Abstract
The mountains of southwest China are one of the hot spots of biodiversity in the world. However, the high-altitude fauna that inhabit these mountains remain a mystery. In this study, the species diversity of the aphids of the genus Cinara from the high-altitude coniferous forests was first assessed and then the processes and the mechanisms of speciation were discussed. Three hundreds and four aphid samples that contained 3040 individuals were collected during fourteen field surveys. The molecular clusters derived from the DNA barcodes were used to explore the species diversity. Notably, the aphid alpha-diversity was high, with as many as 94 candidate species and furthermore, 86.2% of the species collected had not been previously recorded. The centers of aphid species richness corresponded to the distributional pattern of the diversity of the host conifer plant species. The divergence time revealed that following the uplift of the Qinghai-Tibetan Plateau during the Pleistocene, the changes in the climate, ecology and host habitats were likely the most important factors that drove the rapid process of evolutionary radiation in the aphids. Our findings revealed the high species diversity of the aphids with DNA barcoding.
Similar content being viewed by others
Introduction
The mountains of southwest China occupy the area between the easternmost edge of the Qinghai-Tibetan Plateau (QTP) and the Central Chinese Plain and are recognized as one of the 34 hot spots of biodiversity in the world (http://www.biodiversityhotspots.org/xp/Hotspots). Based on the unique fauna, this region has long been considered an independent realm in zoogeography1,2. Therefore, the determination of the species diversity and the distribution patterns of diversity are important to gain a better understanding of the mechanisms that formed and help to maintain the biodiversity of this hot spot3. However, the biodiversity surveys in the high-altitude zones of these mountains face major challenges, because many of the regions are unknown or have rarely or never been visited. In the mountains of southwest China, the coniferous forest is the primary vegetation type in the high-altitude zones. However, studies have rarely focused on the insect species diversity in these high-altitude coniferous forests.
The identification of species based on morphology is frequently used to explore biodiversity. However, such morphology-based taxonomy is often difficult, particularly for a community with largely undescribed biodiversity4. DNA barcoding has become an important tool in the development of a global inventory of biodiversity5,6. The automated DNA-based approaches for OTU (operational taxonomic units) designation have demonstrated their usefulness in probing biodiversity7 and have important advantages8. To estimate biodiversity, DNA barcoding has been successfully used in many insect groups, including ants9, flies10 and wasps11.
Aphids are phloem-feeding insects in the order Hemiptera, with more than 5000 species12. The range of the host plant restricts the distribution of most aphids. As the primary genus of aphids that feeds on conifers, Cinara Curtis is the second largest genus of aphids with 243 described species12; the aphids in this genus feed exclusively on conifers primarily in the temperate and subtropical regions of the Northern Hemisphere. The host plants for this genus of aphid are found in several genera, including Abies, Picea and Larix, which have broad geographic distributions and patterns of species diversity from 2500 to 4500 m in elevation within this region13. The species of Cinara have disjunctive distributions and different allopatric populations because of the specific geographic ranges of the host plants. The dispersal ability of species of Cinara is limited because of the high weight to wing length ratio14 and some species are even recorded without winged morphs15; the absence of dispersal ability might accelerate the genetic diversification of species in an area with such complex topography16. However, previous investigations on aphids in the high-altitude zones of the mountains of southwest China are rare.
In this paper, we first assessed the aphid diversity and focused on the species in the coniferous forests of the high-altitude zones with field surveys in the last ten years, particularly during three targeted expeditions in 2012–2013. We then used the molecular clusters that were derived from the DNA barcodes to delineate putative species and to explore the diversity of aphids in the mountains of southwest China. The aims of the research were as follows: 1) to identify the genetic diversity of the aphids and to determine the ratio of described to undescribed species, 2) to describe the distribution patterns of the species and to assess the roles of two factors (elevation and host plants) on the patterns of distribution and 3) to estimate evolution of the host associations within the aphid genus and to discuss the processes and mechanisms of speciation for the aphid genus in this region.
Results
Taxonomic assignments and species delineations
A 658-bp PCR product for the COI gene was obtained from 304 samples, among which 373 sites were conserved, 285 were variable and 262 were parsimony-informative. These sequences were heavily biased toward A and T nucleotides, averaging 39.1% for T, 14.9% for C, 35.6% for A and 10.4% for G nucleotides.
Both the clustering method based on the NJ tree and the genetic distances and the ABGD method identified 94 candidate species from the 304 barcodes (Table S1). GMYC model provided evidence of 94(86–101) independent entities. Therefore, we defined 94 candidate species in this research and among these candidate species, 48 species (51.1%) were discovered at only one sample site, whereas 8 species (8.5%) were distributed in more than ten sample sites (Table S1).
Based on morphological taxonomy, only 13 candidate species from 114 samples were successfully identified to the 13 Cinara known species. Among these species, Cinara cuneomaculata (del Guercio) and Cinara piniarmandicola Zhang, Zhang & Zhong were first discovered in this region, whereas seven species that were previously recorded in the region were not found (Table S2). When using our DNA bar-code database, 114 DNA barcodes were matched to the 13 Cinara species, which was consistent with the morphological identifications. However, based on these two methods, the remaining 81 candidate species did not fit the description of any known Cinara species. These species had morphological differences with the known Cinara species, although some of the species shared some near-morphological characters with certain known species. Notably, most of the identified species (9/13) were widely distributed in the mountains of southwest China and were found at relatively low elevations (1500–3000 m), whereas many of the undescribed species (33/81) were found at relatively high elevations (above 3500 m).
