Abstract
This study aims to analyze in depth the role of IFNα in the upregulation of BLyS in different leukocyte populations and the possible relationship of these molecules with IL-17 and other pathogenic cytokines in SLE. Thus, IFNAR1 and membrane BLyS (mBLyS) expression was upregulated on various blood cell types from patients and closely correlated in all individuals. Moreover, BLyS serum levels associated positively with IFNα and IL-17A amounts, as well as with mBLyS on B cells and neutrophils. Interestingly, mBLyS on neutrophils was also correlated with IL-17A levels. Additionally, intracellular IL-17A expression was increased in both CD4+ lymphocytes and neutrophils from patients and IL-17+CD4+ T cell frequency was associated with serum IFNα and IFNRA1 expression on B cells. Finally, in vitro assays support an IFNα role in the activation of Th17 cells in SLE. In conclusion, these data suggest that IFNα, BLyS and IL-17 could form a pathological axis in SLE, involving T and B lymphocytes, monocytes, DCs and neutrophils, which act in a vicious circle that encourage the preexisting inflammation and propagate the disease process.
Similar content being viewed by others
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by heterogeneous clinical manifestations and the presence of multiple cellular and molecular abnormalities in the immune system, including leukocyte activation and cytokine dysregulation. Type I interferons (IFN) and particularly IFNα, are considered to play a central role in SLE etiopathogenesis1,2. Both IFNα serum levels and expression of IFNα-inducible genes are consistently increased in SLE patients and usually correlate with disease activity and clinical manifestations3,4,5,6. Moreover, IFNα from SLE sera can differentiate monocytes into activated dendritic cells (DCs) able to present self-antigens7 supporting that this pleiotropic cytokine could be responsible for initiating development of systemic autoimmunity. Binding of IFNα to the two-chain type I interferon receptor (IFNAR) initiates a signal transduction pathways that results in the expression of IFN-induced genes, most of them with immunoregulatory functions on B, T and NK lymphocytes, monocytes/ macrophages, DCs and neutrophils8. Consequently, anomalous functioning of type I IFN signalling could be an early event in lupus pathogenesis. As a result, several potential treatments blocking IFNα signalling have emerged in recent years9,10,11.
In the same way, much evidence has highlighted the contribution of B-lymphocyte stimulator (BLyS) to autoantibody production and SLE disease exacerbation12. BLyS is produced as a membrane form as well as a soluble protein12,13 by a wide variety of cells, like B lymphocytes, monocytes, neutrophils and plasmacytoid or myeloid DCs (pDCs and mDCs, respectively)14,15. Clinical studies have confirmed both circulating and cell surface BLyS overexpression in SLE patients and its correlation with the disease activity16,17,18,19.
Previous findings from our group revealed that BLyS expression and mobilization from intra to extra cellular compartments in monocytes can be influenced by IFNα, disease activity or anti-dsDNA levels19. Accordingly, there is evidence supporting IFNα as an efficient inducer of BLyS expression. Thus, whereas in vivo and in vitro IFNα treatment prompts BLyS upregulation20,21, the therapy with an anti-IFNα monoclonal antibody reduces BLyS expression in SLE patients22, suggesting a cooperative action between BLyS and IFNα in the aetiology of SLE. Furthermore, treatment with anti-BLyS monoclonal antibodies in lupus patients was associated with improvements in clinical and laboratory parameters23,24. However, such clinical trials have not revealed conclusive results since the efficacy of either IFNα or BLyS therapeutic blockade seemed to be dependent on the patients characteristics. Therefore, additional research into the roles played by IFNα and BLyS is required for the identification of SLE patients appropriated for these treatments.
Similarly, IL-17A pathway inhibitors have been recently proposed as a therapeutic option for SLE patients25, since increased circulating levels of IL-17 correlated with disease activity and a Th17/Th1 imbalance have been reported in SLE26,27,28. Interestingly, it has been described that IL-17, alone or in synergy with BLyS may stimulate B cell survival and differentiation29,30,31, thus leading to the production of autoantibodies and consequently IFNα secretion by pDC activated by the resulting immune-complexes32,33. Indeed, type I IFN has been described to exert a detrimental role in Th17 drive-autoimmune diseases34.
Considering previous evidence, the present study aims to evaluate the role of type I IFN signalling in the upregulation of BLyS in SLE patients by analysing the expression of BLyS and IFNRA1 (the α-chain of the common receptor for type-I IFNs) on the membrane of different leukocyte populations, as well as their possible association with the IL-17 production and the serum levels of other relevant pathogenic cytokines.
