Abstract
Pierce’s disease (PD) of grapevines is caused by Xylella fastidiosa (Xf), a xylem-limited gamma-proteobacterium that is responsible for several economically important crop diseases. The occlusion of xylem elements and interference with water transport by Xf and its associated biofilm have been posited as the main cause of PD symptom development; however, Xf virulence mechanisms have not been described. Analysis of the Xf secretome revealed a putative lipase/esterase (LesA) that was abundantly secreted in bacterial culture supernatant and was characterized as a protein ortholog of the cell wall-degrading enzyme LipA of Xanthomonas strains. LesA was secreted by Xf and associated with a biofilm filamentous network. Additional proteomic analysis revealed its abundant presence in outer membrane vesicles (OMVs). Accumulation of LesA in leaf regions associated positively with PD symptoms and inversely with bacterial titer. The lipase/esterase also elicited a hypersensitive response in grapevine. Xf lesA mutants were significantly deficient for virulence when mechanically inoculated into grapevines. We propose that Xf pathogenesis is caused by LesA secretion mediated by OMV cargos and that its release and accumulation in leaf margins leads to early stages of observed PD symptoms.
Similar content being viewed by others
Introduction
Xylella fastidiosa (Xf) is a fastidious, xylem-limited gamma-proteobacterium that causes several economically important diseases in crop plants, including grapevine, citrus, periwinkle, almond, oleander and coffee1,2. In the field, Xf is vector-transmitted by various xylem sap-feeding sharpshooter insects3,4. The Xf subspecies fastidiosa (Xff), exemplified by the California strain Temecula1, causes Pierce’s disease (PD) in grapevine. PD poses a great threat to the winegrowing regions of California5. However, the Xf life cycle and virulence mechanism are not entirely understood2. A characteristic PD symptom is marginal leaf chlorosis progressing to necrosis (leaf scorch). Three general explanations for PD symptom development have been proposed: (i) occlusion of xylem elements and interference with water transport by Xf and its associated biofilm, (ii) plant systematic responses, e.g., growth regulator imbalance and (iii) Xf-generated phytotoxin5. The occlusion hypothesis extends from early observations of xylem element blockage in PD6. Generally, plant species and regions of the plant body that show the most severe symptoms are those with the greatest proportion of colonized vessels3,7,8,9,10,11, in agreement with the occlusion hypothesis. PD and other Xf diseases are associated with decreased leaf water potential12 and altered carbon isotope incorporation13, also consistent with water stress. Although the occlusion hypothesis widely considered to be supported, other observations are inconsistent with this explanation. The symptoms of wilting and PD are not similar and are additive in effect, Xf accumulation in grapevine leaves does not correlate with local PD symptom severity and in some infections of grapevine and other Xf hosts, severe necrotic symptoms are associated with only minimal vessel occlusion5.
The secretion of virulence factors by pathogens is an important trigger mechanism for many plant diseases. Unlike closely related pathogens from the genus Xanthomonas, Xff lacks the type III secretion system (T3SS)14. However, Xanthomonas and Xf have in common a similar type II secretion system (T2SS) for a battery of important extracellular enzymes involved in nutrient acquisition and virulence15. In Xff, genes have been identified that code for plant cell wall degrading enzymes (CWDEs) such as polygalacturonase, cellulase and proteases14,16,17. These enzymes may aid Xff migration inside xylem vessels by degrading the pit membrane and releasing the carbohydrates necessary for bacterial growth and survival17. Cell wall degradation by CWDEs releases oligosaccharides as a bacterial nutrient source, which can also induce potent plant innate immune responses leading to cell death. Such plant defense responses include production of phytoalexins, fortification of cell walls through callose deposition, oxidative burst and induction of programmed cell death18,19,20.
We report here our analysis of the Xff Temecula1 secretome, including comparison with the bacterial surfaceome and outer membrane and outer membrane vesicle proteomes. The uncharacterized protein PD1703 was identified as the most abundant secreted protein and its characteristics and likely participation in Xff-initiated symptoms of PD are discussed.
