Abstract
B12 belongs to the coumarin class of compounds that have been shown to have various physiological and pharmacological activities including anti-inflammatory, antibacterial and antioxidant. In the present study, we characterised the neuroprotective effects of B12 against H2O2-induced neuronal cell damage in SH-SY5Y cells. Protein expression profiling in combination with pathway analysis was deployed to investigate the molecular events associated with the neuroprotective effects in human neuronal cells using a label-free quantitative proteomics approach. A total of 22 proteins were significantly differentially expressed in H2O2-damaged cells with or without B12 treatment. Bioinformatics analysis using the Cytoscape platform indicated that poly pyrimidine tract binding protein 1 (PTBP1) was highly associated with the protective effect and western blotting verified that PTBP1 was up-regulated in H2O2 + B12 treatment group, compared with the H2O2 treated group. PTBP RNAi experiments knocked down PTBP expression, which cancelled out the protective effect of B12 on cell viability. Thus, we infer that B12 neuroprotective activity involves up-regulation of PTBP1 and its associated signalling networks following H2O2-induced apoptosis in SH-SY5Y cells. B12 or related compounds may prove to be useful therapeutic agents for the treatment of neurodegenerative diseases such as Alzheimer’s and Parkinson’s.
Similar content being viewed by others
Introduction
Neurodegenerative diseases such as Parkinson’s (PD) and Alzheimer’s (AD) are characterized by the progressive loss of specific neuronal cell populations and are associated with the formation of protein aggregates1,2,3. A common feature of these diseases is a connection with oxidative stress, which leads to the dysfunction or death of neuronal cells and contributes to disease pathogenesis3. Oxidative damage is generated following cell lysis and a burst of oxidative activity that results in an accumulation of oxygen free radicals that attack proteins, nucleic acids and lipid membranes, thereby disrupting cellular function and integrity4. Hydrogen peroxide (H2O2) is among the main reactive oxygen species (ROS) and is produced in redox processes5,6. Oxidative stress manifests its toxic effects through different pathways and knowledge of these pathways could assist the development of a number of potential therapeutic strategies that target neurodegenerative diseases4.
Proteomics, the collective study of all expressed proteins in cells, tissues, or biological fluids at a given time or under given conditions, reveals information on not only the individual components (proteins), but also their interplay in complexes, signalling pathways and network modules associated with specific biochemical functions7,8,9. Proteomics studies generally involve high-throughput experimental platforms such as liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS)10,11,12 that measures the abundance of proteins or peptides in different biological conditions by assaying thousands of proteins from complex biological samples simultaneously13. Shotgun proteomics using isotopic labelling can achieve accurate quantitative measurements14, but label-free quantitative proteomics is growing in popularity because it is reliable, versatile and is a cost-effective alternative to labelled quantitation15.
Various compounds have antioxidant activity, including vitamin C, ubiquinone lipoic acid, β-carotene, creatine, melatonin and curcumin1. Sung-Soo Kim et al.16 found that 3,5-dicaffeoylquinic acid (3,5-diCQA) has neuroprotective properties against neuronal cell death caused by H2O2-induced oxidative stress that operate by suppressing caspase-3 activation and restoring glutathione (GSH) levels. Similarly, Venuprasad and colleagues17 showed that a hydroalcoholic extract of holy basil (Ocimum sanctum) ameliorates H2O2-induced neuronal damage by stimulating the antioxidant defence mechanism and could potentially be used to treat oxidative stress-mediated neuronal disorders. B12 (4-methyl-7-hydroxy-8-(1-hydroxyethyl) coumarin; CN patent No. 201210042735.1) is a compound belonging to the coumarin class that have been shown to possess anti-inflammatory, antibacterial and antioxidant activities. In the present study, we characterised the neuroprotective effects of B12 against H2O2-induced neuronal cell damage in SH-SY5Y cells. Moreover, we used protein expression profiling combined with pathway analysis to investigate the molecular events associated with the protective effects and gained insight into the underlying mechanisms of oxidative damage and neuroprotection.
