Abstract
Ixodid ticks transmit several important viral pathogens. We isolated a new virus (Tofla virus: TFLV) from Heamaphysalis flava and Heamaphysalis formsensis in Japan. The full-genome sequences revealed that TFLV belonged to the genus Nairovirus, family Bunyaviridae. Phylogenetic analyses and neutralization tests suggested that TFLV is closely related to the Hazara virus and that it is classified into the Crimean-Congo hemorrhagic fever group. TFLV caused lethal infection in IFNAR KO mice. The TFLV-infected mice exhibited a gastrointestinal disorder and positron emission tomography-computed tomography images showed a significant uptake of 18F-fluorodeoxyglucose in the intestinal tract. TFLV was able to infect and propagate in cultured cells of African green monkey-derived Vero E6 cells and human-derived SK-N-SH, T98-G and HEK-293 cells. Although TFLV infections in humans and animals are currently unknown, our findings may provide clues to understand the potential infectivity and to develop of pre-emptive countermeasures against this new tick-borne Nairovirus.
Similar content being viewed by others
Introduction
Ixodid (hard) ticks transmit several important viral pathogens, including those of the families Bunyaviridae, Flaviviridae, Reoviridae, Rhabdoviridae and Orthomyxoviridae1,2,3. Tick-borne Bunyaviruses include highly pathogenic viruses, such as the Crimean-Congo hemorrhagic fever virus (CCHFV), severe fever with thrombocytopenia syndrome virus (SFTSV) and Heartland virus4,5,6. However, neither efficient vaccines nor specific treatments against these tick-borne diseases are currently available. Instead, epidemiological surveys of ticks provide important information, such as the infection rates in ticks, identification of the tick species that harbor the viruses, distribution of the tick vectors and endemic area, which are used to guide pre-emptive countermeasures to prevent infections with these viruses through tick bites.
CCHFV is categorized as a biosafety level (BSL)-4 pathogen and causes severe hemorrhagic disease with up to 30% mortality in humans4. CCHFV is transmitted by Hyalomma ticks and is broadly distributed in western China, central Asia, the Middle East to southeastern Europe and throughout Africa4. CCHFV belongs to the genus Nairovirus of the family Bunyaviridae4. The principal vector of Nairovirus is ticks and there are seven serogroups in the genus Nairovirus: i) CCHF, ii) Nairobi Sheep Disease (NSD), iii) Dera Ghazi Khan, iv) Hughes, v) Qalyub, vi) Sakhalin and vii) Thiafora. The CCHF and NSD groups include pathogenic viruses in humans and domestic animals4,7.
SFTS is an emerging disease that was first reported in the rural areas of central China in 20095 and has currently been identified in Korea and Japan8,9. The causative agent, SFTSV, belongs to the genus Phlebovirus of the family Bunyaviridae. SFTSV has been detected in ticks in China, including Haemaphysalis longicornis10. An SFTS case was identified in Japan in 2013 and more than 140 cases of SFTS have been identified in western Japan. However, epidemiological information on SFTSV in ticks has not been fully elucidated in Japan. Therefore, we attempted to survey the tick species, infection rates and endemic areas of SFTSV in ticks distributed in Japan.
An epidemiological survey identified a new infectious agent that exhibited a fatal infection in IFNAR KO mice, which is a useful animal for the evaluation of tick-borne Bunyaviruses, including SFTSV, CCHFV and Hazara virus (HAZV)11,12,13. This study demonstrated the experimental findings of the isolation, classification and in vivo and in vitro infectivity of this newly identified infectious agent, Tofla virus (TFLV).
Results
Virus isolation from Haemaphysalis ticks in Japan
Inoculations with homogenized tick pools of H. flava collected in Tokushima in 2013 and H. formosensis collected in Nagasaki in 2014 (Supplementary Figure 1) resulted in fatal outcomes in 129 strain background IFNAR KO (A129) mice at 3 days post-inoculation (pi). Homogenized spleen samples collected from the dead mice were filtrated and re-inoculated in A129 mice and these re-inoculated mice died. Virus-like particles with diameters of approximately 100 nm were detected in the culture fluids (CFs) of Vero E6 cells after inoculation with a spleen sample of a mouse inoculated with a tick homogenate (H. flava in Tokushima) (Fig. 1a). These observations suggested that filterable infectious agents, i.e., TFLV, were included in the homogenized tick samples. Two strains of TFLV from H. flava in Tokushima and H. formosensis in Nagasaki were named Tok-Hfla-2013 and Nag-Hfor-2014, respectively.
TFLV belongs to the genus Nairovirus of the family Bunyaviridae
We next determined the genome sequence of the TFLV using next-generation sequencing (NGS) and general sequencing. The genome had three segments with homologous sequences of the family Bunyaviridae (Fig. 1b). Two strains of Tok-Hfla-2013 and Nag-Hfor-2014 showed the same genome sizes for the L (12,143 bp), M (4,625 bp) and S (1,699 bp) segments. The deduced proteolytic sites in the glycoproteins Gn and Gc (i.e., the M segment) of TFLV were similar to the conserved sites in Nairoviruses, such as HAZV, CCHFV, Dugbe virus (DUGV) and Erve virus (ERVEV) (Fig. 1c). These results suggested that TFLV belongs to the genus Nairovirus.