Mapping species diversity
Based on the 94 Cinara species/candidate species that were identified, the species diversity of Cinara in the mountains of southwest China was concentrated in three areas (Fig. 1): (1) the mountainous areas associated with the Sichuan-Gansu border, the western Qinling and southern Gansu Province, (2) the northern Hengduan Mountains region and (3) northwestern Yunnan Province. These three areas were the centers of distributions in the mountains of southwest China and had the highest species diversity.
Patterns of Cinara species richness along the elevational gradients
The patterns of species richness with elevation in the mountains of southwest China for all Cinara species/candidate species are shown in Fig. 2. The total species richness was highest at the medium elevations (2500–3000 m) and the endemic species richness was also the highest at the 2500–3000 m elevation.
Patterns of Cinara species richness in relation to different host plants
The host plants for the species of Cinara included Abies, Cupressaceae, Larix, Picea, Pinus and Tsuga (Fig. 3). More than one-third of the species of Cinara lived on Picea and Abies was also inhabited by many of the Cinara species. The Cinara species richness on Larix, Tsuga and Cupressaceae was relatively low (6, 6 and 9, respectively).
NJ Tree Structure and COI divergence assessment
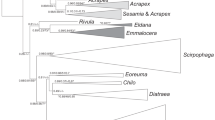
The results of the overall NJ analysis by COI region of the distances among the 304 samples are shown in Fig. 4. The trees represented only the distance matrix and should not be interpreted as phylogenetic hypotheses. Of the Cinara species, almost all were clustered according to a specific host plant. Of the aphids on the different genera of host plants, almost none were found together except for those on Abies and Picea.
For the genetic divergence along the different altitudinal gradients (Table 1), the elevation zone at 3500–4000 m had the highest interspecific divergence (0.1082 ± 0.0243), which was followed by the elevation zones that exceeded 4000 m (0.1062 ± 0.0212) and the 2500–3000 m elevation zone (0.1029 ± 0.0217). Additionally, all elevation zones had low levels of intraspecific variation (0.0005 ± 0.0012 to 0.0025 ± 0.0027).
For the genetic divergence affected by different ecological variables (Table 2), the variable sets concerning allopatric/different feeding site/hosts in the different genera displayed the highest interspecific divergence (0.1352 ± 0.0203). The sets concerning allopatric/different feeding site/hosts in one genus and the sets concerning sympatric/different feeding site/hosts in different genera also showed high interspecific divergence. By contrast, the sets concerning allopatric/same feeding site/hosts in one genus and the sets concerning allopatric/same feeding site/hosts in different genera yielded higher intraspecific divergences than the others.
Divergence times
The ultrametric tree with the divergence times estimated from the BEAST analysis is shown in Fig. 5. All species-level divergences in Cinara occurred less than 3.5 Ma. The Cinara species in this region fed on Abies and Picea early before 3 Ma and then diversified onto different hosts ca. 2 Ma. These results suggested that there were multiple acquisitions of hosts by the Cinara in the mountains of southwest China, including at least five from Pinus, 11 from Picea, three from Larix, four from Abies, one from Tsuga and six from Cupressaceae.
Diversification rates
The LASER results rejected the null hypothesis of temporally homogeneous diversification rates and suggested variable diversification rates within Cinara. The diversification rate-constancy statistic ΔAICRC was 15.89. The yule2rate diversification model was selected as the best fitting model. The Relative Cladogenesis (RC) analyses detected significant shifts of diversification rates within Cinara at ca. 4.35 Ma.
Discussion
High diversity of conifer-feeding aphids in the mountains of southwest China
Our study revealed high genetic diversity of the conifer-feeding aphids in the mountains of southwest China based on species delineation (94 candidate species). The species-level identifications were based on morphological diagnostic features and DNA barcoding, which allowed us to identify 37.5% of the samples (114 of 304 aphids) as species among the 13 known species. However, 86.2% of the candidate species did not fit the description of any known species. Therefore, our results established unequivocally that most of the conifer-feeding aphid species in this region were unknown and undescribed, with a large number of the species documented in this study for the first time.
Significantly, many of the sample locations, which were rarely or never visited, for the conifer-feeding aphid species were in large and unknown regions at elevations above 3000 m in our study area. For example, 34 specimens were collected from the elevation zones over 4000 m and the highest sample elevation was 4782 m. Previously, no conifer-feeding aphid species were found above 4000 m in this region2. The high-elevation “sky island” populations might have remained isolated with little opportunity for gene flow. Additionally, according to previous publications and museum records, 18 Cinara species were distributed in this region and only 6 species were described for Picea or Abies. However, based on our data, more than half of the Cinara species were found on Picea and Abies, which were patchily distributed in the high elevation zones (2500–4000 m) and displayed high diversity13. Therefore, the species richness was unusually high in this region for the Cinara species. This study, based on the novel barcodes, provided a new evidence to evaluate the species richness in the high elevation zones.
The three centers of distribution were predicted
With the uplift of the QTP, the topography and the climate of the mountains of southwest China changed significantly17. An uneven distribution of vegetation and an extremely complex topography codetermined the spatial patterns of aphid diversity in this region2. Three distribution centers for the Cinara species were found in this region: (1) the associated mountainous areas on the Sichuan-Gansu border, in the western Qinling and in southern Gansu Province, (2) northwestern Yunnan Province and (3) the northern Hengduan Mountains region.