Results
Neuroprotective effects of B12 on H2O2-treated SH-SY5Y cells
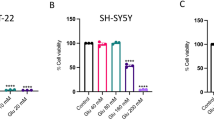
Cell proliferation was observed in SH-SY5Y cells after treatment with H2O2 at a range of doses and cell number decreased with increasing H2O2 concentration. A concentration of 200 μM H2O2 decreased cell number by 50% and this was chosen for subsequent experiments on the neuroprotective effects (Fig. 1A). Clear differences in cell survival rate were apparent following treatment with B12 and survival varied with B12 concentration in a dose-dependent manner. The neuroprotective effects of B12 were prevalent at >2 μM and a 75% cell survival rate was achieved at 200 μM H2O2 (Fig. 1B). Concentrations of 200 μM H2O2 and 20 μM B12 were subsequently used to analyse protein expression in label-free proteomics experiments.
Cell proliferation following H2O2 or B12 treatment.
(A) Cell proliferation of SH-SY5Y cells was monitored following H2O2 treatment at different doses. Death rate increases with increasing H2O2 concentration (*P < 0.05; **P < 0.01, compared with control group). (B) Neuroprotective effects of B12 are apparent at a concentration above 2 μM and the cell survival rate is 75% at an H2O2 concentration of 200 μM (n = 6). Data are mean ± S.E.M. Experiments were repeated in triplicate.
Protein expression analysis
A total of 4,969 proteins were identified using the shotgun method and after applying the label-free algorithm (repeated measurement of at least two peptides of each protein in all six samples), 3,505 proteins were successfully quantified. A logarithmic transformation (base 2) of the LFQ intensity of proteins was pre-performed prior to the identification of differentially expressed proteins (Fig. S2). Statistical analysis with Perseus software was performed to select proteins that were differentially expressed following B12 treatment, using the following criteria: fold change >2, p-value <0.01 (using the Student’s t-test). As a result, we identified 22 significantly differentially expressed proteins in the H2O2 + B12 treatment group (P < 0.01; shown as B12 in figures) compared with the H2O2 treatment group (shown as Ctrl in figures). Of these, 12 proteins were up-regulated and 10 proteins were down-regulated (Table 1). Information on protein intensity and RSD ratio is shown in Table S1. Hierarchical clustering analysis of the 22 significantly differentially expressed proteins was performed (Fig. 2A) and scatter plots were constructed to determine the fold change in protein expression (x-axis = log2 Ratio (B12/Ctrl), y-axis = log p-value; Fig. 2B). Additional MS and Venn diagram data, as well as scatter plots, are shown in Figs S3, 4). The Venn diagram (Fig. S3) shows the number of identified proteins in the three replicates, including their overlap in the replicates. Scatter plots of protein intensity from all replicates against the Pearson correlation coefficient (Fig. S4) showed a higher correlation within the same group than between different groups.
Protein expression profiling using a label-free quantitative proteomics approach.
(A) Unsupervised hierarchical clustering analysis of 22 genes that are differentially expressed following treatment with H2O2 + B12 (B12 group; n = 3) compared to treatment with H2O2 alone (Ctrl group; n = 3). Gene names are listed on the left, while cell culture samples are indicated on top. The colour key beneath the heatmap indicates the level expression level (red = up-regulation, green = downregulation). (B) Scatter plots of fold change (x-axis) against log p-value (y-axis) of all quantified proteins. Up- and down-regulated proteins are coloured red and green, respectively.
Bioinformatics analysis
To construct a protein-protein interaction network associated with B12 regulation, we matched the 22 significantly differently expressed proteins with regulatory data in six publically available databases (DIP, BIOGRID, HPRD, BIND, MINT and INTACT). The resulting network was visualized with Cytoscape 3.1.1 and included 614 nodes and 712 edges (Fig. 3A). The node degree distribution is shown in Fig. 3B. The cluster search algorithm, which considers highly interconnected dense regions within a network, was used to rank network proteins. Protein poly pyrimidine tract binding protein 1 (PTBP1), which was up-regulated in B12-treated cells, was the highest ranked (Fig. 3C), indicating a potential role in the neuroprotective effects of B12. Gene ontology analysis was then performed using the BinGO plugin in Cytoscape and genes influenced by B12 were highly correlated with cellular macromolecule metabolic process, cellular metabolic process, nucleic acid metabolic process and gene expression biological process categories (Fig. 4; Table S2). PTBP1 was also associated with these biological processes (Table S2). The top 10 biological processes are listed in Table S2.
Network analysis using Cytoscape.
(A) Integrated regulatory network constructed using Cytoscape. The network includes two types of nodes: differentially expressed proteins identified from protein profiling (pink squares) and their interaction targets (blue squares) derived from six publically available databases. (B) Node degree distribution of differentially expressed proteins. (C) Cluster analysis ranking of the top hits in the B12 regulatory network using the cluster search algorithm MCODE.