TFLV belongs to the CCHF group
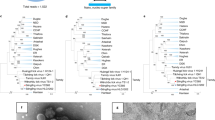
The identities of the nucleotide sequences between the two strains of TFLV were 99.0, 97.2 and 99.1% in the S, M and L segments, respectively. The identities of the amino acid sequences were 100, 99.0 and 99.6% in the S, M and L segments, respectively. The phylogenetic analyses revealed that the two strains of TFLV clustered with HAZV (Fig. 2 and Supplementary Figure 2). The identities of the amino acids between two strains of TFLV and HAZV were 75.7%, 61.2–61.3% and 21.1–21.7% in the S, M and L segments, respectively.
The focus-reduction neutralization test (FRNT) using TFLV (Tok-Hfla-2013) demonstrated that anti-TFLV and anti-HAZV sera had 1:640 and 1:20 neutralization titers against TFLV, respectively (Table 1). The plaque reduction neutralization test (PRNT) using HAZV showed that anti-TFLV and anti-HAZV sera had 1:160 and 1:80 neutralization titers against HAZV (Table 1). These results suggest that the anti-TFLV and anti-HAZV sera exhibited cross-neutralization between TFLV and HAZV infections.
Of note, HAZV belongs to the CCHF serogroup13,14. Therefore, these data suggest that TFLV belongs to the CCHFV serogroup with HAZV.
Pathogenicity of TFLV infection in A129 mice
TFLV was identified from dead A129 mice inoculated with the tick samples. Therefore, we next examined the pathogenicity of the TFLV infection in A129 mice. Following subcutaneous inoculation with TFLV (Tok-Hfla-2013), the mice began to die at 3 days pi and all mice died by day 6 following infection with titers containing more than 10−1 focus-forming units (ffu) infections (Fig. 3a). The LD50 of TFLV was 10−1.7 ffu (Table 2). The mean survival times of the TFLV infections with the 10−1 to 103 ffu that showed 100% mortality were 3.8 ± 0.56 to 4.7 ± 0.68 days (Table 2). The mice that died exhibited acute clinical signs, such as slow movement, ataxia, piloerection, weight loss and loose stool at 2 days pi, whereas the mice that survived did not exhibit any clinical signs.
Viral RNAs were detected in some tissues at 1-day pi and increased to as many as 106 to 107 copies/100 ng of RNA in every tissue (Fig. 3b) at 3 days pi, indicating that TFLV rapidly replicated in these tissues of the A129 mice. There were no significant differences in the viral RNA levels between tissues at 3 days pi (Fig. 3b).
Gross pathology revealed gastric and intestinal distensions, reduced stools in the intestine and reduced cecum size in the TFLV-infected mice (Fig. 3c). The stomach content of TFLV-infected mice was in liquid form, whereas that of mock-infected mice was in solid form (Fig. 3c).
The histopathological examination revealed that the viral genomic RNA was prominently detected in epithelial cells, in particular, in the stomach, duodenum and small intestine (Fig. 4a). Erosive change of the gastric and duodenal mucosae was evident and the viral genome was densely accumulated in these eroded areas. The viral genome was also detected in non-eroded small bowel mucosa and the germinal center of the colonic lymphoid follicles. Esophageal squamous mucosa was also mildly virus-infected. No lesions or signals were demonstrated in the mock-infected mice (Fig. 4b).
The 18F-fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET/CT) images showed 18F-FDG uptake in the intestinal tract (Fig. 5a). No significant uptake of 18F-FDG in the intestinal tract was observed in the mock-infected mice (Fig. 5b). These results suggest that the TFLV infection caused gastrointestinal disorders in A129 mice.
Infectivity of TFLV in human cell lines
We next examined whether TFLV could infect and propagate in human cells. African green monkey-derived Vero E6 and human-derived SK-N-SH, T98-G and HEK-293 cells were inoculated with TFLV (Tok-Hfla-2013) and viral RNAs in the CFs were detected. The viral RNAs in the CFs significantly increased at 1 to 3 days pi in the Vero E6 cells (Fig. 6). Increased viral RNAs were observed in the SK-N-SH, T98-G and HEK-293 cells at 3 to 5 days pi (Fig. 6). These results suggest that TFLV can infect and propagate in these cells, although whether TFLV indeed infects humans is not certain from these results.
Discussion
This study identified a new TFLV from ticks in Japan. The TFLV was closely related to HAZV, which belongs to the CCHF group of the genus Nairovirus. Therefore, the TFLV is suggested to be a new virus in the CCHF group. HAZV was isolated from Ixodes redikorzevi collected in Pakistan in 196415 and no further virus strains have been reported since that time. HAZV infectivity and pathogenicity have not been reported in humans. Thus, HAZV can be used as a BSL-2 pathogen, whereas CCHFV must be used in a BSL-4 facility. We used TFLV in a BSL-3 facility because its pathogenic potential in humans is not clear. Further investigation of human TFLV pathogenicity is required.
