Abstract
MOG1 was initially identified as a protein that interacts with the small GTPase Ran involved in transport of macromolecules into and out of the nucleus. In addition, we have established that MOG1 interacts with the cardiac sodium channel Nav1.5 and regulates cell surface trafficking of Nav1.5. Here we used zebrafish as a model system to study the in vivo physiological role of MOG1. Knockdown of mog1 expression in zebrafish embryos significantly decreased the heart rate (HR). Consistently, the HR increases in embryos with over-expression of human MOG1. Compared with wild type MOG1 or control EGFP, mutant MOG1 with mutation E83D associated with Brugada syndrome significantly decreases the HR. Interestingly, knockdown of mog1 resulted in abnormal cardiac looping during embryogenesis. Mechanistically, knockdown of mog1 decreases expression of hcn4 involved in the regulation of the HR, and reduces expression of nkx2.5, gata4 and hand2 involved in cardiac morphogenesis. These data for the first time revealed a novel role that MOG1, a nucleocytoplasmic transport protein, plays in cardiac physiology and development.
Similar content being viewed by others
Introduction
MOG1 was initially cloned from Saccharomyces cerevisiae while screening for a multi-copy suppressor of conditional growth defect alleles of the Ran gene (GSP1) (thus named as mog1)1. Ran encodes a small GTPase with a critical role in regulating the transport of macromolecules into and out of the nucleus through the nuclear pore complex (NPC)2. MOG1 directly interacts with Ran and can rescue the temperature sensitive growth defect phenotype associated with the yeast Ran mutant3. Similar to Ran mutant, yeast with ΔMOG1 was defective in nuclear-protein import3, suggesting that MOG1 is important for nucleocytoplasmic transport3. The human MOG1 gene was identified by homology database searches. It codes for a protein of 186 amino acid residues with a predicted molecular weight of 20 kDa4. Human MOG1 protein sequence is highly homologous to that of yeast MOG1. Human MOG1 protein was shown to interact directly with Ran, too, and could partially rescue the growth defects of ΔMOG1 yeast cells. The evolutionary conservation of the MOG1 protein sequence suggests that it also plays an important role in animal systems.
During a yeast two-hybrid screen, we identified MOG1 as a protein that also interacts directly with the voltage-gated cardiac sodium channel Nav1.55. Nav1.5, encoded by the SCN5A gene, is required for generation and maintenance of the cardiac action potential (CAP)6,7,8,9,10,11. We have identified the first series of mutations in SCN5A which cause inherited cardiac arrhythmias and sudden death in the young, otherwise healthy, individuals, including long QT syndrome (LQTS) and Brugada syndrome (BrS)6,12,13. Later, SCN5A/Nav1.5 mutations were also found in patients with sick sinus syndrome, cardiac conduction disease, dilated cardiomyopathy and other diseases (together referred to as sodium channelopathies)14,15,16. We have reported that overexpression of MOG1 promotes cell surface trafficking of Nav1.5, thereby increasing the density of peak cardiac sodium current (INa), whereas siRNA knockdown of MOG1 expression reduces cell surface trafficking of Nav1.5, thereby decreasing the density of INa5,17. One dominant-negative mutation, E83D, of MOG1 was found to be associated with BrS and dramatically decrease INa18. We recently found that overexpression of MOG1 can rescue biochemical and electrophysiological defects associated SCN5A/Nav1.5 mutations causing BrS and sick sinus syndrome in mammalian cells17. Therefore, MOG1, delivered by gene therapy, may be used as an effective tool to treat patients with BrS and sick sinus syndrome with SCN5A/Nav1.5 mutations and other cardiac diseases with reduced INa.
Despite the important roles of MOG1 in human diseases and potential utility in treating lethal arrhythmias, little is known about the in vivo function of MOG1. To address this issue, we used zebrafish as a model system to examine the role of MOG1 in the developing heart. Zebrafish embryos are optically transparent and externally fertilized, making them ideal for studying early organogenesis. Moreover, genetic manipulation is easily achieved using antisense morpholinos (MOs) and embryos are permeable to small molecule drugs placed in the medium.
The stages of zebrafish cardiac development have been well-delineated. Cardiac precursors are located at the blastula margin, and development of the zebrafish heart begins at 5 hours post fertilization (hpf)19. These bilateral precursors undergo a complex series of movements that result in the formation of a cardiac cone and subsequently a beating heart tube by 22–24 hpf 20,21. The tube then loops to form a two-chambered heart and the ventricular wall begins to thicken concentrically between 48–72 hpf 20,21. In this study, we identified an important role of mog1 in early cardiac development by regulating the expression of early transcription factor genes nkx2.5, gata4, and hand2. Moreover, we found that Mog1 regulates the heart rate during embryogenesis by regulating the expression of ion channel gene hcn4.
Results
Molecular cloning and expression profile of zebrafish mog1
Using the human MOG1 cDNA sequence, we performed BLAST searches for its homologous genes in the NCBI database (www.ncbi.nlm.nih.gov) and identified a single zebrafish mog1 gene (RefSeqDNA: NM_001099995.1). Its corresponding protein sequence (RefSeq peptide: NP_001093465) showed a high degree of homology to human MOG1 (NP_057576) (46% identity, 62% homology) and mouse Mog1 (NP_001272370) (44% identity, 57% homology) (Fig. 1A and Table 1). Zebrafish mog1 is located on chromosome 5 and contains 5 exons and 4 introns. The zebrafish Mog1 protein contains 183 amino acids.
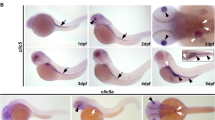
(A) Alignment of amino acid sequences of human MOG1 (GenBankTM accession number NP_057576), mouse Mog1 (GenBankTM accession number NP_001272370) and zebrafish Mog1 (GenBankTM accession number NP_001093465). (B) Whole-mount in situ hybridization analysis of zebrafish mog1 expression at different developmental stages during embryogenesis. Images for embryos at 2.5 hpf, 4 hpf, 6 hpf and 12 hpf are shown with lateral views with the animal pole to the top. Images for embryos at 24 hpf and 48 hpf stages are lateral views with the head to the left. A dorsal view of the 48 hpf embryo was also shown with the head to the top.