As one distribution center of the Cinara species, the associated mountainous areas on the Sichuan-Gansu border, in the western Qinling and in southern Gansu Province are transitional areas from the main body of the plateau to the lower hilly terrains. These areas are the regions that divide the Palearctic and Oriental Realms in central China18, which also have a high diversity of plants. A high abundance of Abies was found in this region13, which provided opportunities for the Cinara species to diversify. Similarly, another center, in northwestern Yunnan Province, is also located in the transitional zone between the Palearctic and the Oriental realms19,20 and has the highest diversity of plants in the Hengduan Mountains. This region was proposed as a refuge during the Pleistocene glaciations18,21. The Cinara that inhabited this region had high levels of species richness because presumably the high plant diversity and the Pleistocene conditions promoted the differentiation and the speciation of the aphids. The third center of Cinara species is in the northern Hengduan Mountains region, which has many mountain ridges and deep gorges. Compared with the rest of the Hengduan Mountains, the northern Hengduan Mountains ranged from 2500 to 4000 m in elevation and formed the center for Abies and Picea pollen abundance13.
From these patterns, we concluded that the centers of distribution of the conifer-feeding aphid species strongly corresponded with the pattern of conifer species richness, particularly for Abies and Picea.
Elevational and host associated patterns of conifer-feeding aphid species richness
The elevation patterns for the total and the endemic species richness of the aphids displayed unimodal patterns, which was similar to previous reports for plants22,23. The Cinara patterns of species richness, for all species and endemics, were highest at the middle elevations (2500–3000 m). Some previous studies highlighted that the peak for endemic species richness was at a higher elevation than the peak for total species richness24,25, whereas other studies found that the total and the endemic species richness peaked at the same elevation23, which was consistent with our results. Our study area, the mountains of southwest China, provided a refuge for many temperate plant taxa during the Pleistocene. Associated with the ever-changing habitat of the rising plateau during the postglacial periods, the plants in this area experienced rapid evolution26. During the alternating processes of glaciation and deglaciation, the middle elevations were more likely to be the center for repeated plant migrations between the high and the low elevations23. Additionally, the middle elevations provided a moist, shady environment for coniferous plants and were not subject to the sharp fluctuations in weather that occurred throughout the year at the higher elevations or to the drying effects of the rain shadows at lower elevations23. Therefore, the suitable conditions for high plant diversity in the coniferous forests of the middle elevations provided more suitable ecological niches for the aphids. Most of the Cinara species evolved in this region and some were limited to only a single mountain (according to our field investigations, unpublished data). The rapid speciation in Cinara resulted in more than one-third of the species restricted to this region and therefore, the expectation was reasonable that elevation patterns for the total and the endemic species richness were strongly correlated.
The species richness of the genus Cinara was diversified in relation to the different host taxa. The number of sample sites on different hosts was not associated with species richness; for example, 78 sites were on Pinus in the mountains of southwest China, but only 13 species/candidate species were discovered on this host plant genus. As a comparison, eight samples were on Tsuga, which had seven species/candidate species. In the mountains of southwest China, Pinus is primarily distributed in the relatively low elevation areas (1500–2500 m) and therefore, this genus might have had little effect on the complex topography of the Cinara species that inhabited this region. By contrast, Tsuga primarily occurs in the 3500–4000 m elevation zones and is distributed sporadically. Thus, Tsuga would provide the geographic isolation and the host isolation that prevented gene flow among the different aphid populations, which would lead to genetic divergence. Additionally, the Cinara on Picea and Abies displayed high species richness. The species richness of Picea and Abies is high in the high elevation zones (2500–4000 m) of the mountains of southwest China13. Thus, the complex topography, the suitable microenvironmental conditions and the high diversity of Picea and Abies provided opportunities for the Cinara species to diversify and to speciate. Furthermore, the Cinara species on the Cupressaceae and the Larix formed genetically distinct lineages that were related to geographical location. The range area is an important explanatory variable for the patterns of species richness27. The distributions of Cupressaceae and Larix in the mountains of southwest China ranged from low elevations (1500 m) to high elevations (4500 m) and exhibited a wide disparity in height. Thus, the local vicariance and complex topography along the elevation gradient were major factors that affected the species richness of aphids.
Rapid evolutionary radiation of the Cinara species
Our analysis suggested variable diversification rates within Cinara and shifts of diversification rates at ca. 4.35 Ma. The first acquired hosts of Cinara were Picea and Abies and the separation of these lineages from the other lineages was assumed to occur ca. 3‒4.5 Ma, following the first and the second phases of the “Tibet movement.” The “Tibet movement” occurred between 3.6 and 1.7 Ma and included three phases that commenced at 3.6, 2.5 and 1.7 Ma28. During this period, the regional environment was relatively warm and humid and there was a subtropical mixed forest of evergreen broadleaf and broadleaf deciduous woodlands29; there were no coniferous forests and only a few fir and spruce trees13. Thus, the early Cinara species fed only on Picea and Abies and maintained a low diversity. The early speciation within Cinara in this region was estimated at ca. 2–3.5 Ma and a more large-scale rapid speciation was estimated to occur at less than 2 Ma. This speciation event occurred approximately at the time of the third phase of the “Tibet movement” and the “Kunhuang movement,” when the plateau was uplifted to an average height of 3000 m, with mountains up to and over 4000 m30. The uplift of the Tibetan Plateau and the surrounding areas caused a climatic and ecological shift; the evergreen broadleaf and broadleaf deciduous forests were replaced with coniferous forests and grasslands, the climate gradually became drier, colder and windier and the development of the glaciers began29. Since the uplift, the plateau has undergone several glaciations31. The dramatic climatic and environmental changes caused by the uplift of the Tibetan Plateau in the Pliocene-Early Quaternary resulted in new habitats for many gymnosperms, which facilitated the evolution of the Cinara species. Therefore, the Pliocene-Early Quaternary might be the period in which the adaptive radiation of Cinara reached a maximum in this region. The pronounced mountain formation at that time led to an isolation of the populations and the formation of many “mountain-island” species.
