, M. F., Crapanzano, J. P. & Saqi, A. Endoscopic ultrasound-guided biopsies of pancreatic masses: comparison between fine needle aspirations and needle core biopsies. Diagn Cytopathol 35, 276–282 (2007).
Sakamoto, H. et al. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge Trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol 24, 384–390 (2009).
Thomas, T., Kaye, P. V., Ragunath, K. & Aithal, G. Efficacy, safety and predictive factors for a positive yield of EUS-guided Trucut biopsy: a large tertiary referral center experience. Am J Gastroenterol 104, 584–591 (2009).
Bang, J. Y., Hebert-Magee, S., Trevino, J. & Ramesh, J. Varadarajulu S. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc 76, 321–327 (2012).
Hucl, T. et al. Feasibility and efficiency of a new 22G core needle: a prospective comparison study. Endoscopy 45, 792–798 (2013).
Iwashita, T. et al. High single-pass diagnostic yield of a new 25-gauge core biopsy needle for EUS-guided FNA biopsy in solid pancreatic lesions. Gastrointest Endosc 77, 909–915 (2013).
Krishnan, K. et al. Rapid on-site evaluation of endoscopic ultrasound core biopsy specimens has excellent specificity and positive predictive value for gastrointestinal lesions. Dig Dis Sci 58, 2007–2012 (2013).
Larghi, A. et al. Feasibility and yield of a novel 22-gauge histology EUS needle in patients with pancreatic masses: a multicenter prospective cohort study. Surg Endosc 27, 3733–3738 (2013).
Lee, Y. N. et al. Core biopsy needle versus standard aspiration needle for endoscopic ultrasound-guided sampling of solid pancreatic masses: a randomized parallel-group study. Endoscopy 46, 1056–1062 (2014).
Vanbiervliet, G. et al. Core needle versus standard needle for endoscopic ultrasound-guided biopsy of solid pancreatic masses: a randomized crossover study. Endoscopy 46, 1063–1070 (2014).
Berzosa, M., Villa, N., El-Serag, H. B., Sejpal, D. V. & Patel, K. K. Comparison of endoscopic ultrasound guided 22-gauge core needle with standard 25-gauge fine-needle aspiration for diagnosing solid pancreatic lesions. Endosc Ultrasound 4, 28–33 (2015).
Ramesh, J. et al. Randomized Trial Comparing the Flexible 19G and 25G Needles for Endoscopic Ultrasound-Guided Fine Needle Aspiration of Solid Pancreatic Mass Lesions. Pancreas 44, 128–133 (2015).
Glas, A. S., Lijmer, J. G., Prins, M. H., Bonsel, G. J. & Bossuyt, P. M. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 56, 1129–1135 (2003).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Yamao, K. et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB): past, present and future. J Gastroenterol 40, 1013–1023 (2005).
Hasegawa, T. et al. Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy 46, 32–38 (2014).
Weynand, B. et al. Pancreatic neuroendocrine tumour grading on endoscopic ultrasound-guided fine needle aspiration: high reproducibility and inter-observer agreement of the Ki-67 labelling index. Cytopathology 25, 389–395 (2014).
Deville, W. L. et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol 2, 9 (2002).
Chen, G., Liu, S., Zhao,Y., Dai, M. & Zhang, T. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for pancreatic cancer: a meta-analysis. Pancreatology 13, 298–304 (2013).
Levy, M. J. & Wiersema, M. J. EUS-guided Trucut biopsy. Gastrointest Endosc 62, 417–426 (2005).
Whiting, P. F. et al. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Med Res Methodol 6, 9 (2006).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003).
Moses, L. E., Shapiro, D. & Littenberg, B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med 12, 1293–1316 (1993).
Rosman, A. S. & Korsten, M. A. Application of summary receiver operating characteristics (sROC) analysis to diagnostic clinical testing. Adv Med Sci 52, 76–82 (2007).
Lijmer, J. G., Bossuyt, P. M. & Heisterkamp, S. H. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med 21, 1525–1537 (2002).
Zamora, J., Abraira, V., Muriel, A., Khan, K. & Coomarasamy, A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 6, 31 (2006).
Acknowledgements
The study was supported by Grants from the Natural Sciences Foundation of the People’s Republic of China (81000154).
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of the study and writing of the manuscript. Y.T.Y. and L.Y.L. undertook the research and performed the analyses. All authors reviewed and approved the final version of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yang, Y., Li, L., Qu, C. et al. Endoscopic ultrasound-guided fine needle core biopsy for the diagnosis of pancreatic malignant lesions: a systematic review and Meta-Analysis. Sci Rep 6, 22978 (2016). https://doi.org/10.1038/srep22978
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep22978
This article is cited by
-
Impact of Biopsy Attempts, Race, and Access on Time to Initiation of Treatment for Pancreatic Cancer
Journal of Gastrointestinal Surgery (2023)
-
The role of diagnostic, prognostic, and predictive biomarkers in the management of early pancreatic cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
Current status and issues in genomic analysis using EUS-FNA/FNB specimens in hepatobiliary–pancreatic cancers
Journal of Gastroenterology (2023)
-
A review of contrast-enhanced harmonic endoscopic ultrasonography for pancreatic solid tumors
Journal of Medical Ultrasonics (2023)
-
Current status of molecular diagnostic approaches using liquid biopsy
Journal of Gastroenterology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.