Gene Ontology (GO) terms associated with proteins interacting with the 22 differentially expressed proteins.
The colour gradient of the cluster distribution network of the GO biological process associated with the neuroprotective effects of B12 shows the p-value of each cluster associated with the term, while the darker (orange) colour indicates a lower p-value.
Western blot verification of differentially expressed protein PTBP1
In view of the important role of PTBP1 in the neuroprotective effect of B12, we used western blotting to verify expression in treatment and control groups and found that PTBP1 was significantly up-regulated in the H2O2 + B12 treatment group (Fig. 5).
PTBP1 knockdown confirmed its involvement in the neuroprotective effect of B12
After validation of PTBP1 expression by western blotting, we further investigated its role using knockdown experiments. Following treatment with 200 μM H2O2 and 20 μM B12, clear differences were observed in the cell survival rate between the PTBP1 knockdown (siPTBP1) and control (siScramble) groups (Fig. 6A). Specifically, cell survival was increased in the siScramble group but not the siPTBP1 group and the number of cells was also increased in cells treated with B12 without H2O2, whereas B12 had no effect on the PTBP1 knockdown group. Knockdown of PTBP1 was confirmed by immunoblotting of transfected cells (Fig. 6B).
PTBP1 contributes to the neuroprotective effects of B12.
(A) Viability of SH-SY5Y cells treated with or without H2O2 and B12 after PTBP1 knockdown. (B) Immunoblot of PTBP1 in SH-SY5Y cells after transfection with PTBP1siRNA to validate the knockdown level. (*P < 0.05; n = 3; mean ± S.E.M). Experiments were repeated in triplicate.
Discussion
H2O2 is a ubiquitous ROS that is produced in cells by superoxide dismutases as a metabolite of the superoxide anion (O22−). H2O2 is a relatively stable and neutrally charged compound that can readily cross cell membranes and these properties have prompted researchers to propose that H2O2 may be involved in oxidative stress signaling3. In the present study, we used H2O2 as a mediator of oxidative stress in the human neuroblastoma SH-SY5Y cell line and H2O2-induced cell damage at a concentration of 200 μM or higher. We discovered that B12 could significantly protect cells from damage induced by H2O2-induced oxidative stress in a dose-dependent manner. B12 could therefore be used as a drug to protect cells from oxidative damage and we further investigated the mechanism underlying the neuroprotective effects.
The results of quantitative proteomics and western blotting revealed that PTBP1 protein levels were up-regulated in cells in which B12 was protecting against oxidative stress-induced apoptosis. PTBP1 belongs to a subfamily of ubiquitously expressed heterogeneous nuclear ribonucleoproteins (hnRNPs), which are RNA-binding proteins that form a complex with heterogeneous nuclear RNA (hnRNA). These proteins are associated with pre-mRNAs in the nucleus and appear to influence pre-mRNA processing and other aspects of mRNA metabolism and transport. Cote et al.18 observed PTBP1 protein degradation in H2O2-treated breast cancer cell lines using western blotting. A recent study by King et al.19 showed that PTBP1 is involved in specific protein sub-complexes that control gene expression during apoptosis induced by TNF-related apoptosis-inducing ligand (TRAIL).
PTBP1 knockdown experiments provided more evidence of the important and potentially pivotal role of PTBP1 in the neuroprotective effects of B12, since B12 could no longer rescue cells damaged by oxidative stress after PTBP1 knockdown. Furthermore, the number of cells increased in cells treated with B12 in the absence of H2O2, indicating an involvement not only in protecting against oxidative stress, but in cells not damaged by H2O2, although this requires further study. In summary, we can infer that pre-mRNA processing is involved in the neuroprotective effects of B12 and expression of PTBP1, the main target of B12, is required to mediate resistance against H2O2-induced oxidative damage.
Comprehensive analysis of protein composition, post-translational modifications and protein dynamics are important goals in cell biology. High performance liquid chromatography mass spectrometry (LC-MS) has established itself as a powerful tool for proteomics studies20. Although comprehensive gene expression profiles can be precisely and comprehensively quantified using transcriptomics approaches, transcript levels often show only a modest correlation with the abundance of their corresponding proteins21. Moreover, proteins are usually more directly responsible for the regulation of cellular pathways than mRNA transcripts. Thus, protein expression profiling is critical for elucidating the mechanisms underlying the cellular response to specific treatments or drugs22. Choi et al.23 used an LC-MS strategy to separate and identify components of the secretory proteome in adipocytes that are responsive to oxidative stress and their results indicated that oxidative stress significantly down-regulated MMP-2 secretion and up-regulated TIMP-2 secretion. MS-based proteomic profiling experiments can therefore provide useful and important information to improve our understanding of complex biological processes.