We isolated two strains, Tok-Hfla-2013 and Nag-Hfor-2014, from different tick species in different areas. The distance between Tokushima and Nagasaki is approximately 500 km. The tick species H. flava and H. formosensis feed on wild animals, such as deer and wild boars and they also feed on humans. H. flava is distributed over a large area of Japan, whereas H. formosensis has been collected in the western and southern regions of Japan, but not in the eastern and northern areas16,17. Therefore, TFLV may be distributed throughout Japan and may naturally circulate within ticks and animals.
TFLV exhibited lethal infection in A129 mice. We also attempted to infect immunocompetent mice with TFLV, such as B6 mice, but these mice did not exhibit any clinical signs (data not shown). Thus, IFN-I responses are likely to be important for protection against TFLV in mice. Previous reports have shown that IFNAR KO mice are useful animal models for in vivo infections with CCHFV and HAZV in the CCHF group12,13 and it has been suggested that inoculating mice with HAZV may act as a surrogate model for testing antiviral agents against CCHFV13,14. Thus, the TFLV infection of mice may also provide a potential model for such testing against CCHFV.
The cause of death in the TFLV-infected A129 mice was uncertain, but a severe gastrointestinal disorder, including indigestion, was observed in the infected mice. Apparently 18F-FDG was taken up in the intestinal tract, but not in the stomach, although pathological lesions and viral infections were similarly observed in both tissues. 18F-FDG is a glucose analog and its uptake is a marker for tissue glucose uptake, which closely correlates with certain types of tissue metabolism18. Thus, glucose metabolism in the intestine may be a key factor of the severe gastrointestinal disorder correlated with the lethal infection in the TFLV-infected mice. Further elucidations of the mechanism of 18F-FDG accumulation in the intestine may provide a novel approach to understanding the disease due to the TFLV-infection in this mouse model.
SFTSV is recently identified emerging virus. The earliest case of all of the reported SFTS cases worldwide was from a patient in Nagasaki, Japan, who was infected in 2005 9. SFTS cases are sporadically distributed in Japan. Therefore, SFTSV has likely existed in this country for a long time, but the disease was not identified because of the rarity of cases. This observation suggests that TFLV might have also caused a disease outbreak that was not identified. Our study demonstrated that TFLV infected and replicated in human cells, but it is unclear if actual viral infections occur in humans. Further investigations, including seroepidemiological surveys using human and animal samples, are an important priority to determine whether TFLV causes infectious diseases in humans and animals. In addition, in vitro experiments using more cell lines, including other tissue-derived human cell lines, such as gastrointestinal cells and other animal-derived cell lines, will provide useful information for understanding the infectivity of TFLV in mammals.
Methods
Virus and cells
The HAZV and the YG-1 strain of SFTSV were kindly provided by Roger Hewson, Public Health England and Ken Maeda, Yamaguchi University, respectively. Stock viruses of TFLV and SFTSV were prepared from a cell culture medium of Vero E6 (African green monkey kidney) cells. The infectious titers of the TFLV and SFTSV stocks were determined using a focus-forming assay. The HAZV stock virus was prepared in SW13 (Human adenocarcinoma) cells. The infectious titer of the HAZV stock was determined using a plaque-forming assay. Vero E6, SW13, SK-N-SH (human neuroblastoma), T98-G (human glioblastoma multiform) and HEK-293 (human embryonic kidney) cells were maintained in Eagle’s Minimal Essential Medium (EMEM; Nissui Pharmaceutical Co., Tokyo, Japan) containing 10% fetal bovine serum (FBS). All experiments using live TFLV and SFTSV were performed in a BSL-3 laboratory at Nagasaki University according to standard BSL-3 guidelines. The experiments using live HAZV were performed in a BSL-2 laboratory.
Focus-forming assay
Confluent Vero E6 cells were inoculated with serially diluted culture supernatants of TFLV and SFTSV stocks and incubated in 2% FCS EMEM containing 1% methyl cellulose 4,000 (Wako Pure Chemical Industries, Ltd., Tokyo, Japan) for 5 days. The viral foci of TFLV were detected using TFLV antiserum from the TFLV-infected B6 mice, peroxidase-conjugated anti-mouse IgG (American Qualex, CA, USA) and DAB substrate (Wako Pure Chemical Industries, Ltd., Tokyo, Japan). The viral foci of SFTSV were detected using an SFTSV antiserum from the recovered SFTS cases, peroxidase-conjugated anti-human IgG (American Qualex, CA, USA) and DAB substrate. Viral titers were expressed as ffu/ml. The experiment using human serum was performed with the approval of the ethics committee of the Institute of Tropical Medicine, Nagasaki University (approval number: 140829129).