Speciation of Cinara in the mountains of southwest China
The processes and mechanisms of speciation were influenced by multiple driving forces32. Most speciation results from isolation combined with divergent selection, such as selection on ecological traits32. In the classic allopatric model of Mayr (1947), different ecological conditions drove the speciation in geographically isolated populations33. Our analyses on the conifer-feeding aphid genus, Cinara, revealed that the high diversity of these aphids was strongly associated with the ecological conditions in the mountains of southwest China. The host plant was an important ecological factor that affected the species richness of Cinara. The different Cinara species had different feeding preferences and most species fed on a single or a few closely related host species, whereas others fed on several species within a genus and sometimes even on hosts in different genera of conifers (i.e., some species inhabited either Picea or Abies). Based on our analysis of the genetic divergence (Table 2), the intra- and interspecific divergence of aphids on hosts of different genera were all greater than those of aphids on a host in one genus, which indicated that closely related species of Cinara tended to feed on closely related host species.
Additionally, the Cinara species had specific feeding sites on the host plants. Some species fed only on young twigs, whereas others fed exclusively on old branches or large trunks34. For the Cinara species in our study, the aphids that used similar feeding sites were more closely related than the aphids that used different feeding sites (Fig. 4, Table 2). Additionally, the species that used similar feeding sites on hosts from different genera were less divergent than the species from different feeding sites on one host (Table 2). These results provided evidence that the speciation in some Cinara species in the mountains of southwest China occurred through a shift to new hosts. Similarly, Favret & Voegtlin (2004) speculated that the Cinara species apparently adapted more easily with a switch to a similar microhabitat on a different host than to feeding on a different part of the same tree species35.
Furthermore, the complex topography in the mountains of southwest China had a strong effect on the acceleration of the diversification of the Cinara species. Our data revealed that the intraspecific divergences from allopatric populations were significantly greater than those from sympatric populations (Table 2). Additionally, the species that inhabited the high elevation zones (>2500 m) had higher interspecific divergence than those from the low elevation zones (<2500 m) (Table 1). These results demonstrated that geographic isolation promoted speciation events in Cinara and that geographical barriers such as high mountains appear to be ‘isolated islands’ that shaped some of the diversity at regional scales.
Consequently, the high diversity of Cinara in the mountains of southwest China was caused by a combination of factors, which included host diversity, a complex topography and historical climatic oscillations. Geographic isolation played a driving force that caused the populations to differentiate, whereas a shift in hosts was a major factor that affected species-level divergence. Ultimately, the host diversity and the niche diversification caused by the uplift of the Tibetan Plateau in the Pliocene-Early Quaternary caused the high species richness of the genus Cinara in this region.
In this study, the patterns of species richness of the genus Cinara in the mountains of southwest China helped us to understand the abundance of Cinara species. The genus Cinara, a conifer-feeding member in the Lachninae, is the key taxon to determine whether the conifer-feeding was ancestral to the extant lachnids, which had the dispute before36. A recent study has suggested that the host of the Lachninae ancestor was an angiosperm and that the diversification of Cinara is concomitant with the adoption of conifer hosts39. Based on our results, a large number of the Cinara species in the mountains of southwest China appeared after the uplift of the Tibetan Plateau and then experienced rapid speciation, which gave a new evidence for the evolution of host affiliation in the lachnids specifically.
Methods
Study area
The mountains of the southwest China hot spot cover over 262,400 km2 and the elevations range from less than 2000 m in some valley floors to 7558 m at the summit of Gongga Mountain. The geographical distributions of the aphids corresponded with the distributions of the conifers. The conifers were absent because of the environmental conditions in some of the mountainous areas. Thus, the area defined for this study was a relatively crude geographical range that corresponded to the distributional ranges of the conifers.
Known species category of Cinara in the mountains of southwest China
Only 18 species of Cinara were described for the mountains of southwest China (Table S2), according to the statistics of Huang et al. 2006 2, the published literature40,41,42,43,44,45 and the information from specimens examined from the following museums: the National Zoological Museum of China, the Institute of Zoology, the Chinese Academy of Sciences (Beijing), the Natural History Museum (London), the French National Museum of Natural History (Paris) and the USDA Systematic Entomology Laboratory (Maryland). Among these described species, ten species fed on Pinus, six species fed on Picea/Abies, one species fed on Larix and one species fed on Cupressaceae.