Before analysing the proteomic data to identify significantly differentially expressed proteins in the present study, we first measured the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a house keeping protein commonly used as an internal control in western blotting experiments. The ratio of GAPDH in B12-treated and control cells (B12/Ctrl) was 1.04, which confirmed the reliability of our label-free quantitative proteomics approach for expression profiling. Our results (Table 1) showed that 12 proteins (MT1F, SUMO3, AKAP8L, PSMG4, DHFR, PYCR1, ALKBH3, DUS3L, PTBP1, APAF1, HSP90AB4P, MRGBP) were up-regulated and 10 proteins (PRKCSH, KHDRBS1, NDUFA5, ANLN, CPSF3L, NME2, COL5A2, TBCEL, DSP, DPM3) were down-regulated in the H2O2 + B12 treatment group. Many of these proteins are reportedly involved in oxidative stress and apoptosis. SANG et al.24 reported oxidative stress-induced down-regulation of SUMO3 at the transcription level, while Yang et al.25 observed activation of SUMO2/3 conjugation as a protective response following brain ischemia/stroke. Our results, together with previous reports, suggest B12 induces the expression of SUMO3 at both mRNA and protein levels and protects cells from H2O2-induced oxidative damage, further linking sumoylation to neuroprotection. Mejia et al.26 reported that Sam68 (KHDRBS1) is recruited into stress granules in response to oxidative stress. In summary, our results provide valuable information on proteins involved in oxidative stress and mediating against oxidative damage.
Interaction networks are useful for studying complex systems in multiple disciplines including economics, sociology and biology27 and can assist the interpretation of the results of proteomics studies. Analysis of proteomics data at the pathway level has become increasingly popular, as demonstrated by Borroto-Escuela et al.28 who used network analysis to study molecular integration in G protein-coupled receptors (GPCRs) to elucidate the overall architecture of GPCR heteromers. Functional network analysis combined with gene ontology revealed that the vast majority of the significantly differentially expressed proteins identified in the present study were involved in cellular macromolecule metabolic process, cellular metabolic process, nucleic acid metabolic process, cellular component organization and gene expression categories (Fig. 4; Table S2). It was reported that both triethylene glycol dimethacrylate (TEGDMA) and 2-hydroxyethyl methacrylate (HEMA) were able to activate the coordinated network of the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI-3K) pathways29. In another study, some of the activated proteins detected functioned in signal transduction pathways associated with the regulation of specific transcription factors14. In the present study, the key regulatory protein PTBP1 was associated with cellular macromolecule metabolic process, nucleic acid metabolic process and gene expression biological process categories, consistent with an important role in the neuroprotective effects of B12. Our network analysis and gene ontology results also highlighted other biological processes that are involved in oxidative stress and the anti-oxidative response. Our results provide information on the mechanism underlying the response of cells to H2O2-induced oxidative stress and this information may prove useful for the development of treatments for neurodegenerative diseases, although further studies are required to determine the exact mechanism.
Conclusions
The present study indicated that B12 (at concentrations higher than 2 μM) can protect cells from H2O2-induced cell damage by inducing specific cellular responses at the protein level. Of the differentially expressed proteins, the pre-mRNA processing protein PTBP1 was found to be pivotal. Functional network analysis and gene ontology indicated the neuroprotective effects of B12 are associated with the metabolism of macromolecules and other cellular metabolic processes. Our results provide information on the mechanisms underlying the cellular response to H2O2-induced oxidative stress, which may prove to be useful for treating chronic neurodegenerative diseases such as Parkinson’s and Alzheimer’s.
Materials and Methods
Materials
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS), ammonium bicarbonate, sodium deoxycholate, iodoacetamide and dithiothreitol were purchased from Sigma (St. Louis, MO, USA). Tris-(2-carboxyethyl) phosphine was acquired from Thermo Scientific (Rockford, Il, USA). Modified sequencing-grade trypsin was obtained from Promega (Madison, WI, USA). All mobile phases and solutions were prepared with HPLC grade solvents (i.e. water, acetonitrile, methanol and formic acid) from Sigma Aldrich. All other reagents were from commercial suppliers and of standard biochemical quality.