The expression profile of mog1 during zebrafish embryogenesis was analyzed using whole-mount in situ hybridization (Fig. 1B). Expression of mog1 was detected as early as in one-cell-stage embryos, suggesting that mog1 is maternally expressed. From 2.5 hpf to 4 hpf, mog1 RNA was localized in blastomeres. From 6 hpf to 12 hpf, mog1 expression was ubiquitous in embryos. At 24 hpf, mog1 expression was over the entire embryo, but more prominently in the brain region and eyes. At 48 hpf, mog1 was expressed predominantly in the brain area, but its expression was also detected in the heart and fins (Fig. 1B).
Knockdown of mog1 expression decreases the heart rate (HR)
To investigate the in vivo role of mog1, we knocked the expression of mog1 down in zebrafish using two independent morpholinos (MOs), one MO targeting the translation start site (MO1) (Fig. 2A) and the other targeting a splicing site spanning intron 2 and exon 3 (MO2) (Fig. 2D). Utilization of two independent MOs may avoid a potential off-target effect of MOs. To examine the effectiveness of MO1, we cloned a mog1 cDNA fragment containing the 5′-UTR, the translation start codon ATG, and the N-terminal coding region (amino acid residues 1 to 57) in the front of EGFP and under the control of the CMV promoter, resulting in a mog1–EGFP reporter (Fig. 2B). The effectiveness of MO1 was examined by injecting MO1 together with the mog1–EGFP reporter plasmid DNA into 1–2 cell embryos. The EGFP signal (mog1 expression) in the embryos injected with MO1 was markedly decreased by 95% (n = 60) compared to that in the embryos injected with control Std MO (n = 69) (Fig. 2C). These data suggest that MO1 is highly effective in knocking mog1 expression down.
(A) Mog1 translation-blocking MO1 was directed against the translation start site. (B) Construction of a pCMV-mog1-EGFP reporter. The 5′-UTR of zebrafish mog1 and a part of the mog1 coding sequence (nucleotide sequences 1–171 starting with A of ATG start codon, amino acid residues 1–57) were fused to the EGFP gene in vector pEGFP-N1, in which expression of the Mog1–EGFP fusion protein (green) is under the control of the CMV promoter. (C) The mog1-EGFP reporter gene was injected into one- to two-cell-stage embryos together with control Std-MO or mog1 MO1. Expression of the Mog1–EGFP fusion protein was examined under a fluorescence microscope at the 10 hpf stage. Note that mog1 MO1 effectively abolished the expression of MOG1–EGFP (lack of green signal) compared with control. (D) Mog1 splicing-blocking MO2 was designed to target the intron 2–exon 3 splicing donor site. (E) Results of RT–PCR analysis with total RNA from 24 hpf embryos treated with MO2 or Std MO. MO2 embryos generated a smaller size of alternatively spliced RT–PCR product (326 bp in MO2 versus 489 bp in Std MO). (F) Sequence analysis of the 326 bp band from MO2-injected embryos revealed that MO2 resulted in an alternatively spliced mog1 transcript with a deletion of exon3 (163 bp).
To test the effectiveness of MO2, we injected MO2 or Std MO into 1–2 cell embryos. RT-PCR analysis of mRNAs from the embryos followed by Sanger sequencing analysis revealed that MO2 injection led to an alternatively spliced transcript without exon 3, resulting in a frameshift mutation and premature translation stop (Fig. 2D–F). The amount of the alternatively spliced transcript (lower 326 bp band) generated by MO2 was considerably less than that of the wild type full-length transcript (upper 489 bp band), but it is clearly visible and consistently identified in every experiment (n > 3) (Fig. 2E). These data suggest that MO2 can knock mog1 expression down.
In control embryos injected with Std MO, the heart exhibited vigorous, rhythmic contractions. However, mog1 morphants with injection of mog1 MO1 showed bradycardia, and the myocardial contraction was obviously weakened. The HR was manually counted. Knockdown of mog1 expression by MO1 significantly decreased the HR compared to control embryos. At 72 hpf, the HR was 18% lower in mog1 MO1 zebrafish (106 ± 4 bpm, n = 31 embryos) than that in control zebrafish (129 ± 2 beats per minute or bpm, n = 35 embryos) (P = 2.27×10−5) (Fig. 3A). Similar but less dramatic results were obtained with mog1 MO2 (115 ± 2 bpm, n = 22 embryos vs. control MO (123 ± 2 bpm, n = 21 embryos) (P = 0.02) (Fig. 3A).
(A) Knockdown of mog1 expression by mog1 MO1 and MO2 caused reduction of the HR at 72 hpf. HR: 107 ± 4 bpm, n = 31 for mog1 MO1 morphants; 129 ± 4 bpm, n = 35 for control Std MO morphants (***P < 0.0001); 115 ± 2 bpm, n = 22 for mog1 MO2 zebrafish; 123 ± 2 bpm, n = 21 for control Std MO zebrafish (*P < 0.05). (B) Overexpression of mog1 in zebrafish embryos caused an increase of the HR at 120 hpf. HR at a dose of 100 pg: 124 ± 1 bpm, n = 33 embryos for mog1 mRNA; 123 ± 1 bpm, n = 35 embryos for control EGFP mRNA (P > 0.05). HR at a dose of 200 pg: 142 ± 1 bpm, n = 44 embryos for mog1 mRNA; 127 ± 2bpm, n = 33 embryos for control EGFP mRNA (***P < 0.0001). HR at a dose of 400 pg: 130 ± 2 bpm, n = 39 embryos for mog1 mRNA, 115 ± 1 bpm, n = 37 embryos for control EGFP mRNA (***P < 0.0001). Data were shown as means ± SEM from three independent experiments. We have noted that the heart rate varies from one experiment to another experiment (probably due to fluctuation of temperature), therefore, the comparison of the heart rate was always made between two groups in the same set of experiments.