Results
IFNAR1 overexpression on blood leukocytes is associated with membrane BLyS levels
To extend the knowledge about the previously reported upregulatory in vitro effect of IFNα on BLyS induction and release19, we wanted to examine the possible relationship between BLyS and IFNAR1 expression on the surface of different circulating fresh cell types. Thus, membrane BLyS (mBLyS) and IFNAR1 levels were quantified by flow cytometry on B cells, neutrophils, monocytes, pDCs and mDCs from SLE patients and healthy controls (HC) (Fig. 1A). As expected, mBLyS expression was upregulated in all studied populations from patients, but especially in neutrophils, monocytes, mDCs and B lymphocytes, the same populations that exhibited higher levels of IFNAR1 compared with HC. Moreover, mBLyS and IFNAR1 levels were closely correlated in all individuals, but more strongly in SLE patients (Fig. 1B).
Expression levels of IFNAR1 and membrane BLyS on blood leukocytes from SLE patients and healthy controls.
Membrane BLyS (mBLyS) and IFNAR1 levels were quantified by flow cytometry on fresh B cells, neutrophils, monocytes, pDCs and mDCs from 67 patients (SLE) and 29 healthy controls (HC). (A) Scatter plots represent MFI levels of BLyS and IFNAR1 in several blood subpopulations and horizontal bars show the median. Statistical significance was assessed by Mann-Whitney U test. (B) Graphs show the correlation between mBLyS and IFNRA1 expression (MFI) in each leukocyte population from SLE patients (circles) and healthy controls (squares). Correlation analyses were evaluated by Spearman test.
Multivariate linear regression analysis showed that mBLyS and IFNAR1 upregulation was independent of the treatment, but significant associations were detected with specific clinical manifestations. Thus, mBLyS and IFNAR1 levels on monocytes were related to the frequency of nephritis (β = 0.275, p = 0.032 and β = 0.270, p = 0.027, respectively) and serositis (β = 0.316, p = 0.012 and β = 0.283, p = 0.028), whereas expression on B cells were associated to the frequency of serositis (β = 0.257, p = 0.025 and β = 0.209, p = 0.021) and neurologic disease (β = 0.336, p = 0.005 and β = 0.289, p = 0.017). Therefore, these data indicate an increase in the expression of IFNAR1 in various cell types linked to the pathological widespread overexpression of mBLyS in SLE patients and associated to specific clinical manifestations.
Circulating BLyS correlated with IFNα and IL-17A serum levels in SLE patients
Next, we analyzed serum levels of BLyS (sBLyS) and IFNα with the aim of determining their possible association with the mBLyS and IFNAR1 overexpression on the different cell types. Interestingly, circulating amounts of sBLyS in SLE patients correlated positively with mBLyS expression on B cells (ρ = 0.273, p = 0.025) and neutrophils (ρ = 0.329, p = 0.007) and the same tendency was observed with IFNAR1 (B cells: ρ = 0.227, p = 0.064; neutrophils: ρ = 0.273, p = 0.026). However, no significant associations were detected between IFNα levels and mBLyS or IFNAR1 expression. Additionally, serum levels of several SLE related cytokines were analyzed in patients and controls (Table 1), showing significant associations of sBLyS with other soluble mediators upregulated in SLE, specifically IFNα, IL-17A, IL-12p70 and MIP-1α (Table 2). Interestingly, in a multiple linear regression model including circulating levels of cytokines, age, gender and disease activity (SLEDAI and anti-dsDNA titer), serum levels of IL-17A remained as an independent predictor of sBLyS (β = 0.252, p = 0.005). Moreover, IL-17A levels correlated with mBLyS expression on neutrophils (ρ = 0.246, p = 0.045), a cell type that is activated by this cytokine in addition to being able to produce it. Therefore, these data suggest a relationship between IFNα, BLyS and IL-17 expression in SLE patients.
IL-17 producing cells are increased and associated with IFNα levels in SLE patients
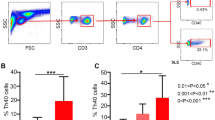
Therefore, in view of the observed results and since IL-17 can be produced by several cell subsets, including activated Th17 cells and neutrophils35, we analyzed the intracellular IL-17 and IFNγ expression in fresh peripheral blood cells from SLE patients and HC (Fig. 2A). The proportion of IL-17+ cells was increased among both neutrophils and CD4+ lymphocytes from patients compared to controls (Fig. 2B), in accordance with the elevated levels of this cytokine detected in the serum. Remarkably, IL-17+CD4+ lymphocytes exhibited a positive correlation with IFNα serum levels as well as with IFNRA1 B cell expression in SLE patients, but not in HC (Fig. 2C). These associations were not displayed by IFNγ+ cells, thus suggesting that IFNα plays a role in the activation of Th17 cells from SLE patients.
