Abstract
Osteoarthritis is a common and progressive joint disorder. Despite its widespread, in clinical practice only late phases of osteoarthritis that are characterized by severe joint damage are routinely detected. Since osteoarthritis cannot be cured but relatively well managed, an early diagnosis and thereby early onset of disease management would lower the burden of osteoarthritis. Here we evaluated if biophysical parameters of small synovial fluid samples extracted by single molecule microscopy can be linked to joint damage. In healthy synovial fluid (ICRS-score < 1) hyaluronan showed a slower diffusion (2.2 μm2/s, N = 5) than in samples from patients with joint damage (ICRS-score > 2) (4.5 μm2/s, N = 16). More strikingly, the diffusion coefficient of hyaluronan in healthy synovial fluid was on average 30% slower than expected by sample viscosity. This effect was diminished or missing in samples from patients with joint damage. Since single molecule microscopy needs only microliters of synovial fluid to extract the viscosity and the specific diffusion coefficient of hyaluronan this method could be of use as diagnostic tool for osteoarthritis.
Similar content being viewed by others
Introduction
Osteoarthritis is the most common joint disorder and a major cause of disability1. It is estimated that 10–15% of the worldwide population over 60 has some degree of osteoarthritis2.
Osteoarthritis is a progressive multifactorial disease, marked by a breakdown of the articular cartilage by heterogeneous causes including abnormal metabolic processes, mechanical injury, joint instability, excessive overweight, peripheral neuropathy and ageing3. Lubrication of healthy joints is provided by the interplay of articular cartilage at the bone ends in combination with the synovial fluid in between4. This composition provides remarkably low friction and low wear5,6. The breakdown of the lubricating properties leads to articular cartilage degradation, osteophyte formation, subchondral sclerosism, meniscal degeneration, bone marrow lesions and synovial proliferation3,7. Patients affected by osteoarthritis suffer from pain, limitations of movement and ultimately loss of joint function. Currently, there is no cure for osteoarthritis available, except total knee replacement, which still leads to loss of joint function8. The symptoms of osteoarthritis can still be managed and progression usually can be slowed down through patient education, weight loss, muscle training and hyaluronan injections9.
An obstacle to disease management strategies is the diagnosis of osteoarthritis7,10. Currently osteoarthritis diagnosis uses radiographic criteria and clinical symptoms11. However, by that only late stages of osteoarthritis are recognized. Therapies that aim to slow down disease progression would benefit from early diagnosis that enables an early onset of disease management.
It is projected that in 2050, 130 million people worldwide will suffer from osteoarthritis and 40 million people will be disabled by osteoarthritis12. This illustrates the increasing burden of osteoarthritis on communities, healthcare systems and the economy.
Next to finding a cure, strong efforts are made to find biomarkers that allow early diagnosis of osteoarthritis and cost effective long-term monitoring of patients. Imaging techniques like quantitative MRI are able to provide better diagnosis of early osteoarthritis, but costs and availability are limiting its widespread use7. Therefore, there is currently an unmet need for biochemical or biophysical markers that can be measured in blood, urine or synovial fluid samples11.
It is known that the overall viscosity of synovial fluid decreases in osteoarthritis. Therefore, the viscosity of synovial fluid was thought to be a potential biomarker for osteoarthritis. However, the viscosity of synovial fluid shows in general a large variance; therefore, its values can be misleading13.
Analyzing the rheological properties at a microscopic scale showed that HA forms a dynamic network in synovial fluid14. The existence of a dynamic network in synovial fluid is also supported by studies showing that interplay between the major macromolecular molecules of synovial fluid hyaluronan, type II collagen fibrils and PRG4 is needed for efficient lubrication15,16. In a previous study, also using single molecule microscopy, a significant reduction of HA diffusion speed could be detected in synovial fluid from a patient without a prior history of osteoarthritis. In contrast, in synovial fluid from a patient with advanced osteoarthritis there was no reduction of HA diffusion speed17. These results suggest a loss of hyaluronan interactions in synovial fluid during osteoarthritis that can be detected via single molecule microscopy.
