Abstract
Tumor necrosis factor α (TNFα) is a major proinflammatory cytokine and its level is elevated in hypertensive states. Inflammation occurs in the kidneys during the development of hypertension. We hypothesized that TNFα specifically in the kidney contributes to the development of hypertension and renal injury in Dahl salt-sensitive (SS) rats, a widely used model of human salt-sensitive hypertension and renal injury. SS rats were chronically instrumented for renal interstitial infusion and blood pressure measurement in conscious, freely moving state. Gene expression was measured using real-time PCR and renal injury assessed with histological analysis. The abundance of TNFα in the renal medulla of SS rats, but not the salt-insensitive congenic SS.13BN26 rats, was significantly increased when rats had been fed a high-salt diet for 7 days (n = 6 or 9, p < 0.01). The abundance of TNFα receptors in the renal medulla was significantly higher in SS rats than SS.13BN26 rats. Renal interstitial administration of Etanercept, an inhibitor of TNFα, significantly attenuated the development of hypertension in SS rats on a high-salt diet (n = 7–8, p < 0.05). Glomerulosclerosis and interstitial fibrosis were also significantly ameliorated. These findings indicate intrarenal TNFα contributes to the development of hypertension and renal injury in SS rats.
Similar content being viewed by others
Introduction
Hypertension is a major public health issue1. Salt is one of the major environmental factors that contribute to the development of hypertension2. The Dahl salt-sensitive (SS) rat is the most commonly used polygenic animal model of human salt-sensitive hypertension and renal injury3.
Inflammation has been proposed as an important contributor to the development of hypertension, including salt-induced hypertension in SS rats. Circulating leukocyte counts were elevated significantly in SS rats fed a high-salt diet compared with Dahl salt-resistant rats4. Renal interstitial inflammation and infiltration of immune cells are prominent in SS rats treated with high-salt diets5,6. Pharmacological or genetic manipulations that inhibit inflammation and immune response reduce renal interstitial infiltration of immune cells and attenuate salt-induced hypertension and renal injury in SS rats as well as in other models7,8,9,10.
Tumor necrosis factor α (TNFα) is a major proinflammatory cytokine that has been reported to be elevated in hypertensive patients11,12. Systemic administration of Etanercept, a soluble recombinant fusion protein that blocks the functional effect of TNFα, attenuates the development of hypertension and renal injury in several models13,14,15.
It is not clear, however, whether TNFα specifically in the kidney contributes to hypertension. In the present study, we analyzed the expression levels of TNFα and its receptors in the kidneys of SS rats. We then examined the contribution of intrarenal TNFα to hypertension by infusing Etanercept directly and locally into the renal interstitium. Renal interstitial infusion or injection is a well-established experimental technique for administering agents specifically into the kidney16,17,18,19. The technique requires sophisticated chronic instrumentation when it is applied in conscious animals.
Methods
Animals
The animal experiments were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal protocols were approved by the Institutional Animal Care and Use Committee at the Medical College of Wisconsin (AUA206). Inbred SS rats and congenic SS.BN-(D13Rat151-D13Rat197)/Mcwi (also called SS.13BN26) rats with reduced blood pressure salt-sensitivity were produced at the Medical College of Wisconsin. Rats were on a 12:12 h light-dark cycle and had unlimited access to water. Male SS and SS.13BN26 rats were maintained on the AIN-76A diet containing 0.4% NaCl as described previously20,21,22,23,24,25.
Real-time PCR
Kidney tissues were collected, RNA extracted, and real-time PCR performed as described previously22,26,27,28. 18S rRNA was used as internal normalizer. Primer sequences are shown in Table 1.
Chronic instrumentation for renal interstitial infusion and blood pressure measurement
Chronic instrumentation of rats for renal interstitial infusion and blood pressure measurement was performed as we described previously24,28,29,30,31. Briefly, 6 weeks old SS rats underwent uninephrectomy to remove the right kidney, allowing the remaining kidney to be the sole determinant of renal function of the rat. Following one week of recovery, an infusion catheter was implanted into the interstitium of the remaining left kidney and another catheter was implanted into the left femoral artery. Both catheters were exteriorized from the back of the neck and connected to an infusion pump or a blood pressure transducer using a swivel. This preparation allows chronic renal interstitial infusion and arterial blood pressure measurement in conscious, freely moving rats. Rats were allowed another week to recover before the initiation of daily blood pressure recording through the arterial catheter. After a stable baseline of blood pressure was obtained for 3 days, continuous renal interstitial infusion of TNFα inhibitor Etanercept (Enbrel, Amgen, Thousand Oaks, CA) at a dose of 0.25 mg/kg/day, or saline control, was initiated. After another 3 days, rats were switched from the 0.4% NaCl diet to a 4% NaCl diet (Dyets). Interstitial infusion and blood pressure measurement continued for another 8 days before the rats were euthanized and tissues collected.