IL-17 producing cells are increased and associated with IFNα in SLE patients.
(A) IL-17 and IFNγ expression was analyzed intracellularly by flow cytometry in fresh peripheral blood neutrophils and CD4+ lymphocytes from SLE patients and HC. Representative dot-plots show positive cells for IL-17 or IFNγ expression, as determined by the fluorescence of cells labelled with the corresponding isotype -matched conjugated monoclonal antibody as a negative control. (B) Scatter-plots represent the percentage of IL-17+ or IFNγ+ cells among CD4+ lymphocytes or neutrophils in SLE patients and HC. Horizontal bars show the median. Statistical differences among groups were evaluated by Mann-Whitney U test. (C) Relationship between IL-17 producing CD4+ T cells and both IFNRA1 and IFNα in SLE patients. Graphs show the proportion of IL-17+ CD4+ cells related to the IFNRA1 expression on B cells (MFI) and the IFNα serum levels. Statistical significance was evaluated by the Spearman’s rank correlation test. (D) IL-17 levels in the culture supernatants of SLE and healthy PBMCs after IFNα treatment. Healthy and SLE PBMCs were treated with IFNα and the release of IL-17 was quantified after 2, 4 or 6 hours of culture. Bars represent median (interquartile range) of the percentage of change in the IL-17 levels in the presence of IFNα compared to unstimulated cultures. Independent experiments were performed with 10 healthy controls and 12 SLE patients. Statistical differences between IFNα-treated and untreated cells at each time of culture were evaluated by the Wilcoxon test for paired data. n.s.: not statically significant differences.
To assess this hypothesis, we performed in vitro cultures with PBMCs isolated from SLE patients and HC to analyze the effect of IFNα treatment on IL-17A and BLyS secretion. As was previously reported19, IFNα stimulation increased BLyS release in both HC and SLE cultures compared to untreated cells. However, IL-17A secretion was increased in the supernatants of PBMCs cultures from patients but not in those with HC cells (Fig. 2D). Moreover, IL-17A and BLyS levels were positively correlated in SLE cultures (ρ = 0.723, p = 0.031) but not with HC cultures, suggesting an anomalous relationship between BLyS, IFNα and IL-17 in SLE patients.
Discussion
In recent years, much evidence has demonstrated the crucial role of IFNα and BLyS in the etiopathogenesis of SLE2,36. Additionally, the upregulation and cellular mobilization of BLyS after IFNα stimulation has been reported19,20,21. Since clinical trials in SLE are conducted with agents that counteract BLyS23, its induction by IFNα in patients becomes an important topic. Consequently, the present study extends the proposed relationship between IFNα signaling and BLyS expression in SLE and reveals a possible pathogenic axis involving both molecules and IL-17 in these patients.
Although prior studies have pointed to a contribution of IFNAR signalling in autoantibody production and renal disease in murine models9,10,11,37,38, no previous works have analysed the expression of IFNAR in SLE patients. The findings of this study revealed an increased expression of IFNAR1 on various SLE leukocyte populations, which were closely related to the enhanced mBLyS levels. Moreover, both molecules were also correlated in healthy individuals, thus suggesting an intrinsic mechanistic connection between these two pathways.
In agreement with the simultaneous overexpression of IFNAR1 and mBLyS, our cohort of patients showed a positive correlation, not previously reported, between serum levels of IFNα and BLyS. Therefore, it becomes clear that IFNα signalling, via the IFNAR1, could be involved in SLE pathogenesis through the upregulation of BLyS in various cell types, including B cells, monocytes, mDCs and neutrophils. However, only the expression on B cells and neutrophils was positively correlated with the circulating amounts of BLyS.
Another interesting finding of this work was the relationship of IL-17 with both BLyS and IFNα in SLE patients, allowing us to propose a new cytokine pathogenic axis in this disease. IL-17 exerts a proinflammatory effect mediated by the recruitment and activation of monocytes and neutrophils, in addition to the induction of cytokines and chemokines39,40,41. Increased frequency of Th17 cells and elevated amounts of IL-17 have been implicated in the pathogenesis of SLE and other autoimmune diseases26,27,28,42,43, although the main cellular source of this cytokine in such patients is uncertain. Accordingly, our ex vivo assays of blood leukocytes indicated an increased proportion of cells expressing IL-17, not only CD4+ lymphocytes but also neutrophils, a population activated in SLE and able to produce this molecule in pathological conditions44,45. Interestingly, IL-17 serum levels in SLE patients correlated with circulating BLyS as well as with mBLyS expression on neutrophils. In line with this, it has been proposed that BLyS could promote the expansion of Th17 cells46, whereas IL-17, alone or in synergy with BLyS may stimulate B cell survival and differentiation29,30,31.