Plaque-forming assay
Confluent SW13 cells were inoculated with serially diluted culture supernatants of the HAZV stock and incubated in 0.7% FBS MEM containing 0.7% agarose for 4 days. The cells were fixed with a methanol/acetic acid mixture (5:1) and stained with 0.3% amido black. The viral titers were expressed as pfu/ml.
Mice
A129 mice were purchased from B&K Universal Limited and were mated in the facility at Nagasaki University. The adult (older than eight weeks old) A129 mice were subcutaneously inoculated with 10−3 to 103 ffu of TFLV (Tok-Hfla-2013 strain) (n = 10) in EMEM containing 2% FBS. The mice were weighed daily and observed for clinical signs of disease. The B6 mice were purchased from CLEA Japan, Inc. The animal experiments were performed in accordance with the recommendations in the Fundamental Guidelines for the Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology. The Animal Care and Use Committee of the Nagasaki University approved all of the experimental protocols (approval number: 1401201115, 1408051166).
TFLV isolation from ticks
H. flava and H. formosensis were collected by flagging in Tokushima in 2013 and in Nagasaki in 2014, respectively. The pooled ticks of H. flava (9 nymphs) and H. formosensis (30 nymphs) were homogenized using Micro SmashTM MS-100 R (TOMY DIGITAL BIOLOGY CO., LTD, Tokyo, Japan) with one stainless bead (4.8 Ø) and 0.5 ml of 2% FBS EMEM per reaction tube at 4,500 rpm for 15 seconds at 4 °C. A total of 100 μl of the samples was used to intraperitoneally inoculate adult A129 mice. The spleens were collected from the dead mice after 3 days and homogenized in 2% FBS EMEM using the same procedure as the tick homogenization. The supernatants of the homogenized spleen samples were inoculated in Vero E6 cells and the CFs were harvested after 6 days.
Electron microscopy
The CFs from the TFLV-infected Vero E6 cells were mixed with PEG 6000 (Wako) and NaCl (Wako, Tokyo, Japan) and stirred at 4 °C for 2 hours. The supernatant was centrifuged at 12,000 G for 1 hour. The PEG and virus pellet was dissolved in 4 ml of STE buffer and centrifuged at 12,000 G for 10 minutes. The supernatant sample was loaded on a sucrose gradient (50% to 15%) and centrifuged at 100,000 G at 4 °C overnight. The turbid band containing virions was collected. The sample was dissolved in STE buffer and centrifuged at 200,000 G for 4 hours. The pellet at the bottom of the tube was dissolved in STE buffer and centrifuged again to completely remove the sucrose. The pellet was dissolved in phosphate-buffered saline (PBS), loaded on a 200-mesh copper grid with carbon-coated plastic film (Nisshin EM, Tokyo, Japan) and negatively stained with 10 μl of a uranyl acetate solution (1.5%, w/v) for 10 s. The morphology of the sample was observed on a JEM-1230 (JEOL Ltd., Tokyo, Japan), with a 80 kV acceleration voltage and imaged using a 2k × 2k Veleta CCD camera (Olympus Soft Imaging Solutions, Lakewood, CO, USA).
TFLV genome sequence
RNA was extracted from the homogenized mouse spleens using a RNeasy Lipid Tissue Mini Kit (Qiagen, Hilden, Germany). Next-generation sequencing (NGS) was performed using a GS Junior 454 (Roche Diagnostic Cooperation, Branford, CT, USA). The viral genome sequences were assembled and analyzed using CLC Genomics Workbench software. The homolog sequences with the Nairovirus were determined using BLAST (http://blast.ncbi.nlm.nih.gov). The terminal regions of the genome were determined using the Sanger method using primers that were designed from the terminal region of the Nairovirus sequence and the 5′-Rapid Amplification of cDNA End (5′-RACE) method was performed using a 5′-Full RACE Core Set (Takara Bio, Inc., Tokyo, Japan). The primers were designated using PRIMER3-web (http://primer3.ut.ee). PCR amplification was performed using Tks Gflex™ DNA Polymerase (Takara Bio, Inc., Tokyo, Japan) and Sanger sequencing was performed using a 3130 × l Genetic Analyzer or 3100 Avanti Genetic Analyzer (Applied Biosciences, CA, USA). The GenBank accession numbers of Tok-Hfla-2013 are LC008510 (S segment), LC0008511 (M segment) and LC0008512 (L segment) and the numbers of Nag-Hfor-2014 are LC030113 (S segment), LC030114 (M segment) and LC030115 (L segment).
