Abstract
To identify the best lung ventilation strategy for acute respiratory distress syndrome (ARDS), we performed a network meta-analysis. The Cochrane Central Register of Controlled Trials, EMBASE, MEDLINE, CINAHL and the Web of Science were searched and 36 eligible articles were included. Compared with higher tidal volumes with FiO2-guided lower positive end-expiratory pressure [PEEP], the hazard ratios (HRs) for mortality were 0.624 (95% confidence interval (CI) 0.419–0.98) for lower tidal volumes with FiO2-guided lower PEEP and prone positioning and 0.572 (0.34–0.968) for pressure-controlled ventilation with FiO2-guided lower PEEP. Lower tidal volumes with FiO2-guided higher PEEP and prone positioning had the greatest potential to reduce mortality and the possibility of receiving the first ranking was 61.6%. Permissive hypercapnia, recruitment maneuver and low airway pressures were most likely to be the worst in terms of all-cause mortality. Compared with higher tidal volumes with FiO2-guided lower PEEP, pressure-controlled ventilation with FiO2-guided lower PEEP and lower tidal volumes with FiO2-guided lower PEEP and prone positioning ventilation are associated with lower mortality in ARDS patients. Lower tidal volumes with FiO2-guided higher PEEP and prone positioning ventilation and lower tidal volumes with pressure-volume (P–V) static curve-guided individual PEEP are potential optimal strategies for ARDS patients.
Similar content being viewed by others
Introduction
Acute respiratory distress syndrome (ARDS) is a common clinical condition with an incidence rate of nearly 9% in the intensive care unit (ICU)1. The mortality of ARDS is relatively high, at approximately 27–45%2. In the United States, there are an estimated 190,600 cases annually, resulting in 74,500 deaths and 3.6 million hospital days3. Mechanical ventilation is the most effective life-saving technique and can save a patient’s life by maintaining adequate tissue oxygenation4. However, the same ventilation interventions have exhibited different effects on mortality concerning ARDS in different clinical trials and the issue remains controversial4,5,6,7,8,9. To compare the different ventilation strategies in the management of ARDS, many studies have attempted to identify optimal strategies for mechanical ventilation10,11. Traditional pairwise meta-analysis performs a systematic review and evaluation of different ventilator parameters, such as positive end-expiratory pressure (PEEP), tidal volume (VT) ventilation, prone positioning and other parameters12,13,14,15,16. However, a standard pairwise meta-analysis can only compare two treatments (or classes) that have been directly compared in head to head trials17. The mechanical ventilation strategies for ARDS patients, however, include many ventilation parameters, such as PEEP, VT, recruitment maneuvers (RM), position and others. Traditional pairwise meta-analysis can only compare a specific parameter between ventilation strategies and is unable to compare the entire set of parameters of different ventilation strategies; therefore, the ability to draw definitive conclusions from the results is limited. Network meta-analysis (also called multiple or mixed treatment comparison meta-analysis, MTC) permits the evaluation of the comparative effectiveness of multiple interventions, even though some pairs may not have been directly compared and has the potential to reduce the uncertainty in treatment effect estimates18,19. By taking advantage of MTC, this study compared the effectiveness and safety of mechanical ventilation strategies with different parameters as follows: different ventilation modes; same ventilation mode with different parameter settings; same ventilation mode and same parameter settings with different parameter value; and same ventilation mode and same parameter settings with different operational techniques. We attempted to identify the optimal mechanical ventilation strategies for ARDS.
Methods
We conducted our systematic review in accordance with the methods recommended in the PRISMA guidelines.
Literature Search
RCTs were identified through electronic and manual searches. We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, EMBASE, MEDLINE, CINAHL and the Web of Science using a combination of MeSH and text words (Appendix 1). We did not restrict our search based on language or year of publication. The last search update was in December 2015. We reviewed the reference lists of published meta-analyses. In addition, we manually searched the Index Medicus of RCTs, meta-analyses and systematic reviews for studies that were missed in the initial electronic search.
Inclusion and Exclusion Criteria
Two groups conducted the literature inclusion and exclusion process separately. When there was a discrepancy between the two groups, the selection committee met to reach an agreement on the inclusion and exclusion of the disputed literature. We first excluded the following literature: review studies, retrospective studies, observational studies, case reports, animal studies, studies conducted on children, studies regarding psychological mechanisms only, unrelated studies (such as studies of mechanical ventilation in patients with non-acute respiratory distress syndrome, or studies using other non-mechanical ventilation treatment strategies, such as medication for ARDS patients), duplicate reports, literature involving repeated experiments (commentary papers on specific studies or secondary analysis on experimental data), non-invasive mechanical ventilation studies, nonrandomized trials and studies focused on comparing the effect of treatment before and after the application of ventilation intervention. Ultimately, randomized controlled trials on mechanical ventilation in adult ARDS patients were included. According to the modified Jadad scale20, all included studies were of relative high quality with a low bias risk (Table 1). No studies were excluded because of quality problems.