Results
Lipases are a highly abundant component of the Xff secretome
The bacterial proteome was investigated to characterize the subcellular localization of Xff proteins as described (Fig. 1A). The identities of soluble supernatant proteins (SSPs) in culture medium were determined after removing bacterial cells and cell fractions through a series of ultracentrifugation and concentration steps. Twenty-four SSPs were identified by LC-MS/MS in the culture supernatant (Table 1). These include many potential plant cell wall-degrading enzymes necessary for nutrient acquisition such as lipases/esterases (PD1703, PD1702 and PD1211), proteases (PD0313, PD0657, PD0950, PD0956 and PD1850) and cellulase (PD0529). SSPs potentially related to cell adhesion (PD1792 and PD0731), bacterial toxicity (PD1427) and pathogenesis (PD1506 and PD0855) were also identified. Separation of SSPs proteins by one-dimensional SDS-PAGE (Fig. 1B) revealed a prominent 40 to 50 KDa band, indicating that a few proteins were highly abundant in Xff secretome. After in-gel digestion, mass spectrometry analysis of this particular band revealed two 42 KDa (PD1703, PD1702) proteins and one 46 KDa (PD1211) putative uncharacterized protein. The high amino acid sequence homology of these three proteins allowed us to estimate their relative abundance from their numbers of assigned spectra (Fig. S1). PD1703 (64.5%; 783) was more abundant in the secretome than PD1702 (34.2%; 268) and PD1211 (1.3%; 10) (Fig. 1B). In silico analysis predicted that PD1703 is a secreted lipase/esterase protein with high sequence similarity to the cell wall-degrading enzyme LipA from Xanthomonas oryzae pv. oryzae (Xoo)21 and Xanthomonas campestris pv. vesicatoria (Xcv)22. To confirm the lipase/esterase functions of PD1703, we evaluated the enzymatic activity of Xff SSPs against short- and long-chain substrates. As reported for Xoo LipA21, Xff SSPs degraded p-nitrophenyl butyrate and the short-chain triacylglyceride tributyrin (C4), but not the long-chain triacylglyceride tricaprin (C10) (Fig. S2).
X. fastidiosa subcellular proteomic analysis.
(A) Workflow describing the procedures used to isolate X. fastidiosa Temecula1 outer membrane proteins (OMPs), outer membrane vesicles (OMVs), surface peptides (surfaceome) and soluble supernatant proteins (SSPs). Outer membrane proteins were extracted using 0.1 M sodium carbonate buffer (pH 11.0) followed by mass spectrometry. To isolate surface peptides, cells (4 × 108 cells/mL) were harvested from a four- to six- day-old culture and subjected to tryptic digestion (cell shaving). Peptides were concentrated (5×) and subjected directly to LC/MSMS. OMVs were purified from the culture supernatant using two ultracentrifugation steps (38,000 × g for 1 h to pellet cell debris followed by 150,000 × g for 3 h for OMVs precipitation). The remaining supernatant was concentrated (∼75 to 100×) using Amicon Ultra-15 3 K filter units to identify SSPs by mass spectrometry. (B) SDS-PAGE 12% resolution of 10 μg Xff total protein (TP), OMP (Table S2) and SSPs (Table 1). Three putative lipase/esterases (PD1703, PD1702 and PD1211) were identified in the highlighted band, which was composed mostly of PD1703 (LesA) and PD1702. (C) Venn diagram quantifying the number of proteins identified in each subcellular proteome. The major outer membrane protein MopB was found in all samples, although it was very poorly represented in the secretome. The lipase/esterase LesA was found in the secretome, surfaceome and OMV proteome.
Xff secretes LesA as cargo in outer membrane vesicles
Gram-negative bacteria produce outer membrane vesicles (OMVs) that contain biologically active proteins and perform diverse biological functions23,24. Production of OMVs by Xff was recently reported elsewhere25. The complete OMV protein cargo has been described for many Gram-negative bacterial species23, including Xff’s closely related pathogen Xanthomonas campestris pv. campestris (Xcc)26, but not for Xff. The OMV fraction was negatively stained and examined using transmission electron microscopy (TEM) (Fig. 2A), revealing the expected vesicles. Our investigation of OMV protein cargo by LC-MS/MS identified 11 proteins (Table 2). Interestingly, the most abundant SSP, LesA (PD1703), was also part of the OMV cargo. Immunoblot analysis confirmed the localization of Xff LesA in the secretome and OMV proteome (Fig. 2C). In addition to LesA, the secreted proteins PD1702, PD0731 and PD1427 were identified in the OMV cargo, indicating that delivery of these proteins in the host could also be mediated by OMVs. Four outer membrane proteins (PD1709, PD1807, PD0313 and PD1283) were identified in the Xff OMV proteome. We previously identified MopB (PD1709) as the major Xff outer membrane protein (OMP)27. PD1807 and PD1283 were also found in our OM preparation (Table S2). The presence of MopB in both the Xff OM and OMV was confirmed by immunoblot (Fig. 2D). The elongation factor Tu (EF-Tu), which is a PAMP in many gram-negative bacteria28,29, was abundant in the Xff outer membrane fraction, but was not identified in Xff secretome or as part of the OMV cargo (Table S2; Fig. 2E).