Phylogenetic analysis
The genome sequences were assembled and analyzed using Phred/Phrap/Consed software. The viral open reading frames and translated amino acid sequences were analyzed using EMBOSS software ver. 6.3.1. The amino acid sequences were aligned using dialign-tx 1.0.2 software19 and processed using TrimAl 1.2 software20. The amino acid sequences based on full-genome sequences were aligned using mafft v7.24521. The phylogenetic trees were constructed based on the conserved region of the amino acid sequences (Fig. 2) and based on the full amino acid sequences (Supplementary Figure 2). The substitution models were determined by Protest v3.222 and Protest v3.4.1. The genetic distance among Nairoviruses was deduced using the maximum likelihood model by PhyML v3.0.123. The phylogenetic trees were processed using FigTree 1.4.2 tree viewer software. The following accession codes were obtained for phylogenetic and homology analyses: Abu Hammad virus (AAQ93043.1), Abu Mina virus (AAQ93044.1), CCHFV (NP_950237.1, YP_325663.1, NP_950235.1), Chim virus (KF801656.1), DUGV (NP_690576.1, NP_690575.1,NP_690574.1), ERVEV (AFH89034.1, AFH89033.1, AFH89032.1), Farallon virus (AAQ93048.1), HAZV (KC344857.1, DQ813514.1, DQ076419.1), Issyk-Kul virus (AII79373.1), Leopards Hill virus (YP_009111284.1), NSD virus (ACH99797.1), Paramushir virus (AHH24352.1), Punte Salinas virus (AAQ93052.1), Qalyub virus (AAQ93053.1), Raza virus (AAQ93054.1), Sakhalin virus (AHH24354.1) and Tillamok virus (AAQ93055.1).
Neutralization test
The B6 mice were subcutaneously infected with 104 ffu of the TFLV (Tok-Hfla-2013), 103 pfu of the HAZV or 106 ffu of the SFTSV and blood samples were collected after 3 weeks pi. The sera were collected from the blood samples. The neutralizing antibody titers against TFLV and SFTSV were determined using 50% FRNT and those against HAZV were determined using 50% PRNT. The serially diluted sera were mixed with TFLV, HAZV or SFTSV and incubated at 37 °C for 1 hour. The Vero E6 cells were inoculated with mixtures of TFLV or SFTSV and incubated for 5 days. The SW13 cells were inoculated with a mixture of HAZV and incubated for 4 days. The focus and plaque staining were performed as described above. The neutralizing titers were determined as the reciprocal of the highest serum dilution that reduced the viral foci of TFLV and SFTSV or the plaque counts of HAZV by 50%.
Copy numbers of TFLV RNA in mouse tissues
The viral replications in the TFLV-infected mice were detected by the viral copy numbers as shown in previous studies for Bunyavirus infections11,13,24,25,26. The mice were subcutaneously infected with 102 ffu of TFLV (Tok-Hfla-2013) and five mice per group were sacrificed at 1 and 3 days pi. The thymus, lungs, spleen, liver, kidneys, small intestine, colon, cecum, brain and spinal cord were removed following perfusion with cold PBS. The small intestine, colon and cecum were washed with cold PBS to remove the stool. The brains were divided into two fractions, the brain cortex and non-cortex tissues. These tissues were immediately submerged in RNAlater (Ambion, CA, USA) and stored at −80 °C until they were used. The total RNA was extracted using an RNeasy Lipid Tissue Mini Kit (Qiagen, Hilden, Germany). The viral copy numbers were examined by using real-time RT-PCR. The TFLV-specific primers and probes were designated based on the M segment using PRIMER3-web. The forward primer was HSKV_M1F: 5′-GCATACACCATGTCCGTGAG-3′, the reverse primer was HSKV_M1R: 5′-GGAAAGTTCCTGAGCTGCAC-3′ and the PrimeTime® qPCR probe was HSKV_M1IN: 5′-/56-FAM/CACCACCAC/ZEN/CTGCATAACAG/3IABkFQ/-3′ (Integrated DNA Technologies, IA, USA). The real-time RT-PCR reactions were performed using a One Step PrimeScript RT-PCR Kit (Takara Bio, Inc., Tokyo, Japan) in a 7500 Real-time PCR System (Applied Biosystems, CA, USA). The copy numbers were calculated as a ratio of the copy numbers of a standard control.