Outcome Measures and Data Extraction
The extracted data included basic study information such as experimental design, experimental time, country of the study, the inclusion criteria, the age and gender of the included patients, detailed experimental procedure, specific parameter settings of the mechanical ventilation, clinical outcome and safety outcomes of the patients. The primary outcome of this study regards the all-cause mortality of ARDS patients. If there were multiple all-cause mortalities calculated in selected studies, the mortality from the most long-term follow-up was extracted for analysis. The secondary outcome of this study regards barotrauma, duration of mechanical ventilation, ICU stay duration and hospital stay duration. Two groups extracted the data separately; data comparison and verification were performed afterwards. If necessary, the extracted table was sent to the paper’s corresponding authors for supplementary data or verification. We also contacted corresponding authors to seek assistance in cases of missing data.
Statistical Analysis
Multiple-treatment meta-analysis or network meta-analysis combines direct and indirect evidence for all relative treatment effects and provides estimates with maximum power21,22,23,24. Multiple-treatment meta-analysis was performed using the GeMTC R package21. As mortality was calculated across different time periods in the majority of the included studies, to maximize accuracy and effectiveness22,25, this study used hazard ratios (HRs) and 95% confidence intervals (CIs) to assess mortality in ARDS patients receiving mechanical ventilation. The statistical analysis was based on Poisson likelihoods with a log link function. We also used the odds ratio (OR) and 95% CIs to assess the incidence of barotrauma.
The statistical analysis was also based on binomial likelihoods with a logit link function. The CI was calculated with statistical methods based on Bayesian probability theory. The CI was considered statistically significant when the CI did not include 1.0. We used a random-effects model within a Bayesian framework using a Markov Chain Monte Carlo simulation to calculate HRs (mortality), ORs (barotrauma) and CI24. The models were run for 150,000 iterations and convergence was assessed using the Brooks-Gelman-Rubin diagnostic26.
We used a technique known as ‘back-calculation’23 to evaluate the consistency of the network meta-analysis findings from direct versus indirect evidence. During this process, three types of model are estimated: unrelated study effects, unrelated mean effects and consistency. The output of the summary function can be plotted for a visual representation. We used visual inspection of the forest plots and the I2 statistic to investigate the possibility of statistical heterogeneity and inconsistency between the direct and indirect effect estimates using the Higgins–Thompson method27 (low heterogeneity 25%, moderate 50% and high 75%).
We also ranked the different interventions in terms of their likelihood of leading to the best results for each outcome. In the Markov chain Monte Carlo cycle, for each of the iterations, regimens were ranked according to the estimated log HR. The probability of a regimen being superior was then defined as the proportion of times a regimen ranked first. Each ventilation strategy was ranked by the estimated effect size. These probabilities sum to 1 for each treatment and each rank. A value of x% means that the strategy achieves x% effectiveness and thus larger percentages denote more effective interventions. However, this denotation only represents one possibility without certainty.
Sensitivity Analysis
We performed two sensitivity analyses, including and excluding specific studies that utilized substantially different study designs and populations. 1) According to Lopez’s trial28, age was independently associated with hospital outcome. In Bollen and co-workers’study29, the mean ages in the high-frequency oscillatory ventilation (HFOV) and traditional ventilation (CV) groups were 81.0 ± 20.5 and 81.7 ± 12.5 years, respectively, which were significantly different from those of the other groups. Age differences can have significant effects on mortality. We performed comparative studies before and after the exclusion of the study29. 2) Sensitivity analyses were performed on the studies’ follow-up times; two studies30,31 were eliminated due to follow-up times greater than 6 months.
Results
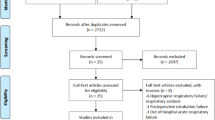
We identified 7,185 studies by reviewing titles and abstracts (Fig. 1). After the initial screening, we retrieved the full texts of potentially eligible articles for detailed assessment. Thirty-six randomized controlled trials were included for meta-analysis (Table 2), with a total of 6,685 patients randomized to receive one of the 26 ventilation strategies (Fig. 2, Table 1). Compared with traditional meta-analysis, we sub-divided the ventilation strategies into 26 ventilation strategies: different ventilation modes; same ventilation mode with different parameter settings; same ventilation mode and same parameter settings with different parameter value; and same ventilation mode and same parameter settings with different operational techniques12,32,33.