Field sampling and data collection
The fieldwork in the mountains of southwest China occurred during four targeted expeditions (April 20, 2006-May 16, 2006, for 27 days; May 21, 2012-June 2, 2012, for 13 days; June 20, 2012-August 9, 2012, for 51 days; and August 5–24, 2013, for 20 days) and on ten general expeditions (July 2002; August 2003; October 2003; August 2004; April-May 2005; September 2005; June-July 2009; November 2009; August 2010; and October 2010) at different times of different years to ensure that the samples covered the entire geographical range of distribution. Because of the unique geographical landforms and the distribution ranges of the host plants, all sample sites were above 1500 m. To avoid the effects of artificial forestation, we tended to select samples from the original forest tree species instead of the planted street and garden trees. For each sample site, we attempted to sample as many species as possible from one or more different host species. The host trees were identified to the genus of conifer and to the species, when possible, using the local flora. Because of the special reproductive mode of aphids (parthenogenesis), a species on a specific host within a certain geographic range might belong to the same clone. Thus, a species on the same host plant at the same sample site within a 10-kilometer radius was sampled only one time, which was regarded as one sample, unless some individuals displayed some differences in biology. In total, we collected 3040 individual aphids from 304 samples (10 individuals per sample) and at least 5 individuals of each sample were sequenced before preparation to ensure that the samples did not contain multiple species and all individuals in one sample belong to the same clone. One to two representative individuals from each clone were sampled for the molecular analyses.
Species delineation
The total DNA was extracted from a single aphid that was preserved in 95% or 100% ethanol and the amplicon size of the COI was approximately 660 bp. The multiple alignments were generated using CLUSTALX46, which were subsequently pruned to lengths of 658 bp (COI). The sequences were deposited in the GenBank and the accession numbers are provided in Table S1.
The species delineation was based on the analysis of the DNA barcodes with automated procedures. Many methods to use DNA data to delimit species were recently proposed6,47 and in this study, we chose three methods for the species delineation.
First, we applied a simple clustering method48, with some improvements. We only considered a ‘candidate species’ when (i) the ‘candidate species’ was in the COI-based NJ tree reconstructions and (ii) the clustering was strongly supported in the COI-based NJ tree (bootstrap support>70). The clades that were identified were well differentiated from one another to prevent minor tip clades from being recognized as distinct species. We applied a threshold difference of at least two percent in the COI sequences between individual specimens of a given clade and those of the nearest branch (a 2% difference in the COI sequence is the smallest difference that is generally found between unambiguously identified aphid species49).
The second method we used was the Automatic Barcode Gap Discovery (ABGD)50. This method was used to objectively delimit the operational taxonomic units considered in this study to be proxies for species, although only an integrative approach (one that combined thorough morphological comparisons with additional markers) could assess the distinctions between species. The results of the ABGD were dependent on the maximum p-threshold that was selected a priori in the group that was studied.
The third method we used was the generalized mixed Yule coalescent (GMYC) model51. The method uses a maximum likelihood approach to optimize the threshold identifying the shift in the branching patterns of the gene tree from interspecific branches (Yule model) to intraspecific branches (coalescent). It identifies clusters of sequences corresponding to independently evolving entities. The maximum likelihood approach also provides a 95% confidence interval around the maximum likelihood solution. We used the conservative approach of identifying the minimum number of entities within the 95% confidence interval. The GMYC model was performed with the R package splits 1.0–11 (https://r-forge.r-project.org/projects/splits/).
Finally, we defined a ‘candidate species’ which was supported by most methods of species delineation. To easily clarify the scientific definition of a species, the ‘candidate species’ defined in this study were considered ‘species’ in our analysis. However, the ‘candidate species’ were not necessarily true species because the genetic diversity was displayed only at the molecular level. The description of a new species requires many hours of morphological work and should not rely only on DNA data.
Based on the results from the species delineations, we used diagnostic morphological features to identify the species. Three to five individual aphids per collection location were mounted on microscope slides for morphological examination. The voucher specimens for each sample were identified by Qiao, who based the identifications on diagnostic morphological features using standard literature-based keys15 and by the comparison with previously identified specimens in the National Zoological Museum of China, Beijing. The samples and the voucher specimens were deposited in this same museum. The complete list of the taxa with the collection data, including the host plants, Feeding sites and the dates, are provided in Supporting Information, Table S1.
Because of the taxonomic impediments and the difficulties of delimiting the morpho-species from a large set of samples in the field, we also identified species with a comparison of the DNA barcodes used in this study with our aphid DNA bar-coding database, which stored large numbers of DNA barcodes from our previous studies52. We compared the DNA barcodes to determine whether the samples matched any known aphid species.
Mapping the distribution pattern
Based on the database from our field collections, we used ArcGIS 9.3 (ESRI co., USA) to map the distribution of the Cinara species in the mountains of southwest China (Figure S1). Additionally, to determine the centers of diversity, a density map of the Cinara species was created with an overlay of the distribution of the candidate species that we defined and the GIS layer of the mountains of southwest China (Fig. 1). To obtain more objective patterns of distribution, the distribution of each species/candidate species was limited to a certain altitudinal range according to the locations of the collection sites and the altitudinal distributions of the host plants (Table S3). The species richness on the map was then estimated based on statistical calculation.