Overexpression of zebrafish mog1 gene increases the HR
To further confirm the finding that mog1 regulates the HR, we overexpressed mog1 in zebrafish embryos by direct injection of mog1 mRNA (200 pg). At 72 hpf, no statistical difference was found on the HR between embryos with injection of mog1 mRNA and those with control EGFP mRNA. However, at 120 hpf, the HR of embryos injected with mog1 mRNA (142 ± 1 bpm, n = 44 embryos) was significantly increased compared to that of control embryos injected with EGFP mRNA (127 ± 2 bpm, n = 33 embryos) (P = 1.12 ×10−8) (Fig. 3B). Similar results were obtained at a higher dose of mog1 mRNA (400 pg) (P = 1.02 ×10−10) (Fig. 3B).
Mog1 regulates expression of hcn4 during embryogenesis in zebrafish
The heart rhythm is generated and maintained by various ionic currents22,23, including If generated by HCN424,25,26,27,28 and the cardiac calcium current generated by CAV1.329,30. Studies with transgenic overexpression mice and genetic studies of human patients with sick sinus syndrome suggested that IK1 generated by KCNJ231 and the cardiac sodium current generated by Nav1.5 (encoded by SCN5A)32,33 were also involved in regulation of the HR, respectively. To explore the molecular mechanism by which Mog1 regulates the HR, we performed real time qRT-PCR analysis for hcn4, cav1.3, kcnj2 and scn5a in zebrafish embryos injected with MO1. Total RNA was isolated from zebrafish embryos injected with mog1 MO1 or control Std MO and used for real-time qRT-PCR analysis. As shown in Fig. 4A, injection of mog1 MO1 selectively reduced the expression level of hcn4 mRNA in zebrafish embryos, but not that of kcnj2, cav1.3, and scn5a (Fig. 4A). Consistently, overexpression of mog1 by injection of mog1 mRNA markedly increased the expression of hcn4, but did not have any effect on expression of kcnj2, cav1.3 or scn5a (Fig. 4B). Together, these data suggest that Mog1 is involved in regulating expression of hcn4.
(A) Real-time quantitative RT-PCR analysis of hcn4, cav1.3, kcnj2, and scn5a in mog1 MO1 morphants or control Std MO morphants. (B) Real-time quantitative RT-PCR analysis of hcn4, cav1.3, kcnj2, and scn5a in zebrafish embryos injected with mog1 mRNA or control EGFP mRNA. Data were shown as means ± SEM from three independent experiments (each in triplicate).
Knockdown of mog1 expression results in defects in cardiac morphogenesis
To determine if knockdown of mog1 affects the structure and/or morphology of the heart, we performed whole-mount in situ hybridization for cmlc2, a marker for cardiomyocytes. Mog1 MOs caused abnormal cardiac looping, a key step in development of the heart (Fig. 5). Co-injection of mog1 MO1 (16 ng) and in vitro synthesized, capped zebrafish mog1 mRNA or human MOG1 mRNA (200 pg) into 1–2 cell embryos rescued the defects of cardiac looping caused by mog1 MO1 at 48 hpf (compare Fig. 5A–D). Similar results were obtained with mog1 MO2 (Fig. 5E,F), although the effect of MO2 was less severe than that of MO1. These results suggest that mog1 plays an important role in heart development during embryogenesis.
(A,B) Whole-mount in situ hybridization with embryos injected with 16 ng of control Std-MO (A) or 16 ng of mog1 MO1 (B) with a cardiac marker, cmlc2, at 48 hpf. (C,D) Whole-mount in situ hybridization with embryos injected with 16 ng of mog1 MO1 together with 200 pg of zebrafish mog1 mRNA (C) or with 200 pg of human MOG1 mRNA (D) with a cardiac marker, cmlc2, at 48 hpf. Note that overexpression of mog1 rescued the defects in mog1 morphants. The construct for making zebrafish mog1 mRNA was mutated at 5 positions (from ATG TCA CGG CCT CTG TTT to ATG TCT CGT CCG CTA TTC) so that mog1 MO1 can knock endogenous zebrafish mog1 mRNA down, but not the in vitro synthesized mog1 mRNA used for injection. The mog1 MO1 cannot bind to human MOG1 mRNA so that it cannot knock the in vitro synthesized human MOG1 mRNA down. (E,F) Whole-mount in situ hybridization with embryos injected with 16 ng of control Std-MO (E) or 16 ng of mog1 MO2 (F) with a cardiac marker, cmlc2, at 48 hpf. (G,H) Overexpression of mog1 did not cause changes of cardiac phenotype compared with embryos injected with EGFP mRNA (negative controls). The numbers in the upper right corner (e.g. 38/42 in A) describe the ratio of the number of embryos with the phenotype (e.g. 38) in the image over the number of total embryos analyzed (e.g. 42). Scale bar = 100 μM.
Overexpression of mog1 by injection of mog1 mRNA into zebrafish embryos did not cause apparent changes in cardiac morphogenesis and development (Fig. 5G,H). This may be due to the finding that the endogenous mog1 level was found to be high4 so that further overexpression did not have any effect on cardiac morphogenesis and development.
Knockdown of mog1 reduces expression of cardiac transcription factors in the anterior lateral mesoderm
To identify the molecular mechanism by which mog1 regulates cardiac morphogenesis and development, we performed whole-mount in situ hybridization to examine expression of important cardiac transcription factors in the heart-forming region of the anterior lateral plate mesoderm (ALPM). The ALPM is a population of undifferentiated cells with a cardiac potential demarcated by the expression of nkx2.534. The gata4 and hand2 genes are also expressed in the ALPM, but their expression regions are larger than that for nkx2.534. Compared to control embryos, mog1 morphants with injection of MO1 displayed a decreased expression level of all three transcription factors in the ALPM at the 6-somite stage (Fig. 6A–D,G–J,M–Q). Somite staging was used to confirm that the observed differences were not the result of developmental delay. Although gata5 is a potent inducer of nkx2.5 expression in zebrafish35, its expression level was indistinguishable between mog1 morphants and control embryos (Fig. 6S–V). These results were independently corroborated by real time qPCR analysis. Knockdown of mog1 led to a significant decrease in the expression levels of nkx2.5, gata4, and hand2 (Fig. 7A). These results indicate that zebrafish mog1 is required for the normal expression of cardiac fate-determining genes in the ALPM and for specification of appropriate numbers of cardiac progenitor cells during development.