Network of the comparisons for the Bayesian network meta-analysis.
The size of the nodes is proportional to the number of patients (in parentheses) randomized to receive the treatment. The width of the lines is proportional to the number of trials (beside the line) comparing the connected treatments.
All 36 trials reported information on all-cause mortality and were included for meta-analysis. Two trials were three-arm randomized studies and the remaining trials were two-arm randomized studies. Compared with the ventilation strategy HVT + FiO2-LPEEP (higher tidal volumes with FiO2-guided lower PEEP), LVT + FiO2-LPEEP + PRONE (lower tidal volumes with FiO2-guided lower PEEP and prone positioning) and PCV + FiO2-LPEEP (pressure-controlled ventilation with FiO2-guided lower PEEP) were associated with lower mortality: the HRs and 95% CIs were 0.62 (0.42–0.98) and 0.57 (0.34–0.97) (Fig. 3), respectively. In addition, the HR and 95% CI between LVT + FiO2-LPEEP (lower tidal volumes with FiO2-guided lower PEEP) and LVT + FiO2-LPEEP + PRONE (lower tidal volumes with FiO2-guided lower PEEP and prone positioning) was 0.73 (0.53–1), although this was not statistically significant. All HR and 95% CIs of the ventilation strategies are shown in Table 3.
Most of the comparisons showed little or no heterogeneity. The endpoint of the I2 value of all-cause mortality exceeded 50% (I2 = 51.8%) in only one of the comparisons, ventilation strategy HFOV (high-frequency oscillatory ventilation) vs. ventilation strategy LVT + FiO2-HPEEP (lower tidal volumes with FiO2-guided higher PEEP), indicating the presence of moderate heterogeneity. Comparing the all-cause mortality results from traditional pairwise meta-analysis and network meta-analysis did not suggest any inconsistency between direct and indirect evidence (Appendix 3). The sensitivity analyses regarding age and follow-up period did not affect the results of the meta-analyses on mortality in ARDS patients. After the exclusion of three studies29,30,31, the ventilation strategies PCV + FiO2-LPEEP (pressure controlled ventilation with FiO2-guided lower PEEP) and LVT + FiO2-LPEEP + PRONE (lower tidal volumes with FiO2-guided lower PEEP and prone positioning) still had statistically significant reductions in mortality in ARDS patients compared with HVT + FiO2-LPEEP (higher tidal volumes with FiO2-guided lower PEEP).
Twenty-two included studies reported the incidence of barotrauma as a secondary outcome. Barotrauma was involved in 15 ventilation strategies. Using OR as the “combining effect size,” ventilation strategy PV-PEEP and VT + RM (static pressure-volume [P–V] curve was measured daily, PEEP and VT were set based on the P–V variation and the open-lung potential was evaluated before recruitment maneuvers) was associated with a lower incidence of barotrauma compared to other ventilation strategies. The ORs and 95% CIs of different types of ventilation strategies are shown in Table 3. Twelve studies4,7,29,30,34,35,36,37,38,39,40,41 reported the lengths of mechanical ventilation, 147,31,36,38,39,40,41,42,43,44,45,46,47,48 reported ICU stay durations and 838,40,41,42,44,45,46,47 reported hospital stay durations. Unfortunately, certain treatment strategies in these studies were isolated and distinctive from other treatment strategies and thus cannot be included in network-meta-analysis.
In Fig. 4, we summarize the rankings of the different competing treatment strategies in terms of all-cause mortality and the incidence of barotrauma, with details provided in Appendices 4 and 5. Ventilation strategy LVT + FiO2-HPEEP + PRONE (lower tidal volumes with FiO2-guided higher PEEP and prone positioning) had the greatest potential to reduce mortality and the possibility of its receiving the first ranking was 61.6%. The second ranking was ventilation strategy LVT + PV individual PEEP (lower tidal volumes with P–V static curve-guided individual PEEP), with a possibility of 18.4%. Permissive hypercapnia + RM + LAP (permissive hypercapnia, recruitment maneuvers and low airway pressures) was the worst in terms of all-cause mortality. In terms of reducing the incidence of barotrauma, ventilation strategy PV-PEEP and VT + RM (static pressure-volume [P–V] curve was measured daily, PEEP and VT were set based on P–V variation and the open-lung potential was evaluated before recruitment maneuvers) was ranked highest, with a possibility of 63.4%. Ventilation strategy LVT + FiO2-HPEEP + HDPLV (lower tidal volumes with FiO2-guided higher PEEP and higher-dose partial liquid ventilation) had the highest probability of causing barotrauma.