Cell culture and treatment
Cell culture: Human neuroblastoma SH-SY5Y cells were maintained in minimum essential medium (MEM) supplemented with 10% fetal calf serum, 100 U/ml penicillin and 100 U/ml streptomycin in a humidified atmosphere with 5% CO2 at 37 °C.
H2O2 treatment: SH-SY5Y cells were placed in plates and pretreated with various concentrations (200, 400, 500, 600, 800, 1000 and 2000 μM) of H2O2 for 6 h. Control cells were grown in MEM containing 0.5% fetal calf serum, but without H2O2.
B12 treatment: SH-SY5Y cells were placed in plates and pretreated with various concentrations (0.2, 2, 20 and 200 μM) of B12 for 12 h, followed by exposure to 200 μM of H2O2 for another 24 h. To induce oxidative stress, H2O2 was freshly prepared from 30% stock solution prior to each experiment. Control cells were grown in MEM containing 0.5% fetal calf serum, but without H2O2 and B12.
Analysis of cell viability
SH-SY5Y cells were treated and cell viability was determined using the conventional MTS reduction assay. Briefly, after treatment, 40 μl of MTS (2 mg/ml in PBS) was added to each well and cells were incubated at 37 °C for 4 h. Supernatants were aspirated carefully and the absorbance at 490 nm was measured (in triplicate) with a microplate reader (BIO-RAD Model 3550, CA, USA). Experiments were repeated three times.
Sample preparation for shotgun analysis
In this study, cells treated with 200 μM H2O2 and no B12 were chosen as the control group (H2O2 treated group). Cells treated with 200 μM H2O2 and 20 μM B12 comprised the H2O2 + B12 treatment group. After determination of the density of the harvested SH-SY5Y cells, aliquots containing 1000 to 10000 cells were collected and placed in individual tubes. Cell pellets were flash frozen in liquid nitrogen and stored at −80 °C. Proteins were extracted with RIPA lysis buffer and each group was analyzed in triplicate. A workflow flowchart is shown in Fig. S1. Protein samples (200 μg) from each group were processed according to the manufacturer’s protocol for filter-aided sample preparation (FASP)30. Proteins were concentrated using Vivacon 500 filtration tubes (Cat No. VNO1HO2, Sartorius Stedim Biotech, UK), mixed with 100 μL of 8 M urea in 0.1 M Tris/HCL (pH 8.5) and centrifuged at 14000 g for 15 min at 20 °C. This step was performed twice, after which 10 μL of 0.05 M Tris-(2-carboxyethyl) phosphine (TCEP) in water was added to the filters and samples were incubated at 37 °C for 1 h. Then, 10 μL of 0.1 M iodoacetamide (IAA) was added to the filters, after which the samples were incubated in darkness for 30 min. Filters were washed twice with 200 μL of 50 mM NH4HCO3. Finally, 4 μg of trypsin (Promega, Madison, WI) in 100 μL of 50 mM NH4HCO3 was added to each filter. The protein to enzyme ratio was 50:1. Samples were incubated overnight at 37 °C and released peptides were collected by centrifugation.
High pH reverse phase chromatography was performed using the Dionex Ultimate 3000 Micro Binary HPLC Pump system31. The digested peptide mixture was reconstituted with 600 μL buffer A (20 mM ammonium formate in water, pH 10) and loaded onto a 2.1 mm × 150 mm Waters BEH130 C-18 column containing 3.5 μm particles. Peptides were eluted at a flow rate of 230 μL/min with a gradient of 5% buffer B (20 mM ammonium formate in 80% acetonitrile, pH 10) for 5 min, 5% to 15% buffer B for 15 min, 15% to 25% buffer B for 10 min, 25% to 55% buffer B for 10 min and 55% to 95% buffer B for 5 min. The system was then maintained in 95% buffer B for 5 min before equilibrating with 5% buffer B for 10 min prior to the next injection. Elution was monitored by measuring the absorbance at 214 nm and fractions were collected every 2 min. Fractions containing eluted peptides were pooled into 15 fractions based on peptide density31 and vacuum-dried for nano-ESI-LC-MS/MS analysis.