(A–D) Knockdown of mog1 expression reduces intensity of the nkx2.5 signal. Zebrafish embryos (1–2 cell stage) were injected with 16 ng of Std-MO (A,C), 16 ng of mog1 MO1 (B) or 16 ng of mog1 MO2 (D) and used for whole-mount in situ hybridization at 12 hpf with an antisense probe for nkx2.5. (G–J) Knockdown of mog1 expression reduces intensity of the gata4 signal. The embryos were injected and processed as in (A–D), but probed with an antisense probe for gata4. (M–P) Knockdown of mog1 expression reduces expression of hand2. (S–V) Knockdown of mog1 expression does not affect expression of gata5. (E,F,K,L,Q,R,W,X) Over-expression of zebrafish mog1 did not affect expression of cardiac transcriptional factors in the anterior lateral mesoderm at the 12 hpf stage. (A–R1,S–X) Embryos are shown at a dorsal view with the anterior at the top. (M2–R2) embryos are shown in a lateral view with the anterior on the left. The numbers in the upper right corner (e.g. 27/31 in A) describe the ratio of the number of embryos with the phenotype (e.g. 27) in the image over the number of total embryos analyzed (e.g. 31). Scale bar = 100 μM.
(A) Knockdown of mog1 expression significantly reduced the expression level of nkx2.5 (***P < 0.0001), gata4 (*P < 0.05) and hand2 (*P < 0.05), but not that of gata5 (P > 0.05). (B) Overexpression of zebrafish mog1 by injection of 200 pg mRNA did not affect the expression level of nkx2.5 (P > 0.05), gata4 (P > 0.05), hand2 (P > 0.05) or gata5 (P > 0.05). Std MO (A) was used as control for MO1 and EGFP mRNA (B) was used as control for mog1 mRNA. Data were shown as means ± SEM from three independent experiments (each in triplicate).
Over-expression of zebrafish mog1 with injection of mog1 mRNA, however, did not cause any changes in expression of nkx2.5, gata4, hand2, and gata5 in the ALPM (Fig. 6E,F,K,L,Q,R,W,X). Real time qPCR analysis did not identify any effect of mog1 overexpression on expression of nkx2.5, gata4, hand2, and gata5, either (Fig. 7B).
To determine if abnormal cardiac looping in embryos injected with the mog1 MO was caused by decreased expression of cardiac transcriptional factors, we co-injected zebrafish nkx2.5 mRNA (80 pg) together with mog1 MO1 into 1–2 cells stage embryos. At 48 hpf, co-injection of human nkx2.5 mRNA rescued the defects of cardiac morphogenesis caused by mog1 MO1 (Fig. 8E–G). Furthermore, the reduced expression level of nkx2.5 at the 6 somite stage caused by mog1 MO1 was partially rescued by both human MOG1 or zebrafish mog1 mRNA injection (Fig. 8A–D). These results suggest that mog1 acts upstream of nkx2.5 to regulate specification of cardiac morphogenesis.
Zebrafish embryos were injected with Std-MO (16 ng; A,E), mog1 MO1 (16 ng; B,F), mog1 MO1 (16 ng) together with in vitro synthesized full-length zebrafish (C) or human MOG1 mRNA (200 pg) (D,G) and used for whole-mount in situ hybridization at 12 somites with antisense probes for nkx2.5 (A–D) or cmlc2 (E–G). Reduced expression of nkx2.5 by knockdown of mog1 was rescued by injection of mog1 mRNA (A–D). Co-injection of nkx2.5 mRNA (G) rescued the cardiac developmental defects in mog1 MO1 morphants (whole-mount in situ hybridization signal for cmlc2). (A–D) Dorsal view with the anterior at the top. (E–G) Anterior view with the ventral side at the top. The numbers in the upper right corner (e.g. 33/37 in A) describe the ratio of the number of embryos with the phenotype (e.g. 33) in the image over the number of total embryos analyzed (e.g. 37).
Brugada syndrome mutation E83D in mog1 reduces the heart rate
Previously, a mutation in human MOG1 was identified in a symptomatic female patient with BrS, a cardiac disorder characterized by ST segment elevation in right precordial leads on electrocardiograms (ECGs), syncope, ventricular arrhythmia, and sudden cardiac death (SCD)6,18. Here we tested whether MOG1 mutation E83D affected the HR in zebrafish embryos. We injected mRNA samples transcribed from a wild type human MOG1 expression plasmid or from a mutant human MOG1 expression plasmid with the E83D mutation into 1–2 cells stage embryos, respectively. At the dose of 200 pg mRNA, no statistical difference was found on the HR between the embryos over-expressing the E83D mutation and those over-expressing wild type MOG1 or with injection of control EGFP mRNA (153 ± 1 bpm, n = 36 E83D embryos vs. 157 ± 2 bpm, n = 34 WT embryos or 158 ± 2, n = 29 EGFP embryos, P = 0.275) at day 5 (Fig. 9A). However, at the dose of 400 pg mRNA, embryos with overexpression of the E83D mutant MOG1 showed a significantly reduced HR compared to that of the embryos with overexpression of wild type MOG1 (143 ± 1 bpm, n = 30 E83D embryos vs. 186 ± 2 bpm, n = 29 embryos P = 1.27×10−36) or to embryos with injection of control EGFP mRNA (143 ± 1 bpm, n = 30 E83D embryos vs. 156 ± 2 bpm, n = 32 embryos, P = 2.09×10−9) (Fig. 9B). Thus, mutant MOG1 with the BrS-associated E83D mutation caused a decrease of the HR in zebrafish.