Discussion
A complete ventilation strategy for ARDS includes various different respiratory parameters. However, because of its limitations, conventional pair-wise meta-analysis can only compare two different parameters, or different values of one specific parameter, but cannot be used to compare two complete ventilation strategies. Therefore, only one ventilatory parameter can be used as the primary study variable and all other ventilatory parameters can only be considered as background variables. However, clinical studies have shown that other ventilatory parameters might also affect the outcome of ARDS patients4,46. Santa Cruz R and colleagues12 included 7 studies in a meta-analysis study on the effect of higher-PEEP and lower-PEEP on the mortality and barotrauma incidence of ARDS patients. In their study, PEEP value was the only comparative variable. Other respiratory parameters, such as the PEEP setting mode and tidal volume, were not considered. For example, in the original 3 studies32,39,49, the PEEP values, tidal volumes and PEEP setting modes were all set differently. In the study49, the ventilation mode of higher tidal volumes with FiO2-guided PEEP was set in the control group; the ventilation mode of P–V-guided lower PEEP was set in the experimental group. The tidal volume was consistently set lower in both of the studies32,39. Similarly, Hodgson and co-workers50 conducted a conventional meta-analysis to study the effect of recruitment maneuvers on mild ARDS patients. The included studies by Amato49, Brower51 and Meade52 had different higher or lower initial tidal volume settings and different PEEP setting modes; however, these two different parameter settings were ignored in Hodgson C’s study. Some studies4 have shown that a difference in tidal volume can also produce different mortality in ARDS patients. Lower tidal volume ventilation could reduce mortality in ARDS patients. Studies46 have also indicated that the PEEP setting mode has different effects on ARDS patients compared with the FiO2-guided group. Multiple-organ-dysfunction-free days, respiratory-failure-free days and hemodynamic-failure-free days at 28 days were significantly lower in subjects with compliance-guided PEEP settings. Conventional meta-analysis cannot simultaneously study various ventilation parameters as a complete treatment strategy due to its methodological limitations; therefore, the study results have limited reference significance. The greatest difference between our study and conventional meta-analysis is that this study not only examined individual parameters but also simultaneously examined various parameters as a complete treatment strategy. Our method is more reasonable, more scientific and able to provide a more direct reference standard for clinical practitioners.
The results of this meta-analysis showed that compared with the ventilation strategy (higher tidal volumes with FiO2-guided lower PEEP), both (lower tidal volumes with FiO2-guided lower PEEP and prone positioning) and (pressure-controlled ventilation with FiO2-guided lower PEEP) were associated with lower mortality in patients and the difference was statistically significant.
When summarizing the possible rankings of the different ventilation strategies on ARDS patients’ mortality, we found that (lower tidal volumes with FiO2-guided higher PEEP and prone positioning) was the optimal ventilation strategy and (lower tidal volumes with P–V static curve guided individual PEEP) ranked second. In addition, (permissive hypercapnia, recruitment maneuvers and low airway pressures) had the highest potential mortality among all ventilation strategies.
The major cause of death in ARDS is multiple organ failure resulting from systemic inflammatory mediator release53. Ventilator-associated pneumonia (VAP) may contribute to the mortality associated with ARDS54. Lung volume was significantly reduced in patients with ARDS. The number of alveoli participating in normal ventilation function, which is referred to as “baby lung,” was also reduced55. Conventional tidal volume can lead to increased tension in the walls of alveoli or stress in the alveoli56,57. In comparison, lower tidal volume can avoid the overexpansion of residual normal alveoli, alleviate lung injury and reduce the release and spread of inflammatory mediators58, which can improve tissue oxygenation and simultaneously significantly reduce the incidence of ventilator-associated lung injury49,59. By reducing the overexpansion of alveoli, improving ventilation-perfusion matching (V/Q) and lung mechanics60, promoting lung recruitment61 and improving the excretion of airway secretions62, prone positioning can simultaneously improve tissue oxygenation6 and reduce the incidence of VAP63. Pressure-controlled ventilation can also produce relatively good physiological effects, such as increased static lung compliance, reduced mechanical ventilation time64 and improved tissue oxygenation65. The above theories might be potential mechanisms through which (lower tidal volumes with FiO2-guided lower PEEP and prone positioning) and (pressure-controlled ventilation with FiO2-guided lower PEEP) are associated with lower mortality. Because the study sample sizes of (lower tidal volumes with FiO2-guided higher PEEP and prone positioning) and (lower tidal volumes with P–V static curve-guided individual PEEP) were relatively small, the results were more likely to have bias66. Therefore, regarding possible ranking, combining the direct and indirect evidence analysis on overall mortality has more reference significance. In our study, we divided ventilation strategies into groups in detail, avoiding the limit effect of single parameters and we integrated the combined actions of different ventilation parameters or same ventilation modes with different parameter settings, which can more comprehensively account for the effectiveness of the entire mechanical ventilation strategy.