Likewise, IFNα serum levels and IFNRA1 expression on B cells were related to the proportion of CD4+ T cells producing IL-17 in patients, suggesting that high IFNα levels, maybe indirectly, could induce IL-17 secretion in SLE. In fact, our in vitro experiments support this hypothesis. Whereas our results agree with the observed blocking effect of type I IFNs on the IL-17 production by human PBMCs47,48,49,50, IFNα treatment of such cells from SLE patients induced IL-17 secretion, which was positively correlated with BLyS release. In agreement with this, IL-17 has been positively correlated with IFNα and MxA expression, a sensitive marker of type I IFNs activity51,52, in cutaneous lesions of lupus erythematosus53. This effect of IFNα on SLE cells could be due to an a non-canonical IFNAR signalling that can activate the transcription factor STAT3 or the induction of IL-6, both of them critical factors for the Th17 differentiation54,55,56. Moreover, higher percentages of T-helper cells producing IL-17 have been described in SLE patients expressing type I IFN inducible genes, supporting the hypothesis that type I IFN co-acts with Th17 cytokines in SLE pathogenesis57.
In summary, the present study has prompted us to propose the existence of a pathological axis in SLE involving IFNα, BLyS and IL-17, all of which are cytokines with well-known deleterious roles in these patients (Fig. 3). First, the high IFNα levels usually present in SLE patients could be responsible for widespread leukocyte activation and induction of BLyS, as was suggested by the coordinated overexpression of IFNAR1 and BLyS on the membrane of B cells, monocytes, mDCs and neutrophils and by the positive correlation detected between serum levels of both cytokines. Furthermore, IFNα could promote IL-17 secretion by Th17 cells, increased in SLE patients, probably through the co-stimulation provided by activated antigen-presenting cells, such as B cells, monocytes or mDCs, thus explaining the parallel release of BLyS and IL-17 in SLE cultures after IFNα treatment. Moreover, IFNα-stimulated mDCs secrete IL-2358, a cytokine that amplifies Th17 differentiation. Likewise, the altered neutrophils present in SLE patients, over-activated with IFNα, could be also a relevant source of IL-1759. Finally, the vicious circle is closed by the effect of IL-17 and BLyS promoting B cell survival and differentiation, which lead to autoantibody production and IFNα secretion by pDCs32,33. Also, IL-17 and BLyS can activate neutrophils, thus encouraging the preexisting inflammation. Furthermore, SLE neutrophils and especially the recently described low-density granulocyte subset60,61, could secrete increased levels of IFNα and form neutrophil extracellular traps (NETs), which in turn would lead to more IFNα synthesis by pDCs leading to a self-perpetuating cycle. Therefore, the concomitant participation of IFNα, BLyS and IL-17 in a pathogenic axis could be a key factor underlying the incomplete response to therapies based on the blockade of these cytokines in a subset of SLE patients. Hence, given the heterogeneity of the disease, determination of the individual cytokine profile could be useful for the identification of SLE patients candidates for biological therapies blocking such targets, alone or in combination.
Proposed IFNα, BLyS and IL-17 pathogenic axis in SLE.
The high IFNα levels present in most SLE patients could promote the activation and induction of BLyS in B cells, monocytes, mDCs and neutrophils, including the low-density granulocyte (LDG) subset. Thus, the co-stimulation provided by these activated antigen-presenting cells - such as B cells, monocytes or mDCs-, to the Th17 subset enhanced in SLE patients can induce the release of IL-17. Moreover, IL-23 secretion by IFNα-activated mDCs may amplify Th17 differentiation. Also, IFNα-activated neutrophils under the inflammatory conditions presented in SLE are able to secrete IL-17. Then, this vicious circle is closed by the combined effect of IL-17 and BLyS. First, they promoted B cell survival and differentiation, which lead to autoantibody production and further generation of the immune complexes able to induce IFNα secretion by pDCs. In addition, both cytokines can activate neutrophils, thus sustaining the preexisting SLE inflammation. Finally, IFNα secretion by neutrophils and especially by the LDG subset, propagates the disease process.
Methods
Patients and controls
Patients included in the study were not hospitalized individuals fulfilling at least four American College of Rheumatology (ACR) revised criteria for the SLE classification62 and that were sequentially recruited from the outpatient clinic of the Autoimmune Disease Unit (Hospital Universitario Central de Asturias, HUCA). Information on clinical features during the disease course was obtained after a retrospective review of clinical histories. At the time of sampling, patients were asked precise questions regarding the treatment received over the previous 3 months and anti-dsDNA titer and SLE disease activity index (SLEDAI) were determined. Ex vivo cytometric analysis were performed in sixty-seven patients (Table 3) and twenty-nine healthy controls (mean age ± SD: 47.73 ± 6.56 years; female/male: 21/8) recruited from the same population. For cytokine quantification, additional serum samples from 199 SLE patients (Supplementary Table S1) and 90 sex and age-matched healthy controls were collected (mean age ± SD: 45.37 ± 10.31 years; female/male: 78/21), including those previously recruited for cytometry analysis.