Histopathological study and In situ hybridization (ISH)
The mice were subcutaneously infected with 102 ffu of TFLV (Tok-Hfla-2013) and five mice per group of the A129 mice were anesthetized and perfused with 10% phosphate-buffered formalin. The fixed tissues were routinely embedded in paraffin, sectioned and stained with hematoxylin and eosin. The viral RNA was detected using ISH in formalin-fixed, paraffin-embedded (FFPE) sections using the AT-tailing method for high-sensitivity detection27. The FFPE sections mounted on aminosilane-coated glass slides (Matsunami, Tokyo, Japan) were heated in a pressure pan cooker in 10 mM citrate buffer, pH 6.0 for 10 min, followed by digestion with 0.1 μg/mL proteinase K for 15 min at 37 °C. The endogenous biotin activity was quenched using a biotin blocking system (Dako, Carpinteria, CA, USA) and the sections were hybridized overnight at 50 °C with 0.01 pmol/mL AT-tailed oligonucleotide cocktail sense or antisense probes against the L segment (sense: 5′-GTCTGAGTGGCCAGACTCAAGAGTGGTCACAC TAGTAGTGATATATATATATATATATAT-3′, antisense: 5′-CACTACTAGTGTGACCA CTCTTGAGTCTGGCCACTCAGACATATATATATATATATATAT-3′), M segment (sense: 5′-TGGCTTCACTTCACTACCCTCTGTTATGCAGGTGGTGGTGATATATAT ATATATATATAT-3′, antisense: 5′-CACCACCACCTGCATAACAGAGGGTAGTGAA GTGAAGCCAATATATATATATATATATAT-3′) and S segment (sense: 5′-GGAGTTCC ACGCCTGGTTCAAGGCCTATTCAGATAAGCACATATATATATATATATATAT-3′, antisense: 5′-GTGCTTATCTGAATAGGCCTTGAACCAGGCGTGGAACTCCATATA TATATATATATATAT-3′) segments of the viral RNA. The sections were rinsed in 1× saline sodium citrate (SSC) after hybridization followed by 0.1× SSC for 10 min at 55 °C. “Gene Frame” (Thermo Fisher Scientific, Yokohama, Japan) was attached to each slide for exposure to the AT-tailing mixture containing nucleotides, biotin-16-dUTP and Gene Taq DNA polymerase for 10 min at 60 °C. The Gen Point System (Dako, Carpinteria, CA, USA) was employed for signal amplification as follows. One thousand-fold diluted streptavidin-horseradish peroxidase (SA-HRP) was applied to the sections for 15 min at room temperature (RT). The biotinylated tyramides were reacted for 15 min at RT. SA-HRP was challenged again for 15 min at RT. The reaction products were visualized in 20 mg/dL diaminobenzidine tetrahydrochloride and 0.006% hydrogen peroxide in 50 mM Tris-HCl buffer, pH 7.6 and the nuclei were lightly counterstained with hematoxylin.
PET/CT imaging
PET/CT images were acquired using Triumph combined PET/SPECT/CT systems (TriFoil Imaging, Inc., CA, USA). The A129 mice were subcutaneously infected with 102 ffu of TFLV (Tok-Hfla-2013) and administered 10.2–11.4 of MBq 18F-FDG (Nihon Medi-Physics Co., Ltd. Kurume, Japan) intravenously in the tail vein 3 days pi. The mice were anesthetized with 1.5% isoflurane and CT acquisitions were performed for anatomical reference. Subsequently, PET acquisitions were performed for 30 min starting 30 min after 18F-FDG injection. The PET data were reconstructed using a 3D-Maximum-Likelihood Expectation Maximization (MLEM) algorithm (60 iterations). The acquired PET and CT data were processed using VIVID™ (TriFoil Imaging, Inc.).
Growth curves of TFLV in cultured cells
A total of 105 cells per well of the Vero E6, SK-N-SH, T98-G and HEK-293 cells were inoculated with 103 ffu of TFLV (Tok-Hfla-2013) at a multiplicity of infection of 0.01 in 12-well plates. The experiment was performed in triplicate. The CFs and cells were harvested at 0, 1, 3, 5 and 7 days pi. RNAs were extracted from the CFs using a QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany). The viral copy numbers were examined using real-time RT-PCR as described above.
Additional Information
How to cite this article: Shimada, S. et al. Tofla virus: A newly identified Nairovirus of the Crimean-Congo hemorrhagic fever group isolated from ticks in Japan. Sci. Rep. 6, 20213; doi: 10.1038/srep20213 (2016).
References
Lani, R. et al. Tick-borne viruses: a review from the perspective of therapeutic approaches. Ticks and tick-borne diseases 5, 457–465, 10.1016/j.ttbdis.2014.04.001 (2014).
Butenko, A. M., Gromashevsky, V. L., L’Vov, D. K. & Popov, V. F. First isolations of Barur virus (Rhabdoviridae) from ticks (Acari: Ixodidae) in Africa. J Med Entomol 18, 232–234 (1981).
Davies, C. R., Jones, L. D. & Nuttall, P. A. Experimental studies on the transmission cycle of Thogoto virus, a candidate orthomyxovirus, in Rhipicephalus appendiculatus. Am J Trop Med Hyg 35, 1256–1262 (1986).
Bente, D. A. et al. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res 100, 159–189, 10.1016/j.antiviral.2013.07.006 (2013).
Yu, X. J. et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med 364, 1523–1532, 10.1056/NEJMoa1010095 (2011).
McMullan, L. K. et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med 367, 834–841, 10.1056/NEJMoa1203378 (2012).
Marczinke, B. I. & Nichol, S. T. Nairobi sheep disease virus, an important tick-borne pathogen of sheep and goats in Africa, is also present in Asia. Virology 303, 146–151 (2002).
Kim, K. H. et al. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg Infect Dis 19, 1892–1894, 10.3201/eid1911.130792 (2013).
Takahashi, T. et al. The first identification and retrospective study of Severe Fever with Thrombocytopenia Syndrome in Japan. J Infect Dis 209, 816–827, 10.1093/infdis/jit603 (2014).
