Abstract
The nocioceptive information carried by neurons of the pontine parabrachial nucleus to neurons of the lateral division of the central amydala (CeA-L) is thought to contribute to the affective components of pain and is required for the formation of conditioned-fear memories. Importantly, excitatory transmission between parabrachial axon terminals and CeA-L neurons can be inhibited by a number of presynaptic receptors linked to Gi/o-type G-proteins, including α2-adrenoceptors and GABAB receptors. While the intracellular signalling pathway responsible for α2-adrenoceptor inhibition of synaptic transmission at this synapse is known, the mechanism by which GABAB receptors inhibits transmission has not been determined. The present study demonstrates that activation of presynaptic GABAB receptors reduces excitatory transmission between parabrachial axon terminals and CeA-L neurons by inhibiting N-type calcium channels. While the involvement of Gβγ subunits in mediating the inhibitory effects of GABAB receptors on N-type calcium channels is unclear, this inhibition does not involve Gβγ-independent activation of pp60C-src tyrosine kinase. The results of this study further enhance our understanding of the modulation of the excitatory input from parabrachial axon terminals to CeA-L neurons and indicate that presynaptic GABAB receptors at this synapse could be valuable therapeutic targets for the treatment of fear- and pain-related disorders.
Similar content being viewed by others
Introduction
The laterocapsular division of the central amygdala (CeA-LC) is referred to as the ‘nocioceptive amygdala’1. This region receives nocioceptor-related information from spinal cord lamina I neurons, via neurons of the pontine parabrachial nucleus2,3 and contains a high proportion of neurons that respond to peripherally applied noxious stimuli4. As the central amygdala has a well-established role in the generation and modulation of emotional responses, the processing of nocioceptive information within the CeA-LC is thought to underpin the generation of the emotional/affective components of the pain experience1,2. In support of this suggestion, disruption of the parabrachial connection to CeA-LC neurons blocks the processing of the aversive component of footshock-related nocioceptor-input required for the formation of conditioned fear memories5.
Parabrachial neurons send direct projections to CeA-LC neurons3,6 and the release of glutamate from parabrachial axon terminals excites CeA-LC neurons via the activation of post-synaptic AMPA receptors7,8. However, glutamate release from parabrachial axon terminals is modulated by a number of presynaptic, G-protein-linked receptors. Activation of presynaptic Group I metabotropic glutamate receptors enhances transmission through an action to increase the probability of synaptic vesicle release8, whereas activation of Group II or III metabotropic glutamate receptors inhibits transmission by reducing release probability9,10. Similarly, activation of presynaptic α2-adrenoceptors and GABAB receptors inhibits synaptic transmission between parabrachial axons and CeA-LC neurons7.
Binding of noradrenaline to α2-adrenoceptors on parabrachial axon terminals activates a Gi/o class of G-protein, releasing Gα and Gβγ subunits. Inhibition of the glutamate release from parabrachial axon terminals then occurs via a direct action of Gβγ on release machinery (i.e. SNARE complexes) to inhibit fusion of synaptic vesicles and subsequent release of neurotransmitter (i.e. glutamate), reducing the number of released vesicles but not release probability7. In contrast, GABAB receptor-mediated inhibition of release targets calcium influx through the voltage-gated calcium channels, resulting in a reduction in release probability7. However, the intra-cellular signalling pathway responsible for GABAB-mediated inhibition of voltage-gated calcium channels has not been determined. As such, the present study sought to elucidate the intracellular mechanism by which the activation of presynaptic GABAB receptors inhibits transmission between parabrachial axons and CeA-LC neurons.
Results
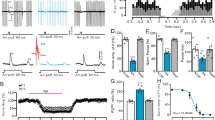
The axons of parabrachial neurons form perisomatic basket-synapses onto neurons of the lateral central amygdala (CeA-L)6. These synapses provide a significant, AMPA-mediated, excitatory input onto CeA-L neurons that is inhibited by the activation of pre-synaptic α2-adrenoceptors (by noradrenaline) and GABAB-receptors (by baclofen) (Fig. 1A,B; see also7). To determine the G proteins coupled to GABAB-receptors and α2-adrenoceptors located on parabrachial axon terminals, slice were pre-exposed to the sulfhydryl alkylating agent N-ethylmaleimide (NEM) at a concentration that selectively inhibits signalling by pertussis-toxin sensitive Gi/o-type G proteins (50 μM, 10 min bath application)11. The pre-incubation of slices with NEM blocked the inhibitory effects of both baclofen and noradrenaline at these synapses (Fig. 1C,D, n = 5 and 4 respectively), a result consistent with the coupling of GABAB-receptors and α2-adrenoceptors to Gi/o-type G proteins.