(Permissive hypercapnia, recruitment maneuvers and low airway pressures) has the highest potential mortality among all ventilation strategies. During RM, it can increase the resistance of lung vessels and transiently decrease cardiac output and mean arterial pressure67,68. It can also decrease tissue oxygen saturation51,69, causing harmful hemodynamic effects. The death of the patient can also be caused by RM-induced complications, such as hemodynamic compromise or pneumothorax. Therefore, (permissive hypercapnia, recruitment maneuvers and low airway pressures) is the ventilation strategy with the highest potential mortality.
In comparing different models of mechanical ventilation-induced barotrauma incidence, there is significant inconsistency between the direct and indirect evidence (Appendix 6). After carefully analyzing the incidence data of mechanical ventilation-induced barotrauma across all mechanical ventilatory strategies, we found that a ventilation strategy can have significantly different incidences of barotrauma in different experiments. Among them, the variability of (higher tidal volumes with FiO2-guided lower PEEP) in the incidence of barotrauma was the largest, with a range from 3.8% to 41.6%8,49. After careful comparison of the included studies, we found that the inclusion criteria for barotrauma was not exactly the same. For example, barotrauma was defined in the study as any new pneumothorax, pneumomediastinum, subcutaneous emphysema, or pneumatocele with a diameter of more than 2 cm after randomization32. Barotrauma has also been defined as including pneumothorax, pneumomediastinum, pneumoperitoneum, pneumopericardium, or subcutaneous emphysema42. However, other studies10,34,35 have recorded the incidence of barotrauma as including only pneumothorax. In addition, we speculate that due to the different limitations of medical conditions at the experimental sites and the difference in diagnostic experience of the clinical practitioners, certain barotrauma might not be detected, leading to relatively the large difference in the incidence of barotrauma in different clinical experiments. These phenomena are the most likely causes of inconsistencies between analysis from the direct and indirect evidence and these inconsistencies mean the network meta-analysis of the incidence of barotrauma in ARDS patients might have very limited reference value. Therefore, we suggest that the inclusion of barotrauma should be performed according to identical diagnostic procedures and diagnostic standards in future studies, to obtain more unified clinical data and assist in further comparison analysis.
Limitations
As an innovative study, this study also has certain limitations. 1) The included trials numbers of (lower tidal volumes with FiO2-guided higher PEEP and prone positioning), (lower tidal volumes with P–V static curve-guided individual PEEP) and (permissive hypercapnia, recruitment maneuvers and low airway pressure) ventilation were limited. In addition, there are few direct head-to-head comparisons of related treatment strategies. Therefore, the results can easily exhibit deviation. Future large sample size, multi-center, parallel-group and direct comparison studies are needed to validate the results of our study. For example, in the original studies on mechanical ventilation, Villar70 and Mercat10 studied LVT + PV-HPEEP vs. HVT + FiO2-LPEEP and LVT + PV-HPEEP vs. LVT + FiO2-LPEEP, respectively. Based on transitivity, we could obtain comparison results of HVT + FiO2-LPEEP and LVT + FiO2-LPEEP; however, we could not establish a relationship between LVT + FiO2-HPEEP and LVT + PV-HPEEP and we could not obtain comparison results of LVT + PV-HPEEP and LVT + FiO2-HPEEP by network meta-analysis. 2) Because the original experimental results are not complete, no network meta-analysis was conducted on the time of mechanical ventilation, ICU stay duration and hospital stay duration. Therefore, the results of this study are relatively simple and lack a comprehensive conclusion.
Conclusion
We conducted a network meta-analysis based on direct and indirect evidence to compare the currently applied invasive mechanical ventilation strategies with respect to all-cause mortality in ARDS patients. The results indicated that the ventilation strategies (higher tidal volumes with FiO2-guided lower PEEP), (pressure-controlled ventilation with FiO2-guided lower PEEP) and (lower tidal volumes with FiO2-guided lower PEEP and prone positioning) were associated with lower mortality in ARDS patients. Ventilation strategies W (lower tidal volumes with FiO2-guided higher PEEP and prone positioning) and T (lower tidal volumes with P–V static curve-guided individual PEEP) are potential optimal ventilation strategies for ARDS patients.
Key messages
Mechanical ventilation is the most effective life-saving technique and can save an ARDS patient’s life.
We attempted to identify optimal strategies for mechanical ventilation of patients with ARDS by taking advantage of a network meta-analysis.