(A) HR at the dose of 200 pg mRNA (158 ± 2 bpm, n = 29 for EGFP embryos vs. 153 ± 1 bpm, n = 36 for E83D embryos vs. 157 ± 2 bpm, n = 34 for WT embryos, P > 0.05 at day 5). (B) HR at the dose of 400 pg mRNA (156 ± 2 bpm, n = 32 for EGFP embryos vs. 143 ± 1 bpm, n = 30 for E83D embryos, ***P < 0.0001; 143 ± 1 bpm, n = 30 for E83D embryos vs. 186 ± 2 bpm, n = 29 for WT embryos ***P < 0.0001). Data were shown as means ± SEM from three independent experiments.
Discussion
Using zebrafish as an in vivo system, we have identified a novel role for Mog1 in cardiac rhythm and morphogenesis. Knockdown of mog1 by two independent morpholinos significantly decreased the HR, whereas overexpression of mog1 by direct injection of mog1 mRNA into the embryos increased the HR. We then showed that knockdown of mog1 expression in zebrafish caused a decrease of hcn4 expression without affecting expression of other ion channel genes, including kcnj2, cav1.3 or scn5a. Furthermore, overexpression of mog1 in zebrafish led to an increased level of hcn4 expression. Hcn4 is the dominant Hcn isoform in the adult sinoatrial node of all species investigated (rabbit, mouse, dog)25. This channel mediates the sympathetic stimulation of the HR in embryos. It contributes significantly to the generation of the regular pacemaker potential and the determination of the basal HR24,26,27. Mutations in HCN4 cause sick sinus syndrome in humans27,28. Loss of Hcn4 led to severe bradycardia and chronotropic incompetence in the embryos of knockout mice26. Results of these studies are in line with our observations and suggest that Mog1 regulates the cardiac rhythm in zebrafish through regulation of hcn4 expression.
However, the molecular mechanism underlying the regulation of hcn4 expression by mog1 is unknown. Mog1 is involved in regulating nucleocytoplasmic transport of proteins, and it may regulate nuclear import of transcriptional factors and RNA splicing factors and nuclear export of regulators of translation, some of which may be directly involved in the regulation of hcn4 transcription, splicing or translation. Future studies should focus on detailed investigations of these interesting possibilities.
Unexpectedly, we found that knockdown of mog1 by MOs significantly altered cardiac morphogenesis and development, resulting in abnormal hearts failing to complete looping. In zebrafish, both gata4 and hand2 are expressed in larger domains of the ALPM than nkx2.534,36,37. Prior studies in zebrafish revealed essential roles for Nkx2.5 in limiting the atrial cell number, promoting the ventricular cell number, and preserving chamber-specific identity in differentiated myocardium38. Nkx2.5 is essential during cardiac progenitor differentiation to maintain ventricular and atrial chamber morphology and cellular traits later in development39. Reduced gata4 expression was observed to cause defects in cardiac chamber growth and looping, while loss of hand2 resulted in embryos with significantly fewer embryonic cardiomyocytes36,37. We have demonstrated that knockdown of mog1 expression led to a significant decrease of the expression levels of multiple cardiac transcription factor genes, including nkx2.5, gata4, and hand2 in the ALPM (Fig. 6). Consistently, the expression of mog1 was identified in the gastrulating embryo, prior to the differentiation of excitable tissues (Fig. 1). By 12 hpf, the cardiac-specification gene Nkx2.5 identifies bilateral stripes of cardiac progenitors in the ALPM. Zebrafish in which mog1 was knocked down exhibited defects in initial specification of the pre-cardiac mesoderm as shown by marked reduction in early expression of Nkx2.5. Previous studies in zebrafish have demonstrated that gata5 is a potent positive regulator of nkx2.5 expression35,40. Notably, we found that although mog1 regulated nkx2.5 expression, it did not affect the expression level of gata5. On the other hand, we found that mog1 regulated expression of gata4, which may be a key to regulation of nkx2.5. All together, these data suggest that mog1 may regulate cardiac morphogenesis and development by modulating the expression levels of nkx2.5, gata4, and/or hand2.
The abnormal cardiac looping detected in mog1 morphants was remarkably similar to that observed in embryos with knockdown of scn5a expression by MOs reported previously32. When the findings in scn5a morphants were reported, it was puzzling why down-regulation of an ion channel gene scn5a caused abnormalities in cardiac morphogenesis and development. The puzzle may be partially resolved with our findings in this study that knockdown of mog1 expression also caused abnormal cardiac morphogenesis and development. We have identified MOG1 as an interacting protein of Nav1.5 by a yeast two hybrid screen5. MOG1 regulates the function of Nav1.5. Overexpression of MOG1 increases peak cardiac sodium current density, whereas knockdown of MOG1 decreased the density of peak sodium current5,17. Mechanistically, MOG1 facilitates trafficking of Nav1.5 to plasma membrane and caveolae on the cell surface17. Conversely, it is likely that knockdown of scn5a expression by MOs may affect the function of Mog1 because both of them interact strongly and may cross-regulate their functions. Deregulation of functions of mog1 by scn5a MO may affect expression of cardiac transcriptional factors nkx2.5, gata4 and hand2, resulting in abnormal cardiac morphogenesis and development.
Kattygnarath et al. identified a missense mutation, E83D, in MOG1 in a BrS patient18. Wild type MOG1 significantly increased the peak sodium current density (−171.0 ± 19.0 pA/pF at −20 mV for WT-MOG1 vs. −91.5 ± 11.6 pA/pF at −20 mV for empty vector)18. The E83D mutation significantly reduced the sodium current density (−89.5 ± 28.4 pA/pF) compared with wild type MOG1, and co-expression of both wild type and mutant MOG1 failed to increase sodium density (−106.7 ± 6.1 pA/pF)18. Despite the strong functional data, Olesen et al.41 raised a cautious note about the causal nature of the mutation due to identification of the mutation in a single BrS patient and lack of co-segregation analysis. In this study, we found that injection of wild type human MOG1 mRNA increased the HR in zebrafish embryos, but injection of mutant MOG1 mRNA with the E83D mutation resulted in a significantly lower HR than wild type MOG1 mRNA. Our data provides further evidence that the E83D mutation in MOG1 is a functional mutation affecting MOG1 function.