Single molecule microscopy is a very powerful technique to probe the properties of a single molecular entity such as hyaluronan in complex biological environments using selective labeling. Single molecule microscopy provides a high spatial and temporal resolution. This method allows extracting even coarse structural information of single molecules. By using this method, it could be confirmed that hyaluronan molecules form random coil structures in buffer solution17,18.
Here we measured the diffusion coefficient of fluorescent hyaluronan (fl-HA) and compared it to the diffusion of fluorescent dextran (fl-dextran), an inert tracer, in human synovial fluid. We analyzed synovial fluid samples of patients without joint damage (w/o-JD) and with joint damage (w/-JD).
The diffusion coefficient of spherical objects depends on its hydrodynamic radius, the viscosity of the surrounding medium and temperature. The Stokes-Einstein equation describes the dependency of diffusion coefficient and these three variables (see M&M) eq. 2). Higher viscosities or larger hydrodynamic radii result in a lower diffusion coefficient. Higher temperatures result in a higher diffusion coefficient.
The diffusion coefficient of fl-dextran corresponds to the viscosity of the sample17. For hyaluronan it is known that it can undergoes interactions in synovial fluid. The hydrodynamics radius of such conjugates would be larger and therefore its diffusion coefficient smaller.
Single molecule microscopy observation of tracer molecules in human synovial fluid samples, as conducted here (Fig. 1), would be feasible with standard microscopy equipment. With minimal (microliter) amounts of synovial fluid accurate quantitative measurements of diffusion coefficients is possible.
Results
Viscosity of synovial fluid shows large variability
First, the diffusion coefficient of fl-Dextran (Ddex) and fl-HA (DHA) was determined in buffer solution (Supplementary Fig. S1). Due to its lower molecular weight fl-HA has a higher diffusion coefficient as fl-Dextran in buffer solution.
Fl-Dextran is a commonly used inert probe in single molecule microscopy and its diffusion coefficient (DDex) corresponds to the viscosity of the sample. DDex scatter over a wide range for both patient cohorts (Fig. 2). DDex of the w/o-JD-group and w/-JD -group show a large overlap and do not differ significantly. Because of their large overlap, it is impossible to distinguish the w/-JD -group from the w/o-JD-group based only on DDex respectively synovial fluid viscosity.
The viscosity of synovial fluid sample of patients affected by joint damage (w/-JD) tends to be lower, as indicated by the on average higher diffusion coefficient of fl-dextran. The overlap between both patient cohorts makes it unfeasible to diagnose joint damage based on synovial fluid-viscosity. Whereas, the diffusion coefficient of fl-HA is significantly reduced in the w/o-JD-group compared to samples collected from patients affected by joint damage. Nevertheless, the HA diffusion coefficients still show an overlap between patients with and without joint damage.
Three patients showed joint damage (ICRS > 2) during knee arthroscopy. The samples of these patients were assigned to the w/-JD-group. Interestingly, DHA and Ddex of these three patients (Fig 2. encircled data points) did not differ from the other subjects in the w/-JD-group, which were in need of total knee arthrosplasty.
Slowed hyaluronan diffusion is a characteristic feature of healthy synovial fluid
In contrast to DDex, DHA between patients with and without joint damage differs. The diffusion of fl-HA is significantly slower in the w/o-JD-group (2.2 μm2/s) compared to the w/-JD-group (4.6 μm2/s) (P = 0.01) (Fig. 2).
While average DHA is slower in the w/-JD-group compared to the w/o-JD-group, the distributions of DHA still show a great overlap. Therefore, DHA alone is no suitable marker to detect joint damage, either.
While DHA and DDex of both patient cohorts overlap at least to some extent, the w/o-JD-group becomes distinguishable from the w/-JD-group by correlating DHA and DDex (Fig. 3).
(A) Linear regression of DHA vs. DDex shows a selective reduction of HA diffusion coefficient in synovial fluid from patients without joint damage (slope = 0.4 +/− 0.1). This reduction is missing synovial fluid from patients affected by joint damage (slope 1.3 +/− 0.2). The slope of the w/-JD-group resembles the ratio of DHA and DDex found in buffer solution and indicates that in patients with osteoarthritis, HA undergoes no or less interactions. The encircled data points represent patients not in need of total knee arthroplasty but with an ICRS-score > 2. (B) Analyzing DHA/DDex makes patients with joint damage distinguishable from patients without joint damage.