Ventilation strategies (higher tidal volumes with FiO2-guided lower PEEP, pressure-controlled ventilation with FiO2-guided lower PEEP and lower tidal volumes with FiO2-guided lower PEEP and prone positioning) were associated with lower mortality in ARDS patients.
The ventilation strategies LVT + FiO2-HPEEP + PRONE (lower tidal volumes with FiO2-guided higher PEEP and prone positioning) and LVT + PV individual PEEP (lower tidal volumes with P–V static curve-guided individual PEEP) are potential optimal ventilation strategies for ARDS patients.
Additional Information
How to cite this article: Wang, C. et al. Lung ventilation strategies for acute respiratory distress syndrome: a systematic review and network meta-analysis. Sci. Rep. 6, 22855; doi: 10.1038/srep22855 (2016).
References
Roupie, E. et al. Prevalence, etiologies and outcome of the acute respiratory distress syndrome among hypoxemic ventilated patients. Intensive Care Med. 25, 920–9 (1999).
Force, A. D. T. Acute respiratory distress syndrome. JAMA. 307, 2526–2533 (2012).
Rubenfeld, G. D., Caldwell, E. & Peabody, E. Incidence and outcomes of acute lung injury. N Engl J Med. 353, 1685–93 (2005).
The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 342, 1301–8 (2000).
Guérin, C. et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 368, 2159–68 (2013).
Gattinoni, L. et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 345, 568–73(2001).
Brochard, L. et al. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. The Multicenter Trial Group on Tidal Volume Reduction in ARDS. Am J Respir Crit Care Med. 158, 1831–8 (1998).
Brower, R. G. et al. Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit Care Med. 27, 1492–8 (1999).
Amato, M. B. P. et al. Improved survival in ARDS: beneficial effects of a lung protective strategy (abstract). Am J Respir Crit Care Med. 153, A531 (1996).
Mercat, A. et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 299, 646–55 (2008).
Malhotra, A. Low-tidal-volume ventilation in the acute respiratory distress syndrome. N Engl J Med. 357, 1113–20 (2007).
Santa, C. R. et al. High versus low positive end-expiratory pressure (PEEP) levels for mechanically ventilated adult patients with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev. 6, CD009098 (2013).
Galvin, I. M. et al. Partial liquid ventilation for preventing death and morbidity in adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev. 23, 7:CD003707 (2013).
Sud, S. et al. High frequency oscillation in patients with acute lung injury and acute respiratory distress syndrome (ARDS): systematic review and meta-analysis. BMJ. 18, 340:c2327 (2010).
Petrucci, N. & De, F. C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev. 2, CD003844 (2013).
Beitler, J. R. et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med. 40, 332–41 (2014).
Haas, D. M. et al. Tocolytic therapy for preterm delivery: systematic review and network meta-analysis. BMJ. 9, 345:e6226 (2012).
Mills, E. J. et al. Multiple treatment comparison meta-analyses: a step forward into complexity. Clin Epidemiol. 3, 193–202 (2011).
Caldwell, D. M., Ades, A. E. & Higgins, J. P. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 331, 897–900 (2005).
Jadad. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 17, 1–12. (1996).
Van, V. G., Lu, G. & Brock, B. Automating network meta-analysis. Res Syn Meth. 3, 285–99 (2012).
Woods, B. S., Hawkins, N. & Scott, D. A. Network meta-analysis on the log-hazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: a tutorial. BMC Med Res Methodol. 10, 54–62 (2010).
Dias, S., Welton, N. J., Caldwell, D. M. & Ades, A. E. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 29, 932–44 (2010).
Salanti, G., Ades, A. E. & Ioannidis, J. P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 64, 163–71 (2011).
Dogliotti, A., Paolasso, E. & Giugliano, R. P. Current and new oral antithrombotics in non-valvular atrial fibrillation: a network meta-analysis of 79 808 patients. Heart. 100, 396–405 (2014).
Brooks, S. P. & Gelman, A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 7, 434–55 (1998).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ. 327, 557–60 (2003).
Lopez, S. I. Mortality in patients with respiratory distress syndrome. Med intensiva, 10.1016/j.medin.2015.10.007 (2015).
Bollen, C. W. et al. High frequency oscillatory ventilation compared with conventional mechanical ventilation in adult respiratory distress syndrome: a randomized controlled trial [ISRCTN24242669]. Crit Care. 9, R430–9 (2005).
Derdak, S. et al. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med. 166, 801–8 (2002).
Taccone, P. et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 302, 1977–84 (2009).
Brower, R. G. et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 351, 327–36 (2004).
Schultz, M. J. Lung-protective mechanical ventilation with lower tidal volumes in patients not suffering from acute lung injury: a review of clinical studies. Med Sci Monit. 14, RA22–6 (2008).