LC-MS/MS analysis
LC-MS experiments were performed on a nano-flow HPLC system (Easy-nLC II, Thermo Fisher Scientific, Waltham, MA, USA) connected to a LTQ-Orbitrap Velos Pro (Linear quadrupole ion trap-Orbitrap mass analyser) mass spectrometer (Thermo Fisher Scientific), equipped with a Nanospray Flex Ion Source (Thermo Fisher Scientific). Peptide mixtures (5 μL) were injected at a flow rate of 5 μL/min onto a pre-column (Easy-column C18-A1, 100 μm I.D. × 20 mm, 5 μm, Thermo Fisher Scientific). Chromatographic separation was performed on a reversed phase C18 column (Easy-column C18-A2, 75 μm I.D. × 100 mm, 3 μm, Thermo Fisher Scientific) at a flow rate of 300 nL/min with a 60 min gradient of 2% to 40% acetonitrile in 0.1% formic acid. The electrospray voltage was maintained at 2.2 kV and the capillary temperature was set at 250 °C. The LTQ-Orbitrap was operated in data-dependent mode to simultaneously measure full scan MS spectra (m/z 350–2000) in the Orbitrap with a mass resolution of 60,000 at m/z 400. After full-scan survey, the 15 most abundant ions detected in the full-MS scan were measured in the LTQ-Orbitrap using collision-induced dissociation (CID).
Protein identification and quantitation
Data analysis was performed with MaxQuant software32 version 1.4.1.2 (http://www.maxquant.org/). For protein identification, MS/MS data were submitted to the UniProt human protein database (release 3.43, 72,340 sequences) using the Andromeda search engine33 with the following settings: trypsin cleavage; fixed modification of carbamidomethylation of cysteine; variable modifications of methionine oxidation; a maximum of two missed cleavages; false discovery rate calculated by searching the decoy database. Other parameters were set as default. The results were imported into Microsoft excel for further analysis.
Label-free quantitation (LFQ) was also performed in MaxQuant. The Minimum ratio count for LFQ was set to 2 and the match-between-runs option was enabled. Other parameters were set as default. Up- and down-regulated proteins were defined based on a significantly altered protein ratio (p-values < 0.01). Significance p-values were calculated using Perseus software (version 1.4.1.3; http://www.perseus-framework.org). A 2-fold change in expression and a p-value of 0.01 were used as a combined threshold to define biologically regulated proteins.
Network analysis
Protein-protein interaction networks were constructed from data with or without B12 treatment using the BisoGenet34 plugin in the Cytoscape environment. Differentially expressed proteins were used as initial query terms. Experimentally supported hyperlinks from DIP, BIOGRID, HPRD, BIND, MINT and INTACT were then automatically connected using the SysBiomics platform and visualized in Cytoscape. The MCODE (version 1.4.0-beta2) plugin was used to find clusters (highly interconnected regions) in the resulting networks35. The search parameters were set as follows: network scoring degree cut-off = 2; cluster finding node score cut-off = 0.2; k-core = 2; maximum depth = 100; singly connected nodes were removed from clusters. The BiNGO plugin36 in the Cytoscape environment was used to retrieve the Gene Ontology Consortium (GOC, http://geneontology.org/).
Western blotting
Treated cells (1 × 107 cells/10 ml in 100 mm dishes) were collected and washed with PBS. After centrifugation (1000rpm, 5 min, room temperature), cells were lysed in RIPA buffer (Applygen, Beijing, China) and protein concentration was determined by BCA assay (Pierce, Thermo Fisher Scientific, MA, USA). After the addition of sample loading buffer, protein samples were separated using 12% SDS-PAGE and subsequently transferred to a nitrocellulose membrane (Amersham Pharmacia Biotech, UK). The membrane was incubated in fresh blocking buffer (0.1% Tween 20 in Tris-buffered saline, pH 7.4, containing 5% non-fat dried milk) at room temperature for 30 min and then probed with monoclonal mouse anti-PTBP1 antibody (Abcam, Cambridge, UK) and mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Shanghai Kangchen Biotechnology Co. Ltd, China) in blocking buffer at 4 °C overnight. The membrane was washed three times for 5 min each using PBST (PBS containing 0.1% Tween-20), incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody at room temperature for 1 h and washed three more times in PBST buffer. The membrane was finally incubated with ECL substrate solution (Shanghai Pufei Biotechnology Co., Ltd, China) for 5 min according to the manufacturer’s instructions and visualized with autoradiographic film.