By just increasing the overall viscosity of the fluid, the ratio of the diffusion coefficients of fl-HA and fl-Dextran stay the same (see eq. 2).
DHA and DDex show a linear relationship with a high correlation coefficient in the w/o-JD- and w/-JD-group (0.88 & 0.9). The slope of the linear regression in the w/-JD-group (1.3 +/− 0.2) is concurrent with the ratio of DHA vs. DDex in buffer (1.3 +/− 0.1) (Fig. 3). In contrast to this, the slope of DHA vs. DDex is reduced to 0.4 +/− 0.1 in the w/o-JD group.
Discussion
The ratio of DHA and DDex found in our study allows the distinction of patients with and without joint damage. Furthermore, patients with advanced osteoarthritis had a similar DHA/DDex ratio as patients who needed total knee arthrosplasty. Therefore, we suggest the DHA/DDex ratio could be a suitable marker for osteoarthritis.
A decreased synovial fluid viscosity was linked to pathologic joint conditions of patients suffering from osteoarthritis in several studies. Suitability of synovial fluid viscosity as an indicator for osteoarthritis or joint damage has been discussed controversial13,19. Its viscosity is influenced by several parameters such as age and physical activity. Several studies show a large variance of synovial fluid viscosity between different individuals19,20,21. Our results show a large overlap of synovial fluid viscosity between samples of patients with and without joint damage. The large overlap shows that synovial fluid viscosity alone is not a reliable marker for osteoarthritis.
More strikingly, we found that in synovial fluid samples fl-HA showed a slower diffusion than expected from the sample viscosity. In contrast to that, in samples from patients with joint damage this effect was diminished or missing. The diffusion coefficient of fl-HA in samples from patients with joint damage corresponded well to the sample viscosity.
As previously described, the well-balanced interaction of the main macromolecular synovial fluid components HA, PRG4 and collagen type II fibrils are important for the low coefficient of friction in healthy joints15. Progression of osteoarthritis is linked to a higher coefficient of friction. In line with that results our data show that HA undergoes intermolecular interactions in healthy joints and these interactions are lost in pathological changed joints. The ratio of DHA/DDex presumably allows the discrimination of patients affected by osteoarthritis from patients with no hyaline damage. Therefore, we suggest that the DHA/DDex ratio could be used to monitor pathological changes in this interplay and thereby the detection of early osteoarthritis.
Furthermore, our data raise the question of whether loss of intermolecular interactions of hyaluronan in synovial fluid is cause or consequence of osteoarthritis. This will be addressed in future studies.
Single molecule microscopy requires only small sample volumes (10–100 μl). Furthermore, to its extreme sensitivity only picomolar amounts of tracer molecules are needed. Such low concentrations are not changing the sample properties and reduce the required amount of tracer molecules dramatically. The samples need no further preparation before measurement. The experimental procedure and data analysis can be automated. By using state-of-the-art automation equipment, several hundred samples could be analyzed per microscope per day. Furthermore, the required image analysis algorithms are already freely available helping to keep the costs of this method low.
Thus, single molecule microscopy is a useful method for probing the organization of human synovial fluid and shows potential for diagnosis of early osteoarthritis.
Material and Methods
Single Molecule Microscopy
Single-particle imaging experiments were performed using a custom-built single molecule microscope based on a Zeiss Axiovert 200 TV equipped with a 100 × NA 1.4 oil immersion objective lens (Carl Zeiss Microimaging GmbH, Jena, Germany). Fluorescence was excited at 532 nm by a diode-pumped solid-state laser (Pluto Pegasus Lasersysteme GmbH, Wallenhorst, Germany). Selective plane illumination was achieved via HiLo-illumination. Laser illumination was switched on only during image acquisition by means of an acousto-optical tunable filter (AA Optoelectronics, Orsay Cedex, France). For single-particle image acquisition, we used the iXon DV-860 BI camera (Andor Technology, Belfast, Northern Ireland) in combination with a 4× magnifier and 3x binning yielding a pixel size in the object space of 120 nm. Generally, 2500 frames were recorded in a single movie, with a frame rate of kacq = 200 Hz. All measurements were performed at 22 °C. Since synovial joints are avascular compartments, many of which are located in the periphery of the body, it seems reasonable to assume that the temperature in peripheral joints exposed to the ambient can be substantially lower than 37 °C. It is assumed that the synovial fluid components can still provide their lubricating properties at 22 °C in a physiological relevant manner. The temperature of 22 °C was meant to approximate the conditions in small peripheral joints.