GABAB-receptor and noradrenenergic inhibition of parabrachial-CeA-L EPSCs is blocked by pre-incubation with 50 μM N-ethylmaleimide (NEM) for 10 minutes prior to application of baclofen or noradrenaline.
(A) Parabrachial-CeA-L EPSC amplitudes during the application of 2 μM baclofen (bacl); inset - average of control and baclofen responses. (B) Parabrachial-CeA-L EPSC amplitudes during the application of 10 μM noradrenaline (NAd); inset - average of control and noradrenaline responses. (C,D) Parabrachial-CeA-L EPSC amplitudes in control and with subsequent application of NEM followed by baclofen (bacl) (C) and noradrenaline (NAd) (D). Inset – average traces in NEM (NEM) and NEM + baclofen (NEM+ bacl) or noradrenaline (NEM + NAd) shown.
Given that both pre-synaptic GABAB-receptors and α2-adrenoceptors signal through Gi/o proteins, we tested whether intracellular signalling pathways were shared between these two types of receptors. The specific GABAB-receptor antagonists CGP5535A (3 μM) completely blocked the inhibitory effect of baclofen (2 μM; n = 3 not shown), but not the inhibitory effect of noradrenaline on synaptic transmission between parabrachial axon terminals and CeA-L neurons (10 μM, 63.9 ± 10.3% inhibition in CGP5535A compared to 70.9 ± 3.8% in control, n = 4 and 21 respectively, p < 0.05 for both). Similarly, the α2-adrenoceptor antagonist yohimbine blocked the inhibitory effect of noradrenaline, but not the inhibitory effects of baclofen (n = 5, not shown), on transmission at this synapse (average 79.6 ± 6.0% inhibition in yohimbine compared to 75.8 ± 4.1% in control, n = 3 and 7 respectively). In addition, synaptic transmission was further inhibited by both the application of noradrenaline in the presence of baclofen (44.5 ± 7.0% inhibition, n = 5, data not shown) and the application of baclofen in the presence of noradrenaline (62.6 ± 7.0% inhibition, p < 0.05, n = 5, not shown). These results indicate that while GABAB-receptors and α2-adrenoceptors share a common class of G-proteins (Gi/o) they mediate their effects on vesicular release from parabrachial axon terminals via distinct signal transduction mechanisms.
The application of the myristilated Gβγ binding peptide mSIRK to terminals blocks the inhibitory effect of noradrenaline on vesicular release from parabrachial axons (Fig. 2A; see also7), indicating that Gβγ mediates the α2-adrenoceptor modulation of synaptic transmission between parabrachial axons and CeA-L neurons. In contrast, application of mSRIK failed to block the inhibitory effect of baclofen on synaptic transmission at this synapse (Fig. 2A; 71.1 ± 4.7% inhibition, p < 0.05, n = 4). However, mSIRK did block a baclofen-induced potassium current in neurons of the basolateral amygdala, demonstrating the Gβγ does mediate some of the effects produced by GABAB-receptor activation (Fig. 2B; n = 3). Therefore, while GABAB receptors can use Gβγ to influence downstream targets, the results of this experiment suggest that Gβγ might not be required for GABAB-mediated inhibition of synaptic transmission between parabrachial axons and CeA-L neurons.
GABAB-mediated inhibition does not require Gβγ signalling.
(A) Parabrachial-CeA-L EPSC amplitude recorded from synapses loaded with the myristlated compound mSIRK in control conditions and after the addition of noradrenaline (NAd) and baclofen (bacl). Below - average EPSCs in each condition. (B) Baclofen (bacl) activated post-synaptic currents recorded from CeA-L cells in control conditions and after mSIRK application.