There are several limitations with the current study. First, although two independent mog1 MOs were studied to minimize the confounding of off-target effects of MOs, the second MO, i.e. MO2 targeting a splicing junction, was considerably less effective than MO1 targeting the translation start site. Nevertheless, similar results were obtained for both MO1 and MO2. Second, our studies could not distinguish whether the two major effects of mog1 MOs, i.e. abnormal cardiac looping and a decreased HR, are two independent events or related. Because mog1 MOs reduced the expression levels of hcn4 and transcriptional factor genes nkx2.5, gata4, and hand2, respectively, it is likely that abnormal cardiac looping and a decreased HR caused by MOs may be two independent events. However, we could not exclude the possibility that abnormal cardiac looping may precipitate a decreased HR because knockdown of mog1 expression reduced the expression levels of nkx2.5, gata4, and/or hand2 (causing abnormal cardiac looping), which may decrease expression of hcn4 (reducing the HR).
In conclusion, the data in this study demonstrates that Mog1 regulates heart rhythm through regulating the expression of potassium channel Hcn4. In addition, Mog1 also regulates cardiac morphogenesis and development through regulating expression of cardiac transcriptional factors Nkx2.5, Gata4, and Hand2. We also showed that BrS-associated mutation E83D in MOG1 significantly reduced the HR compared with wild type MOG1, providing in vivo evidence that E83D is a functional mutation. These studies uncover a novel physiological role of Mog1 in regulating heart rhythm and morphogenesis in vivo and further our understanding of molecular mechanisms and biological pathways for maintenance of appropriate heart rhythm and for regulation of cardiac morphogenesis and development.
Methods
Zebrafish breeding and maintenance
Wild type AB strain zebrafish (Danio rerio) were used in the study. Embryos were raised at 28.5 °C, treated with 0.003% 1-phenyl-2-thiourea (PTU, Sigma) to prevent pigment formation, and collected at different developmental stages for analysis42,43. The study was approved by the ethics committee of Huazhong University of Science and Technology, and all procedures were carried out in accordance with the approved guidelines.
Identification and homology analysis of zebrafish mog1
The full-length sequence of zebrafish mog1 gene was identified by searching the NCBI database (http://www.ncbi.nlm.nih.gov). BLAST searches were performed to identify potential homologs, othologs or paralogs of zebrafish mog1. In zebrafish, there is only one copy of the mog1 gene and no paralog was found.
Microinjection of morpholinos (MOs)
To investigate the in vivo role of mog1, we studied two different mog1 MOs to knock expression of mog1 down in zebrafish embryos. The first MO targets the translation start site of mog1 (MO1: 5′- CACCGCCAAACAGAGGCCGTGACAT -3′) (Fig. 1A), and the second MO targets the splicing junction between the second intron and the third exon (MO2: 5-TGATACCTATTACAGCACAGACACA -3′) (Fig. 1B). The negative control MO is a standard control (Std) morpholino that does not match any zebrafish sequence (5′-CCTCTTACCTCAGTTACAATTTATA-3′). MOs (16 ng) were dissolved in water and injected into the yolk sac of one- to two-cell stage zebrafish embryos using a pneumatic picopump (WARNER INSTRUMENTS PLI-90A).
To examine the effectiveness of MO1, we constructed a mog1-EGFP reporter by cloning a mog1 cDNA fragment containing the 5′-UTR, the translation start ATG, and a part of N-terminal coding region (171 bp) in the front of EGFP and under the control of the CMV promoter. The reporter plasmid and MO1 were co-injected into embryos and the EGFP signal was captured under a florescent microscope and analyzed. To test the effectiveness of MO2, we isolated total RNA from mog1 morphants with MO2 or Std MO, converted it into cDNA by reverse transcription, and preformed qRT-PCR analysis with the forward primer in exon 2 and the reverse primer in exon 4 as described previously42. The RT-PCR bands were extracted from agarose gels and sequenced by Sanger sequencing analysis to identity the nature of alternative splicing.
Preparation and microinjection of mRNAs
The coding region of zebrafish mog1 was PCR-amplified from cDNA prepared from embryonic mRNA, subcloned into a pGEM-T easy vector (Promega) and verified by DNA sequence analysis (pGEMT-zmog1). The zebrafish mog1 cDNA was then subcloned into the pcs2+ vector, resulting in plasmid pcs2+-zmog1.
The cDNA for human MOG1 (hMOG1) was amplified by PCR analysis from a mammalian expression plasmid for MOG1 (pcDNA3.1 + MOG1)5,17. The PCR product was subcloned into the pcs2+ vector, resulting in plasmid pcs2+-hMOG1.
The cDNA for the coding region of the EGFP gene (negative control) was amplified by PCR analysis from the pEGFP-N1 vector. The PCR product was subcloned into the pcs2+ vector, resulting in plasmid pcs2+-EGFP.
The cDNA for the coding region of the human NKX2.5 gene was amplified by PCR analysis from the pcDNA3.1−NKX2.5 expression plasmid. The PCR product was subcloned into the pcs2+ vector, resulting in plasmid pcs2+-NKX2.5.
The pcs2+-derived plasmids were linearized by restriction digestion with Not I and used for preparation of capped mRNAs for EGFP, zmog1, hMOG1, and NKX2.5 using SP6 RNA polymerase and the mMESSAGE Mmachine system (Ambion, Austin, TX).
The mRNA samples were dissolved in water and injected into the yolk sac of one- to two-cell stage zebrafish embryos using a pneumatic picopump (WARNER INSTRUMENTS PLI-90A).
Whole-mount in situ hybridization
For generation of antisense RNA probes for whole-mount in situ hybridization, we first used PCR to amplify a 300–600 bp cDNA fragment for a target gene to be analyzed. The cDNA fragment was subcloned into a pGEM-T easy vector. The pGEM-T easy-derived plasmids were linearized with restriction digestion. Antisense RNA probes were prepared by in vitro transcription from linearized templates by T7 or SP6 polymerases (Promega) in the presence of DIG-labeled nucleotides (Roche, Mannheim). Whole-mount in situ hybridization was performed as described by us previously42,43,44.