Histological analysis
The kidneys were fixed in 10% phosphate-buffered formalin for 24 hours at room temperature, and then embedded in paraffin. Sections were prepared with 3 μm thickness and stained with Masson’s trichrome staining. All the slides were scanned by Nanozoomer digital pathology and were analyzed by Metamorph offline version 7.7.0.0 software. Glomeruli (90 to 120 per slide) were evaluated (scored from 0 to 4) on the basis of the degree of glomerulosclerosis as previously described32. Interstitial fibrosis, tubular casts, and urinary protein and albumin excretion was analyzed as we described previously33,34,35.
Immunohistochemistry
Immunohistochemistry was performed in formalin-fixed, paraffin-embedded kidney sections from SS rats (n = 6–9), following procedures similar to what we described previously33,34,35. The primary antibodies, all from Abcam, and the dilution used were ab6671 for TNFα, 1:100, ab19139 for TNF receptor I, 1:1500, and ab109322 for TNF receptor II, 1:100.
Statistics
Student t-test or 2-way RM ANOVA was used to analyze data. P < 0.05 was considered significant. Data are shown as mean ± SEM.
Results
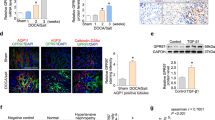
The expression of TNFα and its receptors in the renal medulla of SS rats were examined using real-time PCR. Congenic SS.13BN26 rats, which we have shown to exhibit a lower level of blood pressure salt-sensitivity compared to SS rats25,36, were used as salt-insensitive control. TNFα abundance increased significantly in SS rats treated with 4% (n = 9) or 8% (n = 6) NaCl diet for 7 days, and this change occurred between 3 and 7 days of 8% NaCl diet (Fig. 1A,B). TNFα abundance was not altered by 7 days of 4% NaCl diet in SS.13BN26 rats (Fig. 1A). The mRNA abundance of TNFα receptors Tnfrsf1A and Tnfrsf1B was significantly higher in SS rats than in SS.13BN26 rats (Fig. 1C,D). However, treatment with the 4% NaCl diet for 7 days did not significantly alter the mRNA abundance of Tnfrsf1A or Tnfrsf1B in either rat strain. Immunohistochemistry analysis in the renal medulla of SS rats indicated the presence of TNFα protein in tubular cells and possibly infiltrating inflammatory cells, particularly after the high-salt diet treatment (Fig. 1E). TNF receptor II, encoded by Tnfrsf1B, was detectable in tubular cells in the renal medulla, while TNF receptor I, encoded by Tnfrsf1A, was detectable only in tubules in rats after the high-salt diet treatment.
(A) TNFα mRNA abundance in SS rats increased significantly when treated with a 4% NaCl diet for 7 days compared with SS rats on a 0.4% NaCl diet (n = 9, **P < 0.01) or salt-insensitive SS.13BN26 rats (#P < 0.01). (B) TNFα abundance in SS rats increased significantly after 8% NaCl diet for 7 days (n = 6, **P < 0.01 vs. 0.4% NaCl diet; *P < 0.05 vs. 3 days of 8% NaCl diet). (C,D) Abundance of TNFα receptors Tnfrsf1A and Tnfrsf1B were significantly higher in SS rats than in SS.13BN26 rats (n = 9, #P < 0.01 vs. SS). (E) Immunohistochemistry analysis. Representative images of renal medulla regions from SS rats maintained on the 0.4% NaCl diet or fed the 4% NaCl diet for 7 days (n = 6–9) are shown. The primary antibody was omitted in negative control. Brown color indicates positive staining. The arrow points to cells that appear to be infiltrating inflammatory cells. Scale bar is 50 μm.