X. fastidiosa outer membrane vesicle (OMV) visualization and protein cargo analysis.
(A) Electron micrograph of negatively stained outer membrane vesicles extracted from the Xff Temecula1 cell free supernatant. Both small (left; <200 nm diameter) and large (right; up to 400 nm diameter) OMVs were identified. The black arrow indicates the outer membrane, which was released from the bacterial cell, presumably enclosing periplasmic content to produce the OMV. Large OMVs contained an electrodense material of unknown composition that was not seen in small OMVs. (B) Silver-stained SDS-PAGE 12% separation of OMV cargo showing a band at apparent molecular weight 42 KDa, presumed to be composed of proteins LesA, PD1702 and MopB, all found in the OMV proteomic analysis (Table S1). (C–E) Immunoblot detection of LesA, MopB and EF-Tu in Xff total proteins (TP), outer membrane proteins (OMP), soluble supernatant proteins (SSP) and outer membrane vesicle proteins (OMV). For TP, OMP and SSP (defined in Fig. 1A), 10 μg protein was loaded in the gel; however, only 2 μg protein was loaded in OMV due to the high non-protein content of this sample. This may explain the weak detection of LesA in C.
LesA is localized in the secreted filamentous network
To visualize the distribution pattern of Xff-secreted material, we examined bacterial cells in culture using scanning and transmission electron microscopy (SEM and TEM). Negatively stained cell clusters show that Xff produces cell aggregates embedded in a dense secreted material possibly composed of extracellular polysaccharide (EPS) and proteins that are weakly attached to the bacterial surface (Fig. 3A). The same dense material surrounds planktonic cells (Fig. 3B). SEM analysis of the bacterial culture revealed that Xff secretes a filamentous network of unknown composition similar to the matrix seen surrounding cells aggregates in the xylem vessels of infected grapevines30 (Fig. 3C,D). TEM analysis of the secreted matrix revealed its close localization to Xff cells in vitro (Fig. 3E). Immunogold labeling and TEM revealed that LesA is embedded in the Xff secreted network (Fig. 3F).
Electron microscopy of Xff cells and the secreted filamentous network.
(A) Negative staining of Xff aggregates showing abundant secreted material in which bacterial cells (white arrow) are embedded. (B) Closer view of the secreted material surrounding planktonic Xff cells isolated from the same culture shown in A. (C,D) Scanning electron microscopy of Xff showing bacterial cells surrounded by the secreted filamentous network (white arrows). (E,F) Immunogold detection of LesA in the secreted filamentous network (black arrows) surrounding Xff cells (E). LesA was abundant in the secreted network as shown in F (black arrows).
LesA accumulates abundantly in leaf regions with minimal Xff titer and is associated with early stages of PD symptom development
Although LesA accumulated in both OMVs and the secreted matrix of Xff cells, its localization in Xff-infected host tissue was unknown. To search for LesA and other potential secreted virulence factors in the infected host, we compared the total leaf proteome of Xff-infected and non-infected grapevines. Of the 524 proteins found, six were of Xff origin (Table 3). LesA (the most abundant secreted protein) and MopB (the most abundant outer membrane protein) were identified in infected host tissue. The surface protein Hsf (PD0744) was found in infected grapevine leaves, although it was not previously identified in the secretome of cultured Xff. This suggests that both expression and secretion of Hsf may be triggered during infection of grapevine leaves. As expected, no Xff protein was found in non-infected grapevine leaves.