Down-regulation of PTBP1 by siRNA
PTBP1 small interfering RNA (PTBP1 siRNA; Abnova) and negative control siRNA were transiently transfected into SH-SY5Y cells using siRNA transfection medium (Opti-MEM) and siRNA transfection reagent (lipofectin 2000) according to the manufacturer’s instructions. Primer sequences were as follows: Sense = CCUCUAGAGUGAU (ccacatcc); Antisense = (atgtgg) AUCACUCUAGAGGGG. In brief, 2 × 106 cells were seeded in 10 cm plates in fresh culture medium and cultured for 24 h. Transfection medium containing siRNA and siRNA transfection reagent was mixed with non-FBS culture medium and added to cells. Culture medium was replaced after 12 h and cells were incubated for further 72 h. Cells were collected and lysed and PTBP1 expression was then measured. To assess cell viability, cells were incubated for a further 36 h after transfection and treatment with B12 and H2O2.
Statistical analysis
Results are expressed as mean ± S.E.M. Statistical evaluation was performed using the Student’s t-test (for comparing two value sets). P < 0.05 was considered statistically significant (*P < 0.05: **P < 0.01).
Data availability
Mass spectrometry proteomics data have been deposited at the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the MassiVE partner repository with the dataset identifier PXD003391.
Additional Information
How to cite this article: Zhong, L. et al. Quantitative proteomics study of the neuroprotective effects of B12 on hydrogen peroxide-induced apoptosis in SH-SY5Y cells. Sci. Rep. 6, 22635; doi: 10.1038/srep22635 (2016).
References
Finkel, T. & Holbrook, N. J. Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247 (2000).
Markesbery, W. R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radical Bio Med 23, 134–147 (1997).
Barnham, K. J., Masters, C. L. & Bush, A. I. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 3, 205–214 (2004).
Gorman, A., McGowan, A., O’Neill, C. & Cotter, T. Oxidative stress and apoptosis in neurodegeneration. J Neurol Sci 139, 45–52 (1996).
Zhang, L. et al. Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur J Pharmacol 564, 18–25 (2007).
Rhee, S. G. Redox signaling: hydrogen peroxide as intracellular messenger. Exp Mol Med 31, 53–59 (1999).
Wasinger, V. C. et al. Progress with gene—product mapping of the Mollicutes: mycoplasma genitalium. Electrophoresis 16, 1090–1094 (1995).
Wilkins, M. R. et al. Progress with proteome projects: why all proteins expressed by a genome should be identified and how to do it. Biotechnol Genet Eng Rev 13, 19–50 (1996).
Walther, T. C. & Mann, M. Mass spectrometry-based proteomics in cell biology. J Cell Biol 190, 491–500 (2010).
Ono, M. et al. Label-free quantitative proteomics using large peptide data sets generated by nanoflow liquid chromatography and mass spectrometry. Mol Cell Proteomics 5, 1338–1347 (2006).
Altelaar, A. M., Munoz, J. & Heck, A. J. Next-generation proteomics: towards an integrative view of proteome dynamics. Nat Rev Genet 14, 35–48 (2013).
Kingsmore, S. F. Multiplexed protein measurement: technologies and applications of protein and antibody arrays. Nat Rev Drug Discov 5, 310–321 (2006).
Aebersold, R. & Mann, M. Mass spectrometry-based proteomics. Nature 422, 198–207 (2003).
Ong, S. E. & Mann, M. Mass spectrometry–based proteomics turns quantitative. Nat Chem Biol 1, 252–262 (2005).
Neilson, K. A. et al. Less label, more free: approaches in label-free quantitative mass spectrometry. Proteomics 11, 535–553 (2011).
Kim, S. S., Park, R. Y., Jeon, H. J., Kwon, Y. S. & Chun, W. Neuroprotective effects of 3,5-dicaffeoylquinic acid on hydrogen peroxide-induced cell death in SH-SY5Y cells. Phytother Res 19, 243–245 (2005).
Venuprasad, M. P., Hemanth Kumar, K. & Khanum, F. Neuroprotective effects of hydroalcoholic extract of Ocimum sanctum against H2O2 induced neuronal cell damage in SH-SY5Y cells via its antioxidative defence mechanism. Neurochem Res 38, 2190–2200 (2013).
Cote, G. J. et al. Hydrogen peroxide alters splicing of soluble guanylyl cyclase and selectively modulates expression of splicing regulators in human cancer cells. Plos One 7, e41099 (2012).
King, H. A. et al. Remodelling of a polypyrimidine tract-binding protein complex during apoptosis activates cellular IRESs. Cell Death Differ 21, 161–171 (2014).