Image acquisition
The images of embryos were visualized and captured under an Olympus SZX16 microscope with an Olympus DP72 digital camera with cellSensversion1.6 software, and processed with Adobe Photoshop CS4 soft-ware. Identical modifications and adjustments were applied to all images in the same experiment42,43,44.
Quantitative real time RT-PCR analysis (qPCR)
Embryos were collected at different developmental time points (e.g. 48 hpf, 72 hpf) and used for preparation of total RNA with TRIzol reagent (TaKaRa). Genomic DNA was removed by digestion with DNA-free TMDNase (ABI, Foster City, CA). Extracted RNA samples were quantified by spectrometry. Two micrograms of RNA samples were reverse-transcribed into cDNA by reverse transcription using a cDNA Synthesis kit (Invitrogen) with random primers. The cDNA products generated using random primers were used for real time qPCR analyses of scn5A, hcn4, kcnj2 and cav1.3 using a FastStart Universal SYBRGreen Master kit (Roche). Real time qPCR analysis was carried out in a 10 ul reaction volume on an ABI 7900Genome Analyzer System. The β-actin gene was used as internal control. Data analysis was performed using the 2−△Ct(RQ value) method as described by us previously45,46. The sequences of primers used for real time qPCR are listed in online supplementary material (Table S1).
Statistical analysis
An unpaired Student’s t test was used to compare the means from two different groups. The data were presented as means ± SEM. A P value of ≤0.05 was considered to be statistically significant.
Additional Information
How to cite this article: Zhou, J. et al. Cardiac sodium channel regulator MOG1 regulates cardiac morphogenesis and rhythm. Sci. Rep. 6, 21538; doi: 10.1038/srep21538 (2016).
References
Baker, R. P., Harreman, M. T., Eccleston, J. F., Corbett, A. H. & Stewart, M. Interaction between Ran and Mog1 is required for efficient nuclear protein import. J Biol Chem 276, 41255–41262 (2001).
Moore, M. S. & Blobel, G. The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature 365, 661–663 (1993).
Oki, M. & Nishimoto, T. A protein required for nuclear-protein import, Mog1p, directly interacts with GTP-Gsp1p, the Saccharomyces cerevisiae Ran homologue. P Natl Acad Sci USA 95, 15388–15393 (1998).
Marfatia, K. A., Harreman, M. T., Fanara, P., Vertino, P. M. & Corbett, A. H. Identification and characterization of the human MOG1 gene. Gene 266, 45–56 (2001).
Wu, L. et al. Identification of a New Co-factor, MOG1, required for the full function of cardiac sodium channel Nav1.5. J Biol Chem 283, 6968–6978 (2008).
Chen, Q. Y. et al. Genetic basis and molecular mechanism for idiopathic: ventricular fibrillation. Nature 392, 293–296 (1998).
Gellens, M. E. et al. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. P Natl Acad Sci USA 89, 554–558 (1992).
Tian, X. L. et al. Mechanisms by which SCN5A mutation N1325S causes cardiac arrhythmias and sudden death in vivo . Cardiovasc Res 61, 256–267 (2004).
Tian, X. L. et al. Optical mapping of ventricular arrhythmias in LQTS mice with SCN5A mutation N1325S. Biochem Bioph Res Comm 352, 879–883 (2007).
Yong, S. L. et al. Characterization of the cardiac sodium channel SCN5A mutation, N1325S, in single murine ventricular myocytes. Biochem Bioph Res Comm 352, 378–383 (2007).
Zhang, T. et al. LQTS mutation N1325S in cardiac sodium channel gene SCN5A causes cardiomyocyte apoptosis, cardiac fibrosis and contractile dysfunction in mice. Int J Cardiol 147, 239–245 (2011).
Wang, Q. et al. Cardiac sodium channel mutations in patients with long QT syndrome, an inherited cardiac arrhythmia. Hum Mol Genet 4, 1603–1607 (1995).
Wang, Q. et al. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell 8, 805–811 (1995).
Pambrun, T. et al. Myotonic dystrophy type 1 mimics and exacerbates Brugada phenotype induced by Na(v)1.5 sodium channel loss-of-function mutation. Heart Rhythm 11, 1393–1400 (2014).
Ziyadeh-Isleem, A. et al. A truncating SCN5A mutation combined with genetic variability causes sick sinus syndrome and early atrial fibrillation. Heart Rhythm 11, 1015–1023 (2014).
Zumhagen, S. et al. A heterozygous deletion mutation in the cardiac sodium channel gene SCN5A with loss- and gain-of-function characteristics manifests as isolated conduction disease, without signs of Brugada or long QT syndrome. PLoS ONE 8, e67963 (2013).
Chakrabarti, S. et al. MOG1 rescues defective trafficking of Na(v)1.5 mutations in Brugada syndrome and sick sinus syndrome. Circ Arrhythm Electrophysiol 6, 392–401 (2013).
Kattygnarath, D. et al. MOG1: a new susceptibility gene for Brugada syndrome. Circ Cardiovasc Genet 4, 261–268 (2011).
Keegan, B. R., Meyer, D. & Yelon, D. Organization of cardiac chamber progenitors in the zebrafish blastula. Development 131, 3081–3091 (2004).
Glickman, N. S. & Yelon, D. Cardiac development in zebrafish: coordination of form and function. Semin Cell Dev Biol 13, 507–513 (2002).
Stainier, D. Y. Zebrafish genetics and vertebrate heart formation. Nat Rev Genet 2, 39–48 (2001).
Wang, Q., Chen, Q. Y., Li, H. & Towbin, J. A. Molecular genetics of long QT syndrome from genes to patients. Curr Opin Cardiol 12, 310–320 (1997).
Wang, Q., Chen, Q. Y. & Towbin, J. A. Genetics, molecular mechanisms and management of long QT syndrome. Ann Med 30, 58–65 (1998).