Image analysis
Identification of the single-molecule signals and tracking was done with Matlab using a self-written single particle-tracking algorithm. Analysis of the trajectory data as described before was done with a in house built Matlab program17.
Mathematical analysis of the jump distance distribution
The jump distance distribution of a molecule with diffusion coefficient D is given by

While the diffusion coefficient D of spherical objects is described by the Stokes-Einstein equation

Whereas, kb denotes the Boltzmann constant, T absolute temperature, η the viscosity and R0 the hydrodynamic radius.
Statistical Testing
For calculation of all P values, two-tailed Mann-Whitney test was used.
Preparation of fluorescent probes
Fluorescent HA (fl-HA) was prepared from rooster comb HA (average molecular weight 400 kDa) as described with modifications18. Instead, we used TRITC, due to its superior photo stability. Fl-HA was used at a concentration of ≈170 pm. As an inert macromolecular tracer fluorescent, 500-kDa dextran (fl-Dextran) at ≈170 pm was used.
Sample collection
The sample collection has been performed by the department of Orthopedic Surgery and Traumatology of the University Hospital in Bonn, Germany.
Samples have been taken of patients that underwent surgical procedures such as knee-Arthroscopy and Implantation of knee-Arthroplasty. The study was approved by the ethics committee of the University of Bonn, Germany, conducted in accordance with the approved guidelines and informed consent was obtained from the patients.
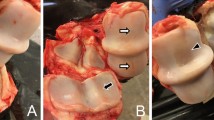
Patients who underwent knee-arthroscopy due to damage of the meniscus (medial and lateral were assigned to the appropriate group according to their joint status. After implanting the trocar of the arthroscope and before starting the lavage, samples of synovial fluid (maximum 5 ml) have been taken.
After inserting the arthroscope, the status of the cartilage has been analyzed. The patients have been structured in two different groups. If there was no severe cartilage damage (ICRS-score < 1) the patient was assigned to Group 1 (w/o-JD N = 5). If there have been severe cartilage damages (ICRS-score > 2) the patient has been assigned to Group 2 (w/-JD).
In patients that underwent implantation of a knee arthroplasty due to severe osteoarthritis, synovial fluid has been taken before opening joint capsula by using a cannula. These patients were grouped in Group 2(w/-JD total N = 16, N = 3 with ICRS-score > 2 and N = 13 with TKA)
Collected samples have been stored for a maximum of 48 h at 4 °C before single molecule measurement.
Additional Information
How to cite this article: Kohlhof, H. et al. Single Molecule Microscopy Reveals an Increased Hyaluronan Diffusion Rate in Synovial Fluid from Knees Affected by Osteoarthritis. Sci. Rep. 6, 21616; doi: 10.1038/srep21616 (2016).
References
Gamble, R., Wyeth-Ayerst, J., Johnson, E. L., Searle, W. & Beecham, S. Recommendations for the medical management of osteoarthritis of the hip and knee. Arthritis Rheum. 43, 1905–1915 (2000).
Lim, K. & Lau, C. S. Perception is everything: OA is exciting. Int. J. Rheum. Dis. 14, 111–112 (2011).
Goldring, S. R. & Goldring, M. B. Clinical aspects, pathology and pathophysiology of osteoarthritis. J. Musculoskelet. Neuronal Interact. 6, 376–378 (2006).
Jay, G. D. & Waller, K. A. The biology of Lubricin: Near frictionless joint motion. Matrix Biol. 39, 17–24 (2014).
Charnley, J. The lubrication of animal joints Symposium on biomechanics (pp. 12–22). Institution of Mechanical Engineers London (1959).
Schmidt, T. A., Gastelum, N. S., Nguyen, Q. T., Schumacher, B. L. & Sah, R. L. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 56, 882–891 (2007).