Baclofen reduces transmitter release from parabrachial axons by reducing voltage-dependent calcium influx into terminals resulting in a decrease in the probability of action-potential-driven vesicle fusion and neurotransmitter release7 and the previous experiments indicated that this GABAB-mediated inhibition might not involve Gβγ signalling. Indeed, activation of GABAB receptors can produce a voltage-independent block of the N-type calcium channels via a Gβγ-independent activation of the pp60C-src tyrosine kinase12. To determine whether a similar intracellular signalling process was responsible for the GABAB-mediated inhibition of vesicle release from parabrachial axon terminals the specific N-type calcium channel antagonist ω-conotoxin CVID13 (200 nM) was applied to slices. This revealed that N-type calcium channels were largely responsible for mediating neurotransmitter release from the parabrachial axon terminals (69.6 ± 8.0% inhibition; Fig. 3A, n = 5, p < 0.5). In addition, application baclofen in the presence ω-conotoxin CIVD did not produce any further inhibition of synaptic transmission (Fig. 3A, 9.2 ± 22.3% change, n = 4). Thus, activation of presynaptic GABAB receptor on parabrachial axon terminals inhibits neurotransmitter release by inactivating N-type calcium channels. However, pre-incubation of the slices in a src-kinase antagonist (PP2; 10μM) did not block GABAB-mediated inhibition of transmission between parabrachial axons and CeA-L neurons (63.5 ± 6.2% inhibition p < 0.01, n = 8; Fig. 3B). Therefore, inhibition of neurotransmitter release from parabrachial axon terminals does not involve a voltage-independent block of the N-type calcium channels via a Gβγ-independent activation of the pp60C-src tyrosine kinase.
GABAB-mediated inhibition of parabrachial-CeA-L EPSCs by baclofen is reduced by blocking N-type calcium channels but not by pre-incubation with the selective src-kinase inhibitor PP2.
(A) Parabrachial-CeA-L EPSC amplitudes recorded from synapses in control conditions and following sequential addition of ω-conotoxin CVID (CIVD; 200 nM) and baclofen (bacl; 2 μM). (B) Parabrachial-CeA-L EPSC amplitudes recorded from synapses in slices pre-incubated for 90 min in PP2 in the absence and presence of baclofen. Average EPSCs in control and baclofen are shown on right.
Discussion
Activation of presynaptic GABAB receptors inhibits excitatory transmission between parabrachial axon terminals and CeA-L neurons7. The results of the present study indicate that this inhibition involves the activation of Gi/o-linked G-proteins resulting in the inactivation of N-type calcium channels responsible for action-potential-driven neurotransmitter release. Further, the inactivation of N-type calcium channels within parabrachial axon terminals is not due to the activation of pp60C-src tyrosine kinase.
This study adds to the list of Gi/o-linked receptors that act presynaptically to inhibit synaptic transmission between parabrachial axons and CeA-L neurons. Presynaptic α2-adrenoceptors, GABAB receptors and Group II and Group III metabotropic glutamate receptors all inhibit the release of synaptic vesicles from axon terminals of parabrachial neurons within the CeA-LC. While these four receptors types link to Gi/o-linked G-proteins, they inhibit synaptic transmission in different ways. Binding noradrenaline to α2-adrenoceptors on parabrachial axon terminals activates Gi/o-linked G-proteins, releasing Gα and Gβγ subunits. Inhibition of the glutamate release from axon terminals then occurs via a direct action of Gβγ subunits on release machinery (i.e. SNARE complexes) to inhibit fusion of synaptic vesicles and subsequent release of neurotransmitter (i.e. glutamate). This mechanism reduces the number of vesicles released from the terminal without reducing release probability7. In contrast, activation of GABAB receptors, Group II or Group III metabotropic glutamate receptors reduces the probability of synaptic vesicle release from parabrachial axon terminals. While the mechanism by which metabotropic glutamate receptors influence release probability of parabrachial axon terminals remains to be determined, the present study demonstrates that GABAB receptors reduce release probability at these terminals by inhibiting N-type calcium channels.
In the majority of instances, the activation of Gi/o-linked G-proteins inhibits the opening of presynaptic N-type calcium channels in a voltage-dependent manner14. This voltage-dependent inhibition involves the binding of Gβγ subunits to α1-domain of N-type calcium channels to stabilise the closed state of the channel, making it ‘reluctant’ to open15,16,17. In contrast, the present study found that application of the myristilated Gβγ binding peptide mSIRK did not prevent the GABAB-mediated reduction in synaptic transmission between parabrachial terminals and CeA-L neurons. This result suggests that Gβγ subunits are not involved in the inhibition of N-type calcium channels in parabrachial axon terminals. That mSIRK was able to prevent α2-adrenoceptor-mediated inhibition of synaptic transmission at this synapse demonstrates the ability of mSIRK to infiltrate parabrachial terminals within the CeA-L. However, GABAB-receptors form a complex with G-proteins and N-type calcium channels18 and the vesicular release protein syntaxin 1A form a complex with both Gβγ subunit and the α1-domain of N-type calcium channels19. These two complexes facilitate the interaction between GABAB-receptors, Gβγ subunits and N-type calcium channels and quite possibly limit the ability of mSIRK to bind to Gβγ subunits before they bind to and inhibit N-type calcium channels. Therefore, involvement of Gβγ subunits in GABAB-mediated inhibition of synaptic transmission between parabrachial terminals and CeA-L neurons warrants further investigation.