Zhang, Y. Z. et al. The ecology, genetic diversity and phylogeny of Huaiyangshan virus in China. Journal of virology 86, 2864–2868, 10.1128/JVI.06192-11 (2012).
Liu, Y. et al. The pathogenesis of severe fever with thrombocytopenia syndrome virus infection in alpha/beta interferon knockout mice: insights into the pathologic mechanisms of a new viral hemorrhagic fever. Journal of virology 88, 1781–1786, 10.1128/JVI.02277-13 (2014).
Bereczky, S. et al. Crimean-Congo hemorrhagic fever virus infection is lethal for adult type I interferon receptor-knockout mice. The Journal of general virology 91, 1473–1477, 10.1099/vir.0.019034-0 (2010).
Dowall, S. D. et al. Hazara virus infection is lethal for adult type I interferon receptor-knockout mice and may act as a surrogate for infection with the human-pathogenic Crimean-Congo hemorrhagic fever virus. The Journal of general virology 93, 560–564, 10.1099/vir.0.038455-0 (2012).
Bishop, D. H. L. Bunyaviridae. Intervirology 14, 125–143 (1980).
Begum, F., Wisseman, C. L., Jr. & Casals, J. Tick-borne viruses of West Pakistan. II. Hazara virus, a new agent isolated from Ixodes redikorzevi ticks from the Kaghan Valley, W. Pakistan. Am J Epidemiol 92, 192–194 (1970).
Iwakami, S., Ichikawa, Y. & Inokuma, H. A nationwide survey of ixodid tick species recovered from domestic dogs and cats in Japan in 2011. Ticks and tick-borne diseases 5, 771–779, 10.1016/j.ttbdis.2014.05.008 (2014).
Yamaguti, N., Tipton, V., Keegan, H. & Toshioka, S. TICKS OF JAPAN, KOREA, AND THE RYUKYU ISLANDS. Vol. XV (Brighan Young University, 1971).
Wu, C., Li, F., Niu, G. & Chen, X. PET imaging of inflammation biomarkers. Theranostics 3, 448–466, 10.7150/thno.6592 (2013).
Subramanian, A. R., Kaufmann, M. & Morgenstern, B. DIALIGN-TX: greedy and progressive approaches for segment-based multiple sequence alignment. Algorithms Mol Biol 3, 6, 10.1186/1748-7188-3-6 (2008).
Capella-Gutierrez, S., Silla-Martinez, J. M. & Gabaldon, T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973, 10.1093/bioinformatics/btp348 (2009).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30, 772–780, 10.1093/molbev/mst010 (2013).
Darriba, D., Taboada, G. L., Doallo, R. & Posada, D. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 27, 1164–1165, 10.1093/bioinformatics/btr088 (2011).
Guindon, S. et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59, 307–321, 10.1093/sysbio/syq010 (2010).
Chen, X. P. et al. Infection and pathogenesis of Huaiyangshan virus (a novel tick-borne bunyavirus) in laboratory rodents. The Journal of general virology 93, 1288–1293, 10.1099/vir.0.041053-0 (2012).
Ishii, A. et al. A nairovirus isolated from African bats causes haemorrhagic gastroenteritis and severe hepatic disease in mice. Nature communications 5, 5651, 10.1038/ncomms6651 (2014).
Shimada, S., Posadas-Herrera, G., Aoki, K., Morita, K. & Hayasaka, D. Therapeutic effect of post-exposure treatment with antiserum on severe fever with thrombocytopenia syndrome (SFTS) in a mouse model of SFTS virus infection. Virology 482, 19–27, 10.1016/j.virol.2015.03.010 (2015).
Nakajima, N. et al. In situ hybridization AT-tailing with catalyzed signal amplification for sensitive and specific in situ detection of human immunodeficiency virus-1 mRNA in formalin-fixed and paraffin-embedded tissues. Am J Pathol 162, 381–389, 10.1016/S0002-9440(10)63833-3 (2003).
Acknowledgements
We thank Roger Hewson (Public Health England) for providing the HAZV, Ken Maeda (Joint Faculty of Veterinary Medicine, Yamaguchi University) for providing the SFTSV YG-1, Koichi Izumikawa (Department of Infectious Diseases, Graduate School of Biomedical Sciences, Course of Emerging Infectious Diseases, Nagasaki University) for providing the antiserum against SFTS, Seigo Yamamoto (Miyazaki University) and Fumihiko Mahara (Mahara Hospital, Tokushima) for providing the tick collection and Mya Myat Ngwe Tun (Institute of Tropical Medicine, Nagasaki University) for technical support for the experiments. This work was supported financially by KAKENHI [Grant-in-Aid for Scientific Research (B) (25304045) and Grant-in-Aid for Challenging Exploratory Research (25660229, 15K15126)] from the Japan Society for the Promotion of Science, a Health and Labor Sciences Research Grant on Emerging and Re-emerging Infectious Diseases from the Japanese Ministry of Health, Labor and Welfare, Health and Labor Sciences Research Grants (Grants in aid H25- Shinko-Ippan-007, H25-Shinko-shitei-009), the Cooperative Research Grant of NEKKEN 2015, AMED, J-GRID and the Joint Usage/Research Center on Tropical Disease, Institute of Tropical Medicine, Nagasaki University (2015-Ippan-12).