We examined if intrarenal TNFα contributed to hypertension in SS rats by treating uninephrectomized SS rats with renal interstitial infusion of the TNFα inhibitor Etanercept. Uninephrectomy made the remaining kidney receiving Etanercept the sole determinant of whole body renal function. Renal interstitial administration of Etanercept for 3 days while the rats were maintained on the 0.4% NaCl diet did not have a significant effect on mean arterial pressure. However, Etanercept significantly attenuated hypertension after the rats were switched to the 4% NaCl diet (n = 7–8, P < 0.05 vs. saline control on days 3 to 8 on the 4% NaCl diet, two-way RM ANOVA followed by Holm-Sidak test) (Fig. 2). The effect of Etanercept reached 22 mmHg at 8 days after the rats were switched to the high-salt diet.
Uninephrectomized SS rats received continuous renal interstitial infusion of saline vehicle or Etanercept (0.25 mg/kg/day) and were fed a 0.4% or 4% NaCl diet for the indicated durations. Mean arterial blood pressure (MAP) was measured in conscious, freely moving rats using indwelling femoral arterial catheter. N = 7–8, *p < 0.05 vs. vehicle-treated rats.
SS rats develop substantial renal injury in addition to hypertension, a characteristic that resembles hypertensive African Americans28. Renal interstitial administration of Etanercept significantly reduced the percentage of glomeruli that were severely damaged and the percent area of the renal cortex that were fibrotic (Fig. 3A–C). The Etanercept treatment did not significantly change the percent area of kidney sections occupied by tubular casts or 24 hour urinary excretion of albumin or protein (Fig. 3D,E).
(A) Percent of glomeruli that were severely damaged (scored 4). (B) Percent of renal cortical area that was fibrotic. (C) Representative images of kidney sections. Arrows point to injured glomeruli. (D) Percent of kidney section area occupied by tubular casts. E. Urinary 24 hour excretion of albumin (Ualb) or total protein (Uprot). N = 4–8, *P < 0.05 vs. vehicle-treated rats.
Discussion
The major new finding of the present study was that administration of Etanercept directly into the renal interstitium significantly attenuated salt-induced hypertension in SS rats. The anti-hypertensive effect of Etanercept that we observed is likely the consequence of TNFα inhibition specifically in the kidney, rather than any systemic inhibition of TNFα by any Etanercept spilled over into the systemic circulation. Experiments using radiolabeled compounds have demonstrated that compounds infused into the renal interstitium largely stayed in the infused kidney37. Even if a small fraction of Etanercept did spill over into the systemic circulation, the spill-over is unlikely to account for the blood pressure effect we observed. The doses of Etanercept used for systemic administration are typically 5 to 10 times higher than the entire dose infused into the renal interstitium in the present study13,14,38,39. Note though the renal interstitial administration was used primarily to address mechanistic questions regarding the contribution of the kidney, not as a therapeutic approach.
Prior to the present study, it was not clear whether TNFα specifically in the kidney played a significant role in the development of hypertension. Several studies have reported that systemic treatment with TNFα inhibitor Etanercept altered the progression of hypertension13,40. However, it was not clear whether it was systemic or local TNFα that was involved. Moreover, whether TNFα contributes to increasing or, in some cases, decreasing blood pressure appears to depend on the level of TNFα that is present41. The findings of the current study suggest it would be important to consider levels of TNFα in the kidney locally. TNFα is unlikely to be the only proinflammatory cytokine involved. It is important to consider the role of other proinflammatory factors.
Previous studies have reported an increase of TNFα in the kidneys of SS rats after 5 weeks of 4% NaCl diet42. Our data indicated that up-regulation of renal TNFα in SS rats occurred early within 7 days of exposure to a high-salt diet. TNFα in the kidney could come from several sources including macrophages and lymphocytes that have infiltrated the kidney, resident cells in the kidney, and circulating TNFα. TNFα produced by resident epithelial cells in the kidney may play a physiological role in regulating electrolyte homeostasis43. In the present study, TNFα was detected in tubular cells and possibly infiltrating inflammatory cells in the renal medulla of SS rats particularly after the high-salt diet treatment, although relative contribution of each cell type remains to be determined. We do not know whether or how much TNFα released from the renal medulla contributes to the increase in systemic TNFα. The downstream mechanism by which renal TNFα contributes to hypertension in SS rats also remains to be investigated.
Additional Information
How to cite this article: Huang, B. et al. Renal Tumor Necrosis Factor α Contributes to Hypertension in Dahl Salt-Sensitive Rats. Sci. Rep. 6, 21960; doi: 10.1038/srep21960 (2016).