Hirschl, R. B. et al. Prospective, randomized, controlled pilot study of partial liquid ventilation in adult acute respiratory distress syndrome. Am J Respir Crit Care Med. 165, 781–7 (2002).
Kacmarek, R. M. et al. Partial liquid ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 173, 882–9 (2006).
Long, Y. et al. The application of individualized ventilation strategies in acute respiratory distress syndrome. Zhonghua Jie He He Hu Xi Za Zhi. [Article in Chinese] 29, 549–53 (2006).
Wang, X. Z. et al. Comparison of the effects of BiPAP ventilation combined with lung recruitment maneuvers and low tidal volume A/C ventilation in patients with acute respiratory distress syndrome. [Article in Chinese] Zhonghua Jie He He Hu Xi Za Zhi. 30, 44–7 (2007).
Fernandez, R. et al. Prone positioning in acute respiratory distress syndrome: a multicenter randomized clinical trial. Intensive Care Med. 34, 1487–91 (2008).
Huh, J. W. et al. Efficacy of positive end-expiratory pressure titration after the alveolar recruitment manoeuvre in patients with acute respiratory distress syndrome. Crit Care. 13, R22 (2009).
Hodgson, C. L. et al. A randomised controlled trial of an open lung strategy with staircase recruitment, titrated PEEP and targeted low airway pressures in patients with acute respiratory distress syndrome. Crit Care. 15, R133 (2011).
Agarwal, R., Srinivasan, A., Aggarwal, A. N. & Gupta, D. Adaptive support ventilation for complete ventilatory support in acute respiratory distress syndrome: a pilot, randomized controlled trial. Respirology. 18, 1108–15 (2013).
Esteban, A. et al. Prospective randomized trial comparing pressure-controlled ventilation and volume-controlled ventilation in ARDS. For the Spanish Lung Failure Collaborative Group. Chest. 117, 1690–6 (2000).
Talmor, D. et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 359, 2095–104 (2008).
Xi, X. M. et al. Clinical efficacy and safety of recruitment maneuver in patients with acute respiratory distress syndrome using low tidal volume ventilation: a multicenter randomized controlled clinical trial. Chin Med J (Engl). 123, 3100–5 (2010).
Young, D. et al. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 368, 806–13 (2013).
Pintado, M. C. et al. Individualized PEEP setting in subjects with ARDS: a randomized controlled pilot study. Respir Care. 58, 1416–23 (2013).
Bein, T. et al. Lower tidal volume strategy (≈3 ml/kg) combined with extracorporeal CO2 removal versus ‘conventional’ protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med. 39, 847–56 (2013).
Zhang, N. X., Qin, Y. Z., Xu, L. & Wang, S. P. Clinical comparative study of airway pressure release ventilation and continuous positive airway pressure ventilation. Chin Crit Care Med. 17, 8 (2005).
Amato, M. B. et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 338, 347–54 (1998).
Hodgson, C. et al. Recruitment manoeuvres for adults with acute lung injury receiving mechanical ventilation. Cochrane Database Syst Rev. 2, CD006667 (2009).
Brower, R. G. et al. Effects of recruitment maneuvers in patients with acute lung injury and acute respiratory distress syndrome ventilated with high positive end-expiratory pressure. Crit Care Med. 31, 2592–7 (2003).
Meade, M. O. et al. Ventilation strategy using low tidal volumes, recruitment maneuvers and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 299, 637–45 (2008).
Wiedemann, H. P. & Arroliga, A. C. Acute respiratory distress syndrome: low-stretch ventilation improves survival. Cleve Clin J Med. 67, 435–40 (2000).
Forel, J. M. et al. Ventilator-associated pneumonia and ICU mortality in severe ARDS patients ventilated according to a lung-protective strategy. Crit Care. 16, R65 (2012).
Gattinoni, L. & Pesenti, A. The concept of“baby lungt. ” Intensive Care Med. 31, 776–84 (2005).
Gattinoni, L., Pelosi, P., Crotti, S. & Valenza, F. Effects of positive end-expiratory pressure on regional distribution of tidal volume and recruitment in adult respiratory distress syndrome. Am J Respir Crit Care Med. 151, 1807–14 (1995).
Terragni, P. P. et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 175, 160–6 (2007).
Hickling, K. G, Walsh, J., Henderson, S. & Jackson, R. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med. 22, 1568–78 (1994).
Pinhu, L., Whitehead, T., Evans, T. & Griffiths, M. Ventilator-associated lung injury. Lancet. 361, 332–40 (2003).
Galiatsou, E. et al. Prone position augments recruitment and prevents alveolar overinflation in acute lung injury. Am J Respir Crit Care Med. 174, 187–97 (2006).