Migliore, A. & Massafra, U. Towards the identification of early stage osteoarthritis. Clin Cases Miner. Bone. Metab. 11, 114 (2014).
Van den Berg, W. Osteoarthritis year 2010 in review: pathomechanisms. Osteoarthr. Cartil. 19, 338–341 (2011).
Fibel, K., Hillstrom, H. & Halpern, B. State-of-the-Art Management of Knee Osteoarthritis. World J Clin. Cases 3, 89 (2015).
Symmons, D., Mathers, C. & Pfleger, B. Global burden of osteoarthritis in the year 2000. Geneva: World Health Organization (2003).
Lotz, M. et al. Value of biomarkers in osteoarthritis: current status and perspectives. Ann. Rheum. Dis. 72, 1756–1763 (2013).
Tanna, S. Osteoarthritis “Opportunities to address pharmaceutical gaps”. Priority Medicines for Europe and World: a public health approach to innovation, 3–23, World Health Organization (2004).
Conrad, B. P. et al. Can synovial fluid viscosity be used as a physical marker for osteoarthritis severity. Summer Bioengineering Conference, June. (2003).
Jay, G. D., Torres, J. R., Warman, M. L., Laderer, M. C. & Breuer, K. S. The role of lubricin in the mechanical behavior of synovial fluid. Proc. Natl. Acad. Sci. USA. 104, 6194–6199 (2007).
Majd, S. E. et al. Both Hyaluronan and Collagen Type II Keep Proteoglycan 4 (Lubricin) at the Cartilage Surface in a Condition That Provides Low Friction during Boundary Lubrication. Langmuir 30, 14566–14572 (2014).
Ludwig, T. E., Cowman, M. K., Jay, G. D. & Schmidt, T. A. Effects of concentration and structure on proteoglycan 4 rheology and interaction with hyaluronan. Biorheology 51, 409–422 (2014).
Kappler, J., Kaminski, T. P., Gieselmann, V., Kubitscheck, U. & Jerosch, J. Single-molecule imaging of hyaluronan in human synovial fluid. J. Biomed. Opt. 15, 060504-060504-3 (2010).
Kaminski, T., Siebrasse, J., Gieselmann, V., Kubitscheck, U. & Kappler, J. Imaging and tracking of single hyaluronan molecules diffusing in solution. Glycoconj. J. 25, 555–560 (2008).
Praest, B. M., Greiling, H. & Kock, R. Assay of synovial fluid parameters: hyaluronan concentration as a potential marker for joint diseases. Clin. Chim. Acta 266, 117–128 (1997).
Balazs, E. A., Watson, D., Duff, I. F. & Roseman, S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritic human fluids. Arthritis Rheum. 10, 357–376 (1967).
Balazs, E. A. The physical properties of synovial fluid and the special role of hyaluronic acid in Disorders of the Knee, 61–74 (1974).
Acknowledgements
We thank Molly Chilton for critical proof reading. We thank Debora Kaminski for critical discussions. TPK acknowledges financial support by the German National Academic Foundation.
Author information
Authors and Affiliations
Contributions
T.P.K., H.K. and S.G. designed the study and wrote the manuscript. H.K., S.G., S.K., S.A., T.R., J.S., Y.R. and M.F. conducted sample collection and diagnosed joint status. T.P.K. conducted single molecule measurements. T.K.P., H.K. and S.G. analyzed the data. All authors interpreted the data, critically revised the manuscript and provided final approval.
Corresponding author
Ethics declarations
Competing interests
TPK became after the study employee of AstraZeneca. AstraZeneca was not involved in this study. HK, SG, SK, SA, TR, JS, YR and MF declare that they have no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Kohlhof, H., Gravius, S., Kohl, S. et al. Single Molecule Microscopy Reveals an Increased Hyaluronan Diffusion Rate in Synovial Fluid from Knees Affected by Osteoarthritis. Sci Rep 6, 21616 (2016). https://doi.org/10.1038/srep21616
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep21616
This article is cited by
-
Hyaluronic acid synthesis, degradation, and crosslinking in equine osteoarthritis: TNF-α-TSG-6-mediated HC-HA formation
Arthritis Research & Therapy (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.