The present study did not find any evidence for a voltage-independent block of the N-type calcium channels via a Gβγ-independent activation of the pp60C-src tyrosine kinase12. In addition, the results presented here demonstrate that GABAB-mediated inhibition of N-type calcium channels is not due to G-protein-triggered activation of a number of other kinases that are also blocked by PP2 (e.g. Lck, CSK, Eph-A2, FGF-R1, p38α MAPK, CK1δ and RIP2)20.
Nocioceptive information carried by neurons of the parabrachial nucleus to neurons of the CeA-LC is critical for the formation of conditioned fear memories and is thought to play a key role in generating the affective components of the pain experience2,5. Consistent with this, pharmacological interventions that alter synaptic transmission between parabrachial axon terminals and CeA-LC neurons influence nocioceptive processing and fear-memory formation. For instance, activation of α2-adrenoceptors within the central amygdala appears to decrease the affective component of visceral pain21, while blockade of α2-adrenoceptors within the central amygdala attenuates stress induced analgesia22. Further, the blockade of N-type calcium channels within the central amygdala inhibits the formation of conditioned-fear memories23. The results of the present study reveal that GABAB-receptors reduce synaptic transmission between parabrachial axon terminals and CeA-L neurons by inhibiting N-type calcium channels. Therefore, it is likely that activation of GABAB-receptors within the central amygdala will modulate both fear-memory formation and pain perception, making presynaptic GABAB-receptors within the CeA-L valuable therapeutic targets for the treatment of fear-and pain-related disorders.
Methods
Coronal brain sections were prepared from 21–28 day old Wistar rats. Rats were anaesthetized using isoflurane, decapitated and their brains removed into ice cold artificial cerebrospinal fluid (ACSF) solution containing (in mM): 118 NaCl, 25 NaHCO3, 10 Glucose, 2.5 CaCl2, 1.2 NaH2PO4 and 1.3 MgCl2. Brains were sliced into 350 μm thick coronal sections using a Leica VT1000S vibratome at 0 °C, transferred to a chamber containing ACSF at 33–34 °C for 30 mins, then maintained for several hours in ACSF at room temperature. All procedures were performed with the approval of the Institutional Animal Ethics Committees of The University of Queensland and Charles Sturt University and carried out in accordance with the approved guidelines.
Whole cell recordings were made from brain slices maintained at 32-33 °C in a recording chamber continuously perfused with oxygenated ACSF and visualized using IR/DIC techniques. Recording electrodes (3-5 MΩ) were filled with pipette solution containing (mM): CsMeSO4 135, NaCl 8, HEPES 10, Mg2ATP 2 and Na3GTP 0.3 (pH 7.2 with CsOH, osmolarity 290 mOsm/kg). Whole cell voltage-clamp recordings were made using a patch clamp amplifier (Multiclamp 700A, Axon instruments, Foster City, CA). Current signals were filtered at 4-8 kHz and digitized at 20 kHz (National Instruments, USB-6221 digitiser), acquired, stored and analyzed on Toshiba Satellite Pro L70 PC using Axograph software. Access resistance (5-15 MΩ) was monitored throughout the experiment. In all experiments, fast GABAergic transmission was blocked with picrotoxin (100 μM) added to the external ACSF solution.
Most drugs were added to the ACSF solution used to perfuse the slide i.e. picrotoxin (Sigma); CGP55845A, N-ethylmaleimide (NEM), yohimbine, prazosin and NBQX (all from (Tocris); and conotoxin CIVD (a gift from Prof David Adams). myristilated Gβγ binding peptide, mSIRK, (Tocris) was applied directly to the parabrachial terminals by puffer pipette for 10–15 min prior to recording. The src-kinase-specific antagonist PP2 (Tocris) was applied, in ACSF, to the slices for 30–90 min in a small incubation chamber before being removed to the recording chamber.
Excitatory post-synaptic currents (EPSCs) were evoked using a bipolar stimulating electrode placed onto the surface of the slice. Responses shown are averages of 10–50 individual trials. Student’s t tests were used for statistical comparisons between groups (except where indicated). All results are expressed as mean ± s.e.m.
Additional Information
How to cite this article: Delaney, A.J. and Crane, J.W. Presynaptic GABAB receptors reduce transmission at parabrachial synapses in the lateral central amygdala by inhibiting N-type calcium channels. Sci. Rep. 6, 19255; doi: 10.1038/srep19255 (2016).
References
Neugebauer, V., Li, W., Bird, G. C. & Han, J. S. The amygdala and persistent pain. Neuroscientist 10, 221–34 (2004).