Ethics approval
Ethical approval for this study was obtained from the Regional Ethics Committee for Clinical Research (Servicio de Salud del Principado de Asturias), according to the Declaration of Helsinki. Written informed consent was signed by all individuals prior to participation in the study. All methods were carried out in accordance with the approved guidelines.
Flow cytometry analysis
Peripheral blood was collected with EDTA as anticoagulant. BLyS and IFNRA1 levels were simultaneously quantified by flow cytometry on B cells, neutrophils, monocytes, pDCs and mDCs, whereas IL-17 and IFNγ positive cells were identified among CD4+ lymphocytes and neutrophils. Monoclonal antibodies specific for IFNRA1 (PE) (R&D Systems, USA), CD19 (CF-Blue) (Immunostep, Spain), CD14 (APC-Cy7) (BioLegend, USA), CD303 (BDCA-2) (PECy7), BLyS (FITC), CD123 (PerCP-Cy5.5), CD1c (BDCA-1) (APC), CD4 (APC-Cy7), IL-17 (APC), IFNγ (PerCP-Cy5.5) and isotype, concentration and fluorochrome-matched control antibodies (all from eBioscence, USA) were employed for the cytometry analysis. The specificity of the monoclonal antibody for BLyS (clone 1D6)63 was confirmed in B lymphocytes and peripheral blood mononuclear cells after incubation with 10 or 100 ng/ml of soluble BLyS (eBioscience) at different times (0, 10, 30 and 60 minutes) followed by anti-BLyS (clone 1D6) staining. Blood cells were stained extracellularly with the appropriate monoclonal antibody to identify all the subpopulations for 30 min at 4°C and then cells were washed twice in staining buffer and resuspended in PBS. To determine IL-17 and IFNγ positive leukocytes, blood cells were fixed, permeabilized and intracellularly stained with monoclonal antibodies against these cytokines following the manufacturer’s instructions (Fixation/permeabilization buffer set; eBiosciences). Acquisition of 200,000 events and 10,000 CD4+ cells/tube was performed using a FACSCanto II flow cytometer (BD Biosciences). The analysis was based on cells located in an area of plots termed “the living region” which was defined using forward and side scatter. Then, different leukocyte subpopulations were identified according to the expression of specific surface markers, as previously described19. Thus, pDCs were identified as double positive cells for the expression of BDCA-2 and CD123, mDCs were defined as CD19− BDCA-1+, while monocytes, CD4+ lymphocytes and B cells and were identified by expression of CD14, CD4 or CD19, respectively. Neutrophils were identified according to their distinctive forward and side-scatter signal. Supplementary Table S2 shows the number of events counted in the different cellular subpopulations. Samples were subsequently analyzed using FlowJo software (Scripps Research Institute, San Diego, CA). Results were expressed as the percentage of positive cells or mean fluorescence intensity (MFI) of gated populations after subtracting the fluorescence of the background of the respective isotype control from the total fluorescence.
In vitro cultures
Peripheral blood mononuclear cells (PBMC) from healthy donors and patients were obtained by centrifugation over Ficoll-Hypaque gradients (Lymphoprep, Nycomed). PBMCs, at a density of 2 × 106/ml, were cultured in complete RPMI medium (RPMI 1640 containing 2 mM L-glutamine and 25 mM Hepes, supplemented with 10% heat-inactivated fetal calf serum and the antibiotic streptomycin and ampicillin at 100 μg/ml) at 37 °C and 5% carbon dioxide in presence or absence of human IFNα2b (Intron-A, 1000 U/ml). At different times of culture (2, 4 and 6 hours), cell-free supernatants from these cultures were collected for IL-17A and BLyS quantification.
Cytokine quantification
Culture supernatants and serum samples from SLE patients and HC were maintained at −80°C until cytokine determinations. IFNα, IL-10, IL-12p70, IL-1β, IL-17A, MIP-1α (CCL3) and GM-CSF amounts were quantified by Cytometric Bead Arrays Flex Set in a BD FACS Canto II flow cytometer (both from BD). For IL-1β and IL-10, an Enhanced Sensitivity Flex Set was needed. ELISA kits were used for the quantification of TNFα (Mini EDK kit, PeproTech), IFNγ (OptEIA kit, BD) and BLyS (Human BAFF Instant ELISA, eBioscience) following the manufacturer’s instructions. The lower limits of detection were 1.25 pg/ml for IFNα, 13.7 fg/ml for IL-10, 0.6 pg/ml for IL-12p70, 48.4 fg/ml for IL-1β, 0.3 pg/ml for IL-17A, 0.2 pg/ml for MIP-1α, 0.2 pg/ml for GM-CSF, 3.9 pg/ml for TNFα, 0.58 pg/ml for IFNγ and 0.13 ng/ml for BLyS.