Data Analyses
We divided the range of elevations into 500-m bands between 1500 and 5000 m and we calculated the total number of species/candidate species and the number endemic species (defined as the species/candidate species collected only from one site or from one relatively small area) in each band. Additionally, the total numbers of species/candidate species with the different host plant taxa were calculated. Moreover, the average interspecific and intraspecific divergences along different altitudinal gradients with the effects of different environmental variables were calculated using the Kimura 2-parameter model (K2P). The environmental variables included three factors: allopatric or sympatric populations, the same or different feeding sites and whether the hosts were in one genus or in different genera. The divergence scores were calculated with MEGA 5.0. The neighbor-joining (NJ) analysis53 was used to examine the relationships among the taxa and the population samples. The NJ analyses were conducted with 1000 bootstrap replicates.
Estimate of divergence time
To evaluate the evolution of the host associations within Cinara in the mountains of southwest China, we combined other Cinara samples from Europe and North America16 (Table S4) with our data. We used a Bayesian framework based on the coalescent method to estimate the divergence times in BEAST v.1.7.554,55. The site model that was selected was the GTR + I + G by jModeltest. For each species, the sequences of all haplotypes were used in the analysis. The analysis was conducted with an expansion growth model and an uncorrelated lognormal relaxed clock56, with a proposed insect molecular clock (COI substitution rate = 1.77% per million years)57. The default priors were used. The chains were analyzed for 101.597 million generations, with sampling every 1000 generations. The Tracer v.1.5.058 was used to verify the posterior distribution and the effective sample sizes (ESSs) from the MCMC output. We used the TreeAnnotator v1.7.5 in the BEAST package55 to summarize the tree data with “mean height,” and we discarded the first 25% of the trees as the “burn-in” period, which ended long after the stationary chain likelihood values were established.
Diversification rates
The diversification rate change over time within Cinara in the mountains of southwest China was explored based on R applications. Firstly, we used a maximum likelihood method as implemented in LASER v 2.4–159 to determine whether diversification rates of Cinara have changed over time. The test statistic for diversification rate-constancy was calculated as: ΔAICRC = AICRC − AICRV, where AICRC was the AIC score for the best fitting rate-constant diversification model and AICRV was the AIC for the best fitting rate-variable diversification model. A positive value for ΔAICRC indicates that the data are best explained by a rate-variable model of diversification. Two rate-constant (pure birth and birth–death model) and three rate-flexible diversification models (a logistic density-dependent speciation rate, an exponential density-dependent and a yule2rate model) were evaluated in our study. Secondly, the Relative Cladogenesis (RC) test was performed in GEIGER v 2.0160 to detect shifts of diversification rates within Cinara, using the MCC chronogram generated from BEAST analyses. Bonferroni correction was used to adjust the P-values.
Additional Information
How to cite this article: Chen, R. et al. DNA barcoding reveals a mysterious high species diversity of conifer-feeding aphids in the mountains of southwest China. Sci. Rep. 6, 20123; doi: 10.1038/srep20123 (2016).
References
Chen, Y. Y., Chen, Y. F. & Liu, H. Z. Studies on the position of the Qinghai–Xizang Plateau region in zoogeographic divisions and its eastern demarcation line. Acta Hydrobiological Sinica 20, 97–103 (1996).
Huang, X. L., Qiao, G. X. & Lee, F. Diversity and distribution of aphids in the Qinghai-Tibetan Plateau-Himalayas. Ecol Entomol 31, 608–615, doi: 10.1111/j.1365-2311.2006.00819.x (2006).
Lei, F. M., Qu, Y. H., Song, G., Alström, P. & Fjeldså, J. On mechanism determining high species richness and endemism in east Himalaya-Mountains of Southwest China. Integrative Zoology 10, 171–181 (2015).
Blaxter, M. & Floyd, R. Molecular taxonomics for biodiversity surveys: already a reality. Trends Ecol Evol 18, 268–269, doi: 10.1016/S0169-5347(03)00102-2 (2003).
Monaghan, M. T. et al. Accelerated Species Inventory on Madagascar Using Coalescent-Based Models of Species Delineation. Systematic Biol 58, 298–311, doi: 10.1093/sysbio/syp027 (2009).
Pons, J. et al. Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Systematic Biol 55, 595–609, doi: 10.1080/10635150600852011 (2006).
Blaxter, M., Elsworth, B. & Daub, J. DNA taxonomy of a neglected animal phylum: an unexpected diversity of tardigrades. P Roy Soc B-Biol Sci 271, S189–S192, doi: 10.1098/rsbl.2003.0130 (2004).
Ratnasingham, S. & Hebert, P. D. N. A DNA-Based Registry for All Animal Species: The Barcode Index Number (BIN) System. Plos One 8, doi: 10.1371/journal.pone.0066213 (2013).
Smith, M. A. & Fisher, B. L. Invasions, DNA barcodes and rapid biodiversity assessment using ants of Mauritius. Front Zool 6, doi: 10.1186/1742-9994-6-31 (2009).
Forest, F. et al. Preserving the evolutionary potential of floras in biodiversity hotspots. Nature 445, 757–760, doi: 10.1038/Nature05587 (2007).
Stahlhut, J. K. et al. DNA barcoding reveals diversity of Hymenoptera and the dominance of parasitoids in a sub-arctic environment. Bmc Ecol 13, doi: 10.1186/1472-6785-13-2 (2013).
Favret, C. Aphid Species File. Version 5.0/5.0. [2014–02–14].
Lu, H. Y. et al. Spatial pattern of Abies and Picea surface pollen distribution along the elevation gradient in the Qinghai-Tibetan Plateau and Xinjiang, China. Boreas 37, 254–262, doi: 10.1111/j.1502-3885.2007.00019.x (2008).