Stieber, J., Hofmann, F. & Ludwig, A. Pacemaker channels and sinus node arrhythmia. Trends Cardiovas Med 14, 23–28 (2004).
Ishii, T. M., Takano, M., Xie, L. H., Noma, A. & Ohmori, H. Molecular characterization of the hyperpolarization-activated cation channel in rabbit heart sinoatrial node. J Biol Chem 274, 12835–12839 (1999).
Stieber, J. et al. The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. P Natl Acad Sci USA 100, 15235–15240 (2003).
Schweizer, P. A. et al. The symptom complex of familial sinus node dysfunction and myocardial noncompaction is associated with mutations in the HCN4 channel. J Am Coll Cardiol 64, 757–767 (2014).
Milanesi, R., Baruscotti, M., Gnecchi-Ruscone, T. & DiFrancesco, D. Familial sinus bradycardia associated with a mutation in the cardiac pacemaker channel. New Engl J Med 354, 151–157 (2006).
Zhang, Z. et al. Functional roles of Ca(v)1.3 (alpha(1D)) calcium channel in sinoatrial nodes-Insight gained using gene-targeted null mutant mice. Circ Res 90, 981–987 (2002).
Mangoni, M. E. et al. Functional role of L-type Cav1.3 Ca2 + channels in cardiac pacemaker activity. P Natl Acad Sci USA 100, 5543–5548 (2003).
Li, J., McLerie, M. & Lopatin, A. N. Transgenic upregulation of I K1 in the mouse heart leads to multiple abnormalities of cardiac excitability. Am J Physiol Heart Circ Physiol 287, H2790–H2802 (2004).
Chopra, S. S. et al. Voltage-gated sodium channels are required for heart development in zebrafish. Circ Res 106, 1342–1350 (2010).
Gui, J. H. et al. Multiple loss-of-function mechanisms contribute to SCN5A-related familial sick sinus syndrome. PLoS ONE 5, e10985 (2010).
Schoenebeck, J. J., Keegan, B. R. & Yelon, D. Vessel and blood specification override cardiac potential in anterior mesoderm. Dev Cell 13, 254–267 (2007).
Reiter, J. F. et al. Gata5 is required for the development of the heart and endoderm in zebrafish. Gene Dev 13, 2983–2995 (1999).
Holtzinger, A. & Evans, T. Gata4 regulates the formation of multiple organs. Development 132, 4005–4014 (2005).
Tu, S. & Chi, N. C. Zebrafish models in cardiac development and congenital heart birth defects. Differentiation 84, 4–16 (2012).
Targoff, K. L., Schell, T. & Yelon, D. Nkx genes regulate heart tube extension and exert differential effects on ventricular and atrial cell number. Dev Biol 322, 314–321 (2008).
George, V., Colombo, S. & Targoff, K. L. An early requirement for nkx2.5 ensures the first and second heart field ventricular identity and cardiac function into adulthood. Dev Biol 400, 10–22 (2015).
Reiter, J. F., Verkade, H. & Stainier, Y. R. Bmp2b and Oep promote early myocardial differentiation through their regulation of gata5. Dev Biol 234, 330–338 (2001).
Olesen, M. S., Holst, A. G. & Schmitt, N. Letter by Olesen et al. Regarding Article, “MOG1: a new susceptibility gene for Brugada syndrome”. Circ Cardiovasc Genet 4, e22 (2011).
Chen, D., Li, L., Tu, X., Yin, Z. & Wang, Q. Functional characterization of KlippelTrenaunay syndrome gene AGGF1 identifies a novel angiogenic signaling pathway for specification of vein differentiation and angiogenesis during embryogenesis. Hum Mol Genet 22, 963–976 (2013).
Li, L. et al. Aggf1 acts at the top of the genetic regulatory hierarchy in specification of hemangioblasts in zebrafish. Blood 123, 501–508 (2014).
Su, Z. H. et al. MiR-144 regulates hematopoiesis and vascular development by targeting meis1 during zebrafish development. Int J Biochem Cell B 49, 53–63 (2014).
Wang, F. et al. Genome-wide association identifies a susceptibility locus for coronary artery disease in the Chinese Han population. Nat Genet 43, 345–349 (2011).
Zhou, B. S. et al. MicroRNA-503 targets FGF2 and VEGFA and inhibits tumor angiogenesis and growth. Cancer Lett 333, 159–169 (2013).
Acknowledgements
We thank the members of Wang laboratory for assistance, help, and advice during the entire period of the study. This study was supported by the Chinese National Basic Research Programs (973 Programs 2013CB531101 and 2012CB517801), the China National Natural Science Foundation Key Program (31430047), Hubei Province’s Outstanding Medical Academic Leader Program, Hubei Province Natural Science Key Program (2014CFA074), the China National Natural Science Foundation grant (91439129, NSFC-J1103514), NIH/NHLBI grants R01 HL126729 and R01 HL121358, Specialized Research Fund for the Doctoral Program of Higher Education from the Ministry of Education, and the “Innovative Development of New Drugs” Key Scientific Project (2011ZX09307-001-09).
Author information
Authors and Affiliations
Contributions
Design of the study: J.Z. and Q.K.W. Experiments and data analysis: J.Z., L.F.W., X.J.W., M.X.Z. and A.S.I.A. Drafting of the manuscript: J.Z. Critical revision of the manuscript: Q.C. and Q.K.W. Obtained funding: Q.C. and Q.K.W. Study supervision: Q.K.W.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhou, J., Wang, L., Zuo, M. et al. Cardiac sodium channel regulator MOG1 regulates cardiac morphogenesis and rhythm. Sci Rep 6, 21538 (2016). https://doi.org/10.1038/srep21538
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep21538
This article is cited by
-
Cardiac-targeted PIASy gene silencing mediates deSUMOylation of caveolin-3 and prevents ischemia/reperfusion-induced Nav1.5 downregulation and ventricular arrhythmias
Military Medical Research (2022)
-
Up-regulation of miR-95-3p in hepatocellular carcinoma promotes tumorigenesis by targeting p21 expression
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.