Statistical analysis
The Kolmogorov-Smirnov test was used to assess the normal distribution of the data and nonparametric testing was used to determine differences between patient and control groups (Mann-Whitney U-test), while correlations were examined by Spearman’s rank correlation test. Multivariate linear regression analyses including treatments, demographic and clinical parameters were performed to determine the influence on the mBLyS and IFNRA1 expression. Variables were log-transformed to achieve normal distribution and standardized linear regression coefficients (beta) were used as an estimate of the association. Data were expressed as the median (interquartile range). A p-value < 0.05 was considered statistically significant. Data were analysed using GraphPad Prism 5 software (GraphPad Software, USA) and SPSS 22 statistical software package (SPSS Inc.).
Additional Information
How to cite this article: López, P. et al. A pathogenic IFNα, BLyS and IL-17 axis in Systemic Lupus Erythematosus patients. Sci. Rep. 6, 20651; doi: 10.1038/srep20651 (2016).
References
Hua, J., Kirou, K., Lee, C. & Crow, M. K. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti–RNA binding protein autoantibodies. Arthritis Rheum. 54, 1906–1916 (2006).
Crow, M. K. & Type I Interferon in the Pathogenesis of Lupus. J. Immunol. 192, 5459–5468 (2014).
Kirou, K. A. et al. Coordinate overexpression of interferon-alpha-induced genes in systemic lupus erythematosus. Arthritis Rheum. 50, 3958–3967 (2004).
Bengtsson, A. A. et al. Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus 9, 664–671 (2000).
Hooks, J. J. et al. Immune Interferon in the Circulation of Patients with Autoimmune Disease. N. Engl. J. Med. 301, 5–8 (1979).
Ytterberg, S. R. & Schnitzer, T. J. Serum interferon levels in patients with systemic lupus erythematosus. Arthritis Rheum. 25, 401–406 (1982).
Blanco, P., Palucka, A. K., Gill, M., Pascual, V. & Banchereau, J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science 294, 1540–1543 (2001).
Theofilopoulos, A. N., Baccala, R., Beutler, B. & Kono, D. H. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu. Rev. Immunol. 23, 307–336 (2005).
Nacionales, D. C. et al. Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis Rheum. 56, 3770–3783 (2007).
Agrawal, H. et al. Deficiency of type I IFN receptor in lupus-prone New Zealand mixed 2328 mice decreases dendritic cell numbers and activation and protects from disease. J. Immunol. Baltim. Md 1950 183, 6021–6029 (2009).
Santiago-Raber, M.-L. et al. Type-I Interferon Receptor Deficiency Reduces Lupus-like Disease in NZB Mice. J. Exp. Med. 197, 777–788 (2003).
Schneider, P. et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J. Exp. Med. 189, 1747–1756 (1999).
Moore, P. A. et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science 285, 260–263 (1999).
Nardelli, B. et al. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood 97, 198–204 (2001).
Chu, V. T., Enghard, P., Riemekasten, G. & Berek, C. In vitro and in vivo activation induces BAFF and APRIL expression in B cells. J. Immunol. Baltim. Md 1950 179, 5947–5957 (2007).
Collins, C. E. et al. B lymphocyte stimulator (BLyS) isoforms in systemic lupus erythematosus: disease activity correlates better with blood leukocyte BLyS mRNA levels than with plasma BLyS protein levels. Arthritis Res. Ther. 8, R6 (2006).
Petri, M. et al. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum. 58, 2453–2459 (2008).
McCarthy, E. M. et al. Elevated B lymphocyte stimulator levels are associated with increased damage in an Irish systemic lupus erythematosus cohort. Rheumatol. Oxf. Engl. 52, 1279–1284 (2013).
López, P. et al. Interferon-α-induced B-lymphocyte stimulator expression and mobilization in healthy and systemic lupus erthymatosus monocytes. Rheumatol. Oxf. Engl. (2014). doi: 10.1093/rheumatology/keu249
Litinskiy, M. B. et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3, 822–829 (2002).
Mathian, A., Weinberg, A., Gallegos, M., Banchereau, J. & Koutouzov, S. IFN-α Induces Early Lethal Lupus in Preautoimmune (New Zealand Black×New Zealand White)F1 but Not in BALB/c Mice. J. Immunol. 174, 2499–2506 (2005).