Guerin, C. et al. Effects of prone position on alveolar recruitment and oxygenation in acute lung injury. Intensive Care Med. 25, 1222–30 (1999).
Mackenzie, C. F. Anatomy, physiology and pathology of the prone position and postural drainage. Crit Care Med. 29, 1084–5 (2001).
Guerin, C. et al. Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA. 292, 2379–87 (2004).
Rappaport, S. H. et al. Randomized, prospective trial of pressure-limited versus volume-controlled ventilation in severe respiratory failure. Crit Care Med. 22, 22–32 (1994).
Armstrong, B. W. & MacIntyre, N. R. Pressure-controlled inverse ratio ventilation that avoids air trapping in the adult respiratory distress syndrome. Crit Care Med. 23, 279–85 (1995).
Chaimani, A. et al. Effects of study precision and risk of bias in networks of interventions: a network meta-epidemiological study. Int J Epidemiol. 42, 1120–31 (2013).
Kloot, T. E. et al. Recruitment maneuvers in three experimental models of acute lung injury. Effect on lung volume and gas exchange. Am J Respir Crit Care Med. 161, 1485–94 (2000).
Lim, S. C. et al. Transient hemodynamic effects of recruitment maneuvers in three experimental models of acute lung injury. Crit Care Med. 32, 2378–84 (2004).
Lapinsky, S. E. et al. Safety and efficacy of a sustained inflation for alveolar recruitment in adults with respiratory failure. Intensive Care Med. 25, 1297–301(1999).
Chen, H. B. Inspiratory plateau pressure controlling mechanical ventilation on traumatic ARDS. Chin J Prim Med Pharm. 11, (2004).
Demory, D. et al. High-frequency oscillatory ventilation following prone positioning prevents a further impairment in oxygenation. Crit Care Med. 35, 106–11 (2007).
Kyle, W. et al. Patients with acute lung injury benefit from airway pressure release ventilation. Am J Respir Crit Care Med. 183, 1, 10.1164/ajrccm (2011)
Ferguson, N. D. et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 368, 795–805 (2013).
Varpula, T. et al. Airway pressure release ventilation as a primary ventilatory mode in acute respiratory distress syndrome. Acta Anaesthesiol Scand. 48, 722–31 (2004).
Varpula, T. et al. The effects of ventilatory mode on lung aeration assessed with computer tomography: a randomized controlled study. J Intensive Care Med. 24, 122–30 (2009).
Villar, J., Kacmarek, R. M. & Pérez, M. L. & Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med. 34, 1311–8 (2006).
Voggenreiter, G. et al. Prone positioning improves oxygenation in post-traumatic lung injury–a prospective randomized trial. J Trauma. 59, 333–41 (2005).
Mentzelopoulos, S. D. et al. Intermittent recruitment with high-frequency oscillation/tracheal gas insufflation in acute respiratory distress syndrome. Eur Respir J. 39, 635–47 (2012).
Sun, J. J. et al. Clinical effects of low-stretch ventilation on acute respiratory distress syndrome. [Article in Chinese] Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 21, 609–12 (2009).
Acknowledgements
Financial support provided by the National Natural Science Foundation of China (No. 81402462) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
C.W. and X.W. participated in the design of the study. C.C., L.G., L.G., N.Z., W.W., X.P. and B.S. performed the statistical analysis, interpreted the data and drafted the manuscript. A.L. and J.S. contributed to the interpretation of the data and critical revision of the manuscript for important intellectual content. E.L. gave final approval of the version to be published and is accountable for all aspects of the work. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, C., Wang, X., Chi, C. et al. Lung ventilation strategies for acute respiratory distress syndrome: a systematic review and network meta-analysis. Sci Rep 6, 22855 (2016). https://doi.org/10.1038/srep22855
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep22855
This article is cited by
-
An appraisal of the methodology and quality of evidence of systematic reviews on the efficacy of prone positional ventilation in adult patients with acute respiratory distress syndrome: an umbrella review
Internal and Emergency Medicine (2023)
-
Lung-protective ventilation worsens ventilator-induced diaphragm atrophy and weakness
Respiratory Research (2020)
-
Effect of PEEP and I:E ratio on cerebral oxygenation in ARDS: an experimental study in anesthetized rabbit
BMC Anesthesiology (2019)
-
Mechanical power normalized to predicted body weight as a predictor of mortality in patients with acute respiratory distress syndrome
Intensive Care Medicine (2019)
-
The role of high airway pressure and dynamic strain on ventilator-induced lung injury in a heterogeneous acute lung injury model
Intensive Care Medicine Experimental (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.