References
Egan, B. M., Zhao, Y. & Axon, R. N. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA 303, 2043–2050 (2010).
Pimenta, E. et al. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension 54, 475–481 (2009).
Rapp, J. P. Dahl salt-susceptible and salt-resistant rats. A review. Hypertension 4, 753–763 (1982).
Shen, K., DeLano, F. A., Zweifach, B. W. & Schmid-Schonbein, G. W. Circulating leukocyte counts, activation, and degranulation in Dahl hypertensive rats. Circ Res 76, 276–283 (1995).
Siegel, A. K. et al. Genetic linkage of albuminuria and renal injury in Dahl salt-sensitive rats on a high-salt diet: comparison with spontaneously hypertensive rats. Physiol Genomics 18, 218–225 (2004).
Mattson, D. L. Infiltrating Immune Cells in the Kidney in Salt-Sensitive Hypertension and Renal Injury. Am J Physiol Renal Physiol 307, 499–508 (2014).
Rodriguez-Iturbe, B. et al. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int 59, 2222–2232 (2001).
Rudemiller, N. et al. CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension 63, 559–564 (2014).
Mattson, D. L. et al. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol. 304, 407–414 (2013).
Mattson, D. L., James, L., Berdan, E. A. & Meister, C. J. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 48, 149–156 (2006).
Bautista, L. E., Vera, L. M., Arenas, I. A. & Gamarra, G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens 19, 149–154, (2005).
Cottone, S. et al. Comparison of tumour necrosis factor and endothelin-1 between essential and renal hypertensive patients. J Hum Hypertens 12, 351–354 (1998).
Elmarakby, A. A., Quigley, J. E., Pollock, D. M. & Imig, J. D. Tumor necrosis factor alpha blockade increases renal Cyp2c23 expression and slows the progression of renal damage in salt-sensitive hypertension. Hypertension 47, 557–562 (2006).
Guzik, T. J. et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204 (2007).
Tran, L. T., MacLeod, K. M. & McNeill, J. H. Chronic etanercept treatment prevents the development of hypertension in fructose-fed rats. Mol Cell Biochem 330, 219–228 (2009).
Gross, J. M., Berndt, T. J. & Knox, F. G. Effect of serotonin receptor antagonist on phosphate excretion. J Am Soc Nephrol: JASN 11, 1002–1007 (2000).
Li, N., Chen, L., Yi, F., Xia, M. & Li, P. L. Salt-sensitive hypertension induced by decoy of transcription factor hypoxia-inducible factor-1alpha in the renal medulla. Circ Res 102, 1101–1108 (2008).
Kemp, B. A. et al. AT(2) receptor activation induces natriuresis and lowers blood pressure. Circ Res 115, 388–399 (2014).
Kassab, S., Novak, J., Miller, T., Kirchner, K. & Granger, J. Role of endothelin in mediating the attenuated renal hemodynamics in Dahl salt-sensitive hypertension. Hypertension 30, 682–686 (1997).
He, X. et al. Ultrastructure of mitochondria and the endoplasmic reticulum in renal tubules of Dahl salt-sensitive rats. Am J Physiol Renal Physiol 306, 1190–1197 (2014).
Cowley, A. W., Jr. et al. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension 37, 456–461 (2001).
Liang, M. et al. Insights into Dahl salt-sensitive hypertension revealed by temporal patterns of renal medullary gene expression. Physiol Genomics 12, 229–237 (2003).
Liu, Y., Liu, P., Yang, C., Cowley, A. W., Jr. & Liang, M. Base-resolution maps of 5-methylcytosine and 5-hydroxymethylcytosine in Dahl S rats: effect of salt and genomic sequence. Hypertension 63, 827–838 (2014).
Liu, Y., Singh, R. J., Usa, K., Netzel, B. C. & Liang, M. Renal medullary 11 beta-hydroxysteroid dehydrogenase type 1 in Dahl salt-sensitive hypertension. Physiol Genomics 36, 52–58 (2008).
Lu, L. et al. Dynamic convergence and divergence of renal genomic and biological pathways in protection from Dahl salt-sensitive hypertension. Physiol Genomics 41, 63–70 (2010).
Liang, M. & Pietrusz, J. L. Thiol-related genes in diabetic complications: a novel protective role for endogenous thioredoxin 2. Arterioscler Thromb Vasc Biol. 27, 77–83 (2007).