Kidd, N. A. C. Does Wingloading Limit Flight Potential in Aphids. J Appl Entomol 112, 27–30 (1991).
Blackman, R. L. & Eastop, V. F. Aphids on the World’s Trees: an identification and information guide. (The Natural History Museum, 1994).
Jousselin, E. et al. Is ecological speciation a major trend in aphids? Insights from a molecular phylogeny of the conifer-feeding genus Cinara. Front Zool 10, doi: 10.1186/1742-9994-10-56 (2013).
Shi, Y. F., Li, J. J. & Li, B. Y. Uplift and environmental change of Qinghai-Xizang (Tibetan) Plateau in the Late Cenozoic. (Guangdong Science and Technology Press, 1998).
Zhang, R. Z. Zoogeography of China. (Science Press, 1999).
Huang, X. L., Lei, F. M. & Qiao, G. X. Areas of endemism and patterns of diversity for aphids of the Qinghai-Tibetan Plateau and the Himalayas. J Biogeogr 35, 230–240, doi: 10.1111/j.1365-2699.2007.01776.x (2008).
Huang, X. L., Ren, S. S. & Qiao, G. X. Composition and characters of the aphid fauna in Hengduan Mountains Region, China. Acta Zootaxonomica Sinica 30, 14–21 (2005).
Li, W. Y. Quaternary vegetation and environment of China. (Science Press, 1998).
Kromer, T., Kessler, M., Gradstein, S. R. & Acebey, A. Diversity patterns of vascular epiphytes along an elevational gradient in the Andes. J Biogeogr 32, 1799–1809, doi: 10.1111/j.1365-2699.2005.01318.x (2005).
Zhang, D. C., Zhang, Y. H., Boufford, D. E. & Sun, H. Elevational patterns of species richness and endemism for some important taxa in the Hengduan Mountains, southwestern China. Biodivers Conserv 18, 699–716, doi: 10.1007/s10531-008-9534-x (2009).
Vetaas, O. R. & Grytnes, J. A. Distribution of vascular plant species richness and endemic richness along the Himalayan elevation gradient in Nepal. Global Ecol Biogeogr 11, 291–301, doi: 10.1046/j.1466-822X.2002.00297.x (2002).
Fu, C. Z. et al. Elevational patterns of frog species richness and endemic richness in the Hengduan Mountains, China: geometric constraints, area and climate effects. Ecography 29, 919–927, doi: 10.1111/j.2006.0906-7590.04802.x (2006).
Sun, H. Evolution of Arctic-Tertiary flora in Himalayan-Hengduan Mountains. Acta Bot Yunnanica 24, 671–688 (2002).
Jankowski, T. & Weyhenmeyer, G. A. The role of spatial scale and area in determining richness-altitude gradients in Swedish lake phytoplankton communities. Oikos 115, 433–442, doi: 10.1111/j.2006.0030-1299.15295.x (2006).
Li, J. J. et al. Geomorphological and environmental evolution in the upper reaches of the Yellow River during the late Cenozoic. Sci China Ser D 39, 380–390 (1996).
Wu, Y. Q. et al. Quaternary geomorphological evolution of the Kunlun Pass area and uplift of the Qinghai-Xizang (Tibet) Plateau. Geomorphology 36, 203–216, doi: 10.1016/S0169-555x(00)00057-X (2001).
Li, J. J. & Fang, X. M. Uplift of the Tibetan Plateau and environmental changes. Chinese Sci Bull 44, 2117–2124 (1999).
Yafeng, S. et al. Studies on altitude and climatic environment in the middle and east parts of Tibetan Plateau during Quaternary Maximum Glaciation. J. Glaciol. Geocryol. 17, 97–112 (1995).
Dobzhansky, T. Genetics and the Origin of Species. (Columbia Univ. Press, 1937).
Mayr, E. Ecological Factors in Speciation. Evolution 1, 263–288, doi: 10.2307/2405327 (1947).
Bradley, G. A. Feeding sites of aphids of the genus Cinara Curtis (Homoptera: Aphididae) in Northwestern Ontario. The Canadian Entomologist 91, 670–671 (1959).
Favret, C. & Voegtlin, D. J. Speciation by host-switching in pinyon Cinara (Insecta : Hemiptera : Aphididae). Molecular phylogenetics and evolution 32, 139–151, doi: 10.1016/j.ympev.2003.12.005 (2004).
Eastop, V. F. Deductions from the present day host plants of aphids and related insects. In “Insect/Plant Relationships”. 157–178 (Wiley, 1973).
Normark, B. B. Molecular systematics and evolution of the aphid family Lachnidae. Molecular phylogenetics and evolution 14, 131–140, doi: 10.1006/mpev.1999.0699 (2000).
Heie, O. E. Palaeontology and phylogeny. In “Aphids: Their Biology, Natural Enemies and Control”. 367–391 (Elsevier, 1987).
Chen, R., Favret, C., Jiang, L. Y., Wang, Z. & Qiao, G. X. An aphid lineage (Aphididae: Lachninae) maintains a bark-feeding niche while switching to and diversifying on conifers. Cladistics, doi: 10.1111/cla.12141 (2015).
Zhang, G. X. & Zhong, T. S. In Insects of Xizang, Vol. I (ed S.X. Chen ) 233–282 (Science Press, 1981).