Yao, Y. et al. Neutralization of interferon-alpha/beta-inducible genes and downstream effect in a phase I trial of an anti-interferon-alpha monoclonal antibody in systemic lupus erythematosus. Arthritis Rheum. 60, 1785–1796 (2009).
Furie, R. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 63, 3918–3930 (2011).
Manzi, S. et al. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Ann. Rheum. Dis. 71, 1833–1838 (2012).
Li, D. et al. Interleukin-17 in systemic lupus erythematosus: A comprehensive review. Autoimmunity 1–9, doi: 10.3109/08916934.2015.1037441 (2015).
Bettelli, E., Oukka, M. & Kuchroo, V. K. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 8, 345–350 (2007).
Shin, M. S., Lee, N. & Kang, I. Effector T-cell subsets in systemic lupus erythematosus: update focusing on Th17 cells. Curr. Opin. Rheumatol. 23, 444–448 (2011).
Shah, K. et al. Dysregulated balance of Th17 and Th1 cells in systemic lupus erythematosus. Arthritis Res. Ther. 12, 1–10 (2010).
Doreau, A. et al. Interleukin 17 acts in synergy with B cell–activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat. Immunol. 10, 778–785 (2009).
Mitsdoerffer, M. et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl. Acad. Sci. 107, 14292–14297 (2010).
Park, S. J. & Shin, J. I. Interleukin-17 and B cell-activating factor in Kawasaki disease and juvenile systemic lupus erythematosus. Lupus 21, 1260 (2012).
Magnusson, M., Magnusson, S., Vallin, H., Rönnblom, L. & Alm, G. V. Importance of CpG dinucleotides in activation of natural IFN-alpha-producing cells by a lupus-related oligodeoxynucleotide. Scand. J. Immunol. 54, 543–550 (2001).
Farkas, L., Beiske, K., Lund-Johansen, F., Brandtzaeg, P. & Jahnsen, F. L. Plasmacytoid dendritic cells (natural interferon- alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am. J. Pathol. 159, 237–243 (2001).
Axtell, R. C., Raman, C. & Steinman, L. Type I interferons: beneficial in Th1 and detrimental in Th17 autoimmunity. Clin. Rev. Allergy Immunol. 44, 114–120 (2013).
Korn, T., Oukka, M., Kuchroo, V. & Bettelli, E. Th17 cells: Effector T cells with inflammatory properties. Semin. Immunol. 19, 362–371 (2007).
Vincent, F. B., Saulep-Easton, D., Figgett, W. A., Fairfax, K. A. & Mackay, F. The BAFF/APRIL system: Emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev. 24, 203–215 (2013).
Jørgensen, T. N., Roper, E., Thurman, J. M., Marrack, P. & Kotzin, B. L. Type I interferon signaling is involved in the spontaneous development of lupus-like disease in B6.Nba2 and (B6.Nba2×NZW)F1 mice. Genes Immun. 8, 653–662 (2007).
Braun, D., Geraldes, P. & Demengeot, J. Type I Interferon controls the onset and severity of autoimmune manifestations in lpr mice. J. Autoimmun. 20, 15–25 (2003).
Witowski, J. et al. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GROα chemokine from mesothelial cells. J. Immunol. 165, 5814–5821 (2000).
Ruddy, M. J., Shen, F., Smith, J. B., Sharma, A. & Gaffen, S. L. Interleukin-17 regulates expression of the CXC chemokine LIX/CXCL5 in osteoblasts: Implications for inflammation and neutrophil recruitment. J. Leukoc. Biol. 76, 135–144 (2004).
Agarwal, S., Misra, R. & Aggarwal, A. Interleukin 17 levels are increased in juvenile idiopathic arthritis synovial fluid and induce synovial fibroblasts to produce proinflammatory cytokines and matrix metalloproteinases. J. Rheumatol. 35, 515–519 (2008).
Wong, C. K. et al. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: Implications for Th17-mediated inflammation in auto-immunity. Clin. Immunol. 127, 385–393 (2008).
Crispín, J. C. et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J. Immunol. 181, 8761–8766 (2008).
Milanova, V., Ivanovska, N. & Dimitrova, P. TLR2 Elicits IL-17-Mediated RANKL Expression, IL-17 and OPG Production in Neutrophils from Arthritic Mice. Mediators Inflamm. 2014, e643406 (2014).
Cua, D. J. & Tato, C. M. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 10, 479–489 (2010).
Lai Kwan Lam, Q., King Hung Ko, O., Zheng, B.-J. & Lu, L. Local BAFF gene silencing suppresses Th17-cell generation and ameliorates autoimmune arthritis. Proc. Natl. Acad. Sci. USA 105, 14993–14998 (2008).