Liu, Y. et al. Suppression of 11beta-hydroxysteroid dehydrogenase type 1 with RNA interference substantially attenuates 3T3-L1 adipogenesis. Physiol Genomics 32, 343–351 (2008).
Liu, Y. et al. Renal medullary microRNAs in Dahl salt-sensitive rats: miR-29b regulates several collagens and related genes. Hypertension 55, 974–982 (2010).
Mattson, D. L., Lu, S., Nakanishi, K., Papanek, P. E. & Cowley, A. W., Jr. Effect of chronic renal medullary nitric oxide inhibition on blood pressure. Am J Physiol 266, 1918–1926 (1994).
Usa, K. et al. Renal interstitial corticosterone and 11-dehydrocorticosterone in conscious rats. Am J Physiol Renal Physiol. 293, 186–192 (2007).
Tian, Z. et al. Novel role of fumarate metabolism in dahl-salt sensitive hypertension. Hypertension 54, 255–260 (2009).
Raij, L., Azar, S. & Keane, W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 26, 137–143 (1984).
Kriegel, A. J., Liu, Y., Cohen, B., Usa, K. & Liang, M. MiR-382 targeting of kallikrein 5 contributes to renal inner medullary interstitial fibrosis. Physiol Genomics 44, 259–267 (2012).
Mori, T. et al. High perfusion pressure accelerates renal injury in salt-sensitive hypertension. J Am Soc Nephrol: JASN 19, 1472–1482 (2008).
Wang F. et al. Antithrombin III/SerpinC1 insufficiency exacerbates renal ischemia/reperfusion injury. Kidney Int. 88, 796–803 (2015).
Feng, D. et al. Increased expression of NAD(P)H oxidase subunit p67(phox) in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metab 15, 201–208 (2012).
Lu, S., Roman, R. J., Mattson, D. L. & Cowley, A. W., Jr. Renal medullary interstitial infusion of diltiazem alters sodium and water excretion in rats. Am J Physiol 263, 1064–1070 (1992).
Muller, D. N. et al. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol 161, 1679–1693 (2002).
Elmarakby, A. A. et al. TNF-alpha inhibition reduces renal injury in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 294, 76–83 (2008).
Venegas-Pont, M. et al. Tumor necrosis factor-alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension 56, 643–649 (2010).
Ramseyer, V. D. & Garvin, J. L. Tumor necrosis factor-alpha: regulation of renal function and blood pressure. Am J Physiol Renal Physiol. 304, 1231–1242 (2013).
Gu, J. W. et al. Renal NF-kappaB activation and TNF-alpha upregulation correlate with salt-sensitive hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 291, 1817–1824 (2006).
Battula, S. et al. Tumor necrosis factor-alpha is an endogenous inhibitor of Na+-K+-2Cl- cotransporter (NKCC2) isoform A in the thick ascending limb. Am J Physiol Renal Physiol. 301, 94–100 (2011).
Acknowledgements
This work was supported by US National Institutes of Health grants HL121233, HL082798, and HL116264. BH was supported in part by the China Scholarship Council.
Author information
Authors and Affiliations
Contributions
B.H., Y.C. and K.U. performed experiments. Y.L., M.A.B., D.L.M. and Y.H. assisted with the experiments. B.H. and Y.C. analyzed the data. B.H. and N.W. participated in study design and data interpretation. M.L. conceived and designed the study. B.H. and M.L. drafted the manuscript. All authors provided input to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Huang, B., Cheng, Y., Usa, K. et al. Renal Tumor Necrosis Factor α Contributes to Hypertension in Dahl Salt-Sensitive Rats. Sci Rep 6, 21960 (2016). https://doi.org/10.1038/srep21960
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep21960
This article is cited by
-
Immune and inflammatory mechanisms in hypertension
Nature Reviews Cardiology (2024)
-
Treatment of male and female spontaneously hypertensive rats with TNF-α inhibitor etanercept increases markers of renal injury independent of an effect on blood pressure
Biology of Sex Differences (2022)
-
TNFα Triggers an Augmented Inflammatory Response in Brain Neurons from Dahl Salt-Sensitive Rats Compared with Normal Sprague Dawley Rats
Cellular and Molecular Neurobiology (2022)
-
Adaptive Immunity in Hypertension
Current Hypertension Reports (2019)
-
Pro-inflammatory Cytokines and Resistant Hypertension: Potential for Novel Treatments?
Current Hypertension Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.