Gauriau, C. & Bernard, J. Pain pathways and parabrachial circuits in the rat. Exp. Physiol. 87, 251–258 (2002).
Bernard, J. F., Peschanski, M. & Besson, J. M. A possible spino (trigemino)-ponto-amygdaloid pathway for pain. Neurosci. Lett. 100, 83–88 (1989).
Neugebauer, V. & Li, W. Processing of nociceptive mechanical and thermal information in central amygdala neurons with knee-joint input. J. Neurophysiol. 87, 103–12 (2002).
Han, S., Soleiman, M. T., Soden, M. E., Zweifel, L. S. & Palmiter, R. D. Elucidating an affective pain circuit that creates a threat memory. Cell 162, 363–374 (2015).
Sarhan, M., Freund-Mercier, M.-J. & Veinante, P. Branching patterns of parabrachial neurons projecting to the central extended amgydala: single axonal reconstructions. J. Comp. Neurol. 491, 418–42 (2005).
Delaney, A. J., Crane, J. W. & Sah, P. Noradrenaline modulates transmission at a central synapse by a presynaptic mechanism. Neuron 56, 880–92 (2007).
Neugebauer, V., Li, W., Bird, G. C., Bhave, G. & Gereau, R. W. Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J. Neurosci. 23, 52–63 (2003).
Han, J. S., Bird, G. C. & Neugebauer, V. Enhanced group III mGluR-mediated inhibition of pain-related synaptic plasticity in the amygdala. Neuropharmacology 46, 918–26 (2004).
Han, J. S., Fu, Y., Bird, G. C. & Neugebauer, V. Enhanced group II mGluR-mediated inhibition of pain-related synaptic plasticity in the amygdala. Mol. Pain 2, 18 (2006).
Shapiro, M. S., Wollmuth, L. P. & Hille, B. Modulation of Ca2+ channels by PTX-sensitive G-proteins is blocked by N-ethylmaleimide in rat sympathetic neurons. J. Neurosci. 14, 7109–7116 (1994).
Raingo, J., Castiglioni, A. J. & Lipscombe, D. Alternative splicing controls G protein-dependent inhibition of N-type calcium channels in nociceptors. Nat. Neurosci. 10, 285–92 (2007).
Lewis, R. J. et al. Novel omega-conotoxins from Conus catus discriminate among neuronal calcium channel subtypes. J. Biol. Chem. 275, 35335–44 (2000).
Zamponi, G. W. & Currie, K. P. M. Regulation of CaV2 calcium channels by G protein coupled receptors. Biochim. Biophys. Acta - Biomembr. 1828, 1629–1643 (2012).
Herlitze, S. et al. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature 380, 258–262 (1996).
Ikeda, S. R. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature 380, 255–258 (1996).
Tedford, H. W. & Zamponi, G. W. Direct G protein modulation of Cav2 calcium channels. Pharmacol. Rev. 58, 837–862 (2006).
Laviv, T. et al. Compartmentalization of the GABAB Receptor Signaling Complex Is Required for Presynaptic Inhibition at Hippocampal Synapses. J. Neurosci. 31, 12523–12532 (2011).
Jarvis, S. E., Magga, J. M., Beedle, A. M., Braun, J. E. A. & Zamponi, G. W. G protein modulation of N-type calcium channels is facilitated by physical interactions between syntaxin 1A and Gβγ. J. Biol. Chem. 275, 6388–6394 (2000).
Bain, J. et al. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 408, 297–315 (2007).
Deyama, S. et al. Roles of β- and α2-adrenoceptors within the central nucleus of the amygdala in the visceral pain–induced aversion in rats. J. Pharmacol. Sci. 114, 123–126 (2010).
Ortiz, J. P., Close, L. N., Heinricher, M. M. & Selden, N. R. α2-Noradrenergic antagonist administration into the central nucleus of the amygdala blocks stress-induced hypoalgesia in awake behaving rats. Neuroscience 157, 223–228 (2008).
Finn, D. P. et al. The role of the central nucleus of the amygdala in nociception and aversion. Neuroreport 14, 981–4 (2003).
Author information
Authors and Affiliations
Contributions
A.J.D. conceived and performed the experiments. J.W.C. and A.J.D. analysed and interpreted the results and prepared the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Delaney, A., Crane, J. Presynaptic GABAB receptors reduce transmission at parabrachial synapses in the lateral central amygdala by inhibiting N-type calcium channels. Sci Rep 6, 19255 (2016). https://doi.org/10.1038/srep19255
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep19255
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.