Author information
Authors and Affiliations
Contributions
D.H. designed the study; D.H. and S.S. wrote the main manuscript text; S.S., Y.F., G.P.H., L.U., Y.T.A., H.F. and D.H. collected the ticks and D.H. performed the virus isolation; S.S., K.A. and T.N. determined the full-sequence of TFLV and prepared Figures 1b and 2a–c; Y.K. prepared Figure 1c; Y.K. and D.H. performed neutralization test and prepared Table 1; M.S. performed the electron microscopy and prepared Figure 1a; D.H. and S.S. performed the animal experiments and prepared Figure 3; K.S., T.O. and Y.T.S. performed the histopathological experiments and prepared Figure 4; T.F., H.O., K.N. and D.H. performed the PET/CT imaging and prepared Figure 5; S.S. and D.H. performed the TFLV infection experiments in cultured cells and prepared Figure 6; J.Y., K.M. and D.H. supervised the project. All authors reviewed the manuscript.
Phylogenetic trees of the genus Nairovirus.
The phylogenetic trees were constructed based on the conserved amino acid sequences (a) Phylogenetic tree based on the L segment (amino acids 2218–2535 in the L segment of TFLV) using the substitution model LG + I + G. (b) Phylogenetic tree based on the M segment (amino acids 791–948 in TFLV) using the substitution model LG + G. (c) Phylogenetic tree based on the sequences of the S segment (2–179 of TFLV) using the substitution model LG + G. Nairoviruses are designated by the following abbreviations: ABHV, Abu Hammad virus; AMV, Abu Mina virus; CCHFV, Crimean–Congo hemorrhagic fever virus; CHIMV, Chim virus; DUGV, Dugbe virus; ERVEV, Erve virus; HAZV, Hazara virus; ISKV, Issyk-Kul virus; KUPEV, Kupe virus; LPHV, Leopards Hill virus; NSDV, Nairobi sheep disease virus; PMRV, Paramushir virus; PSV, Punte Salinas virus; QYBV, Qalyub virus; RAZAV, Raza virus; SAKV, Sakhalin virus; and TLMV, Tillamok virus.
(a) Survival curves of A129 mice (n = 10) subcutaneously inoculated with 10−3 and 103 ffu of TFLV (Tok-Hfla-2013). The mice were observed for 14 days and no mice died after 14 days. (b) Viral loads in the tissues of A129 mice infected with 102 ffu of TFLV (Tok-Hfla-2013). The copy numbers in the thymus (Th), lung (Lu), spleen (Sp), liver (Li), kidney (Ki), brain cortex (Br1) and non-cortex (Br2), spinal cord (SC), stomach (St), small intestine (SI), large intestine (LI) and cecum (Ce) of mice 1- and 3-days post-infection were determined using real-time RT-PCR (n = 5). (c) Gross pathology and viral loads of A129 mice inoculated with TFLV (Tok-Hfla-2013). Internal organs of TFLV-and mock-infected A129 3 days pi.
Histological and ISH features of the gastrointestinal tract.
TFLV (Tok-Hfla-2013)-infected (a) and mock-infected (b) A129 mice were dissected at 3 days pi. Left panels: hematoxylin and eosin. Right panels: ISH using AT-tailed sense and antisense cocktail probes (positive signals are labeled in brown). The gastric and duodenal mucosae highly involved by the viral infection show marked erosive change. Non-eroded small intestinal mucosa reveal positive signals in both the crypts and villi. Some keratinocytes in the esophageal mucosa are also involved. In the large bowel mucosa, the germinal center of the lymphoid follicles represents the site of infection. The gastroduodenal mucosa in mock-infected control animals demonstrates normal histology without viral infection.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Shimada, S., Aoki, K., Nabeshima, T. et al. Tofla virus: A newly identified Nairovirus of the Crimean-Congo hemorrhagic fever group isolated from ticks in Japan. Sci Rep 6, 20213 (2016). https://doi.org/10.1038/srep20213
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep20213
This article is cited by
-
Toyo virus, a novel member of the Kaisodi group in the genus Uukuvirus (family Phenuiviridae) found in Haemaphysalis formosensis ticks in Japan
Archives of Virology (2021)
-
A novel nairovirus associated with acute febrile illness in Hokkaido, Japan
Nature Communications (2021)
-
Lethal infection of embryonated chicken eggs by Hazara virus, a model for Crimean-Congo hemorrhagic fever virus
Archives of Virology (2018)
-
Pathogenic potential and growth kinetics of Muko virus in mice and human-derived cells
Tropical Medicine and Health (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.