Meyers, J. A. et al. Blockade of TLR9 agonist-induced type I interferons promotes inflammatory cytokine IFN-gamma and IL-17 secretion by activated human PBMC. Cytokine 35, 235–246 (2006).
Cui, F., Meng, J., Luo, P. & Chen, P. IFN- alpha blocks IL-17 production by peripheral blood mononuclear cells in patients with chronic active hepatitis B Infection. BMC Infect. Dis. 14, 55 (2014).
Hirohata, S., Shibuya, H. & Tejima, S. Suppressive influences of IFN-alpha on IL-17 expression in human CD4+T cells. Clin. Immunol. Orlando Fla 134, 340–344 (2010).
Liu, X., Yang, P., Wang, C., Li, F. & Kijlstra, A. IFN-alpha blocks IL-17 production by peripheral blood mononuclear cells in Behcet’s disease. Rheumatol. Oxf. Engl. 50, 293–298 (2011).
Roers, A., Hochkeppel, H. K., Horisberger, M. A., Hovanessian, A. & Haller, O. MxA gene expression after live virus vaccination: a sensitive marker for endogenous type I interferon. J. Infect. Dis. 169, 807–813 (1994).
Horisberger, M. A. Interferon-induced human protein MxA is a GTPase which binds transiently to cellular proteins. J. Virol. 66, 4705–4709 (1992).
Oh, S. H. et al. Expression of interleukin-17 is correlated with interferon-α expression in cutaneous lesions of lupus erythematosus. Clin. Exp. Dermatol. 36, 512–520 (2011).
Ambrosi, A., Espinosa, A. & Wahren-Herlenius, M. IL-17: a new actor in IFN-driven systemic autoimmune diseases. Eur. J. Immunol. 42, 2274–2284 (2012).
Harris, T. J. et al. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J. Immunol. Baltim. Md 1950 179, 4313–4317 (2007).
van Boxel-Dezaire, A. H. H., Rani, M. R. S. & Stark, G. R. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity 25, 361–372 (2006).
Brkic, Z. et al. T-helper 17 cell cytokines and interferon type I: partners in crime in systemic lupus erythematosus? Arthritis Res. Ther. 16, R62 (2014).
Farkas, A., Tonel, G. & Nestle, F. o. Interferon-α and viral triggers promote functional maturation of human monocyte-derived dendritic cells. Br. J. Dermatol. 158, 921–929 (2008).
Palanichamy, A. et al. Neutrophil-mediated IFN activation in the bone marrow alters B cell development in human and murine systemic lupus erythematosus. J. Immunol. Baltim. Md 1950 192, 906–918 (2014).
Denny, M. F. et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. Baltim. Md 1950 184, 3284–3297 (2010).
Knight, J. S. & Kaplan, M. J. Lupus neutrophils: ‘NET’ gain in understanding lupus pathogenesis. Curr. Opin. Rheumatol. 24, 441–450 (2012).
Hochberg, M. C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 40, 1725 (1997).
Hase, H. et al. BAFF/BLyS can potentiate B-cell selection with the B-cell coreceptor complex. Blood 103, 2257–2265 (2004).
Acknowledgements
This work was funded by European Union FEDER funds, Fondo de Investigación Sanitaria [FIS, PI12/00523] and FICYT [COF13–12]. J.R.-C. is a recipient of a FPU grant from the Spanish Ministerio de Educación, Cultura y Deporte. The authors would like to show our gratitude to SLE patients and ALAS (Asociación Lúpicos de Asturias) for their continuous encouragement.
Author information
Authors and Affiliations
Contributions
P.L. participated in the study conduct, cellular and cytokine quantification, data analysis/interpretation and manuscript preparation. J.R.-C. and L.M. performed some experimental procedures. L.C.-M. contributed to sample collection as well as review of clinical manifestations, disease activity and therapy of patients. A.S. contributed to study design/conduct, data interpretation and manuscript preparation.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
López, P., Rodríguez-Carrio, J., Caminal-Montero, L. et al. A pathogenic IFNα, BLyS and IL-17 axis in Systemic Lupus Erythematosus patients. Sci Rep 6, 20651 (2016). https://doi.org/10.1038/srep20651
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep20651
This article is cited by
-
The star target in SLE: IL-17
Inflammation Research (2023)
-
Serum protein pattern associated with organ damage and lupus nephritis in systemic lupus erythematosus revealed by PEA immunoassay
Clinical Proteomics (2017)
-
Genetic Polymorphisms of rs3077 and rs9277535 in HLA-DP associated with Systemic lupus erythematosus in a Chinese population
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.