Rodriguez-Aller, M., Gurny, R., Veuthey, J. L. & Guillarme, D. Coupling ultra high-pressure liquid chromatography with mass spectrometry: constraints and possible applications. J Chromatogr A 1292, 2–18 (2013).
Wu, Y. et al. Multilayered genetic and omics dissection of mitochondrial activity in a mouse reference population. Cell 158, 1415–1430 (2014).
Paul, D., Kumar, A., Gajbhiye, A., Santra, M. K. & Srikanth, R. Mass spectrometry-based proteomics in molecular diagnostics: discovery of cancer biomarkers using tissue culture. Biomed Res Int 2013, 783131 (2013).
Choi, S. et al. Targeted label-free quantitative analysis of secretory proteins from adipocytes in response to oxidative stress. Anal Biochem 401, 196–202 (2010).
Sang, J. et al. SUMO2 and SUMO3 transcription is differentially regulated by oxidative stress in an Sp1-dependent manner. Biochem J 435, 489–498 (2011).
Yang, W. et al. Small ubiquitin-like modifier 3-modified proteome regulated by brain ischemia in novel small ubiquitin-like modifier transgenic mice: putative protective proteins/pathways. Stroke 45, 1115–1122 (2014).
Henao-Mejia, J. & He, J. J. Sam68 relocalization into stress granules in response to oxidative stress through complexing with TIA-1. Exp Cell Res 315, 3381–3395 (2009).
Wu, X., Hasan, M. A. & Chen, J. Y. Pathway and network analysis in proteomics. J Theor Biol 362, 44–52 (2014).
Borroto-Escuela, D. O. et al. The G protein-coupled receptor heterodimer network (GPCR-HetNet) and its hub components. Int J Mol Sci 15, 8570–8590 (2014).
Krifka, S., Spagnuolo, G., Schmalz, G. & Schweikl, H. A review of adaptive mechanisms in cell responses towards oxidative stress caused by dental resin monomers. Biomaterials 34, 4555–4563 (2013).
Wiśniewski, J. R., Zougman, A., Nagaraj, N. & Mann, M. Universal sample preparation method for proteome analysis. Nat Methods 6, 359–362 (2009).
Yang, F., Shen, Y., Camp, D. G. & Smith, R. D. High-pH reversed-phase chromatography with fraction concatenation for 2D proteomic analysis. Expert Rev Proteomic 9, 129–134 (2012).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26, 1367–1372 (2008).
Cox, J. et al. Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res 10, 1794–1805 (2011).
Martin, A. et al. BisoGenet: a new tool for gene network building, visualization and analysis. BMC Bioinformatics 11, 91 (2010).
Bandettini, W. P. et al. MultiContrast Delayed Enhancement (MCODE) improves detection of subendocardial myocardial infarction by late gadolinium enhancement cardiovascular magnetic resonance: a clinical validation study. J Cardiovasc Magn R 14, 1–10 (2012).
Maere, S., Heymans, K. & Kuiper, M. BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 21, 3448–3449 (2005).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81270049).
Author information
Authors and Affiliations
Contributions
Y.P. constructed the compound B12. L.Z. performed the proteomic data acquisition and J.Z. performed the analysis of the proteomic data. Y.P., X.C. and Y.L. performed the cell culture and relevant cell treatment. Y.P., Y.Y. and L.Z. conceived the project. L.Z. and J.Z. wrote the paper. Y.P., D.L., X.Z. and B.Y. were involved in editing the paper. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhong, L., Zhou, J., Chen, X. et al. Quantitative proteomics study of the neuroprotective effects of B12 on hydrogen peroxide-induced apoptosis in SH-SY5Y cells. Sci Rep 6, 22635 (2016). https://doi.org/10.1038/srep22635
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep22635
This article is cited by
-
Integrated computational analysis reveals HOX genes cluster as oncogenic drivers in head and neck squamous cell carcinoma
Scientific Reports (2022)
-
Neuroprotective Effect of 3-[(4-Chlorophenyl)selanyl]-1-methyl-1H-indole on Hydrogen Peroxide-Induced Oxidative Stress in SH-SY5Y Cells
Neurochemical Research (2021)
-
The clinically approved antiviral drug sofosbuvir inhibits Zika virus replication
Scientific Reports (2017)
-
Integrated proteomics and metabolomics analysis of rat testis: Mechanism of arsenic-induced male reproductive toxicity
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.