Zhang, G. X. & Zhong, T. S. Economic insect fauna of China, Homoptera, Aphidinea, Part I. (Science Press, 1983).
Zhang, G. X. & Zhong, T. S. in Insects of Mt. Namjagbarwa region of Xizang (ed F. S. Huang ) (Science Press, 1988).
Zhang, G. X., Zhong, T. S. & Zhang, W. Y. In Insects of the Hengduan Mountains Region, Vol. I (ed S. X. Chen ) 360–40.3 (Science Press, 1992).
Ghosh, A. K. The Fauna of India and the Adjacent Countries. Homoptera, Aphidoidea, Part II. Lachninae. (Amra Press, 1982).
Ghosh, L. K. A Conspectus of Aphididae (Homoptera) of Himachal Pradesh in North-West Himalaya, India. (Bani Press, 1986).
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F. & Higgins, D. G. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25, 4876–4882, doi: 10.1093/nar/25.24.4876 (1997).
Monaghan, M. T., Balke, M., Gregory, T. R. & Vogler, A. P. DNA-based species delineation in tropical beetles using mitochondrial and nuclear markers. Philos T R Soc B 360, 1925–1933, doi: 10.1098/rstb.2005.1724 (2005).
Dettman, J. R., Jacobson, D. J. & Taylor, J. W. A multilocus genealogical approach to phylogenetic species recognition in the model eukaryote Neurospora. Evolution 57, 2703–2720, doi: 10.1554/03-073 (2003).
Jousselin, E., Desdevises, Y. & Coeur d’ Acier, A. Fine-scale cospeciation between Brachycaudus and Buchnera aphidicola: bacterial genome helps define species and evolutionary relationships in aphids. P Roy Soc B-Biol Sci 276, 187–196, doi: 10.1098/rspb.2008.0679 (2009).
Puillandre, N., Lambert, A., Brouillet, S. & Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol Ecol 21, 1864–1877, doi: 10.1111/j.1365-294X.2011.05239.x (2012).
Fujisawa, T. & Barraclough, T. G. Delimiting Species Using Single-Locus Data and the Generalized Mixed Yule Coalescent Approach: A Revised Method and Evaluation on Simulated Data Sets. Systematic Biol 62, 707–724, doi: 10.1093/sysbio/syt033 (2013).
Chen, R., Jiang, L. Y. & Qiao, G. X. The Effectiveness of Three Regions in Mitochondrial Genome for Aphid DNA Barcoding: A Case in Lachininae. Plos One 7, doi: 10.1371/journal.pone.0046190 (2012).
Saitou, N. & Nei, M. The Neighbor-Joining Method - a New Method for Reconstructing Phylogenetic Trees. Mol Biol Evol 4, 406–425 (1987).
Drummond, A. J. & Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. Bmc Evol Biol 7, doi: 10.1186/1471-2148-7-214 (2007).
Drummond, A. J., Suchard, M. A., Xie, D. & Rambaut, A. Bayesian Phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29, 1969–1973, doi: 10.1093/molbev/mss075 (2012).
Drummond, A. J., Ho, S. Y. W., Phillips, M. J. & Rambaut, A. Relaxed phylogenetics and dating with confidence. Plos Biol 4, 699–710, doi: 10.1371/journal.pbio.0040088 (2006).
Papadopoulou, A., Anastasiou, I. & Vogler, A. P. Revisiting the Insect Mitochondrial Molecular Clock: The Mid-Aegean Trench Calibration. Mol Biol Evol 27, 1659–1672, doi: 10.1093/molbev/msq051 (2010).
Rambaut, A. & Drummond, A. J. Tracer V1.5. (2009).
Rabosky, D. L. LASER: a maximum likelihood toolkit for detecting temporal shifts in diversification rates from molecular phylogenies. Evolutionary bioinformatics online 2, 273–276 (2006).
Harmon, L. J., Weir, J. T., Brock, C. D., Glor, R. E. & Challenger, W. GEIGER: investigating evolutionary radiations. Bioinformatics 24, 129–131, doi: 10.1093/bioinformatics/btm538 (2008).
Acknowledgements
We gratefully thank all collectors for their assistance in aphid collection, Fen-Di Yang for making slides of the voucher specimens and Zhong-E Hou, Xiang-Qin Yu and Qing-Song Zhou for the help with R. The work was supported by the National Natural Sciences Foundation of China (No. 31430078, No. 31561163002), the External Cooperation Program of Chinese Academy of Sciences (No. 152111KYSB20130012), the Ministry of Science and Technology of the People’s Republic of China (No. 2011FY120200, No. SQ2012FY4910058), the “Strategic Priority Research Program” of the Chinese Academy of Sciences (No. XDA05080700) and National Science Fund for Fostering Talents in Basic Research (No. J1210002).
Author information
Authors and Affiliations
Contributions
R.C. contributed fieldwork, performed most of the laboratory and analytical work and participated in conceptual design and manuscript preparation. G.Q. coordinated the study, contributed fieldwork, performed most of the identifications and participated in conceptual design. L.J. performed identifications and participated in conceptual design; J.C. participated in conceptual design. All authors examined and approved the final version of the manuscript for publication.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chen, R., Jiang, LY., Chen, J. et al. DNA barcoding reveals a mysterious high species diversity of conifer-feeding aphids in the mountains of southwest China. Sci Rep 6, 20123 (2016). https://doi.org/10.1038/srep20123
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep20123
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.