The accumulation of LesA in Xff-infected host tissue confirmed our hypothesis that LesA is highly expressed and secreted not only in vitro but also during the Xff infection/colonization process. We postulated that LesA has an important role in Xff pathogenesis in grapevines similar to that seen in Xoo, where it elicits callose deposition and programmed cell death in rice21,31. We evaluated whether the presence and abundance of LesA correlates with PD symptom development in grapevine leaves 12 weeks post inoculation (wpi). We divided infected leaves into three major areas (inside, middle and outside) corresponding to the pattern of symptom development (Fig. 4A). Viable Xff cells were abundant in the inside portion of infected leaves, but were less abundant in the middle and outside areas, especially close to the leaf margins (Fig. 4B). The most characteristic early PD symptom in grapevines, the marginal leaf necrosis called leaf scorch, was associated with low Xff titer as previously reported32. We postulate that Xff could be releasing either LesA-containing OMVs or SSP, or both, from its colonized region near the petiole. These could deliver LesA to the leaf margins, causing PD symptoms. To test this hypothesis, we localized LesA using a polyclonal antibody in the three areas. LesA was slightly more abundant in the leaf edges than the middle and inside portions (Fig. 4C). When the amount of LesA per viable Xff cell was calculated, a difference of ∼100-fold (p < 0.05) was observed (Fig. 4D).
Detection of LesA movement in grapevine leaves.
(A) Grapevine leaf showing the three segments, each 1 to 2 cm in the radial direction, used to detect LesA: I (inside), M (middle) and O (outside). (B) Viable Xff cell assay of leaf segment (n = 3) extracts revealed ~10-fold reduction in the bacterial population at the leaf edges (outside) than at the center (inside). The bacterial titration was carried out using a standard curve of five dilutions (Fig. S4; *One-way ANOVA test; R2 = 0.9817; p < 0.01). (C,D) The amount of LesA detected in grapevine leaves differs slightly in the inside and outside segments (C) (***Mann-Whitney test; p = 0.1). However, the relative abundance of LesA per number of Xff cells (D) is significantly greater in the outside area than in middle and inside segments (**One-way ANOVA test; p < 0.05), suggesting that the secreted LesA is moving from the Xff-crowded inside area to the symptomatic leaf extremities where the bacteria is scarcely present. This experiment was conducted twice with similar results. Picture of leaf was taken by Rafael Nascimento.
LesA elicits a hypersensitive response in grapevine
LipA from Xoo elicits an innate immune response in rice mediated by cell wall degradation, induces callose deposition and triggers programmed cell death21,31. In addition, LipA is required for wild-type levels of Xcv virulence on tomato22. Analysis of the high-resolution crystal structure of Xoo LipA revealed the canonical catalytic triad residues Ser-176, Asp-336 and His-377. Ser-176 is embedded in the hydrolase conserved motif Gly-X-Ser-X-Gly. Moreover, mutation in the residue Ser-176 reduced Xoo virulence on rice, confirming that the enzymatic activity of LipA, rather than LipA as a protein, is essential for optimal virulence21. In silico analyses mapped LesA from Temecula1 (PD1703) and 9a5c (XF0357) Xf strains on the Xoo LipA structure, assigning the catalytic triad of both Xf LesA molecules to residues Ser-200, Asp-360 and His-402 (Fig. S1). To test whether LesA contributes to Xff virulence in grapevine, we expressed both Xff LesA and its alanine substitution mutant S200A-LesA in E. coli and pressure-infiltrated leaves with protein extract from E. coli. Heterologous expression of both LesA and S200A-LesA constructs was confirmed by immunoblot detection. Extracts from LesA-producing E. coli, but not from the empty-vector control, induced necrosis in the infiltrated area, indicating that LesA may contribute to Xff pathogenesis in grapevines (Fig. 5A,B). Interestingly, infiltrated E. coli extracts producing the Xff S200A-LesA mutant elicited only limited HR-like symptoms compared to wild-type LesA (Fig. 5C), consistent with enzymatic activity of Xff LesA being essential for maximal virulence, as for Xoo LipA.
LesA elicits HR-like symptoms in grapevine leaves.
Crude protein from LesA-expressing E. coli was isolated and syringe-infiltrated in grapevine leaves of greenhouse-grown plants. (A) HR-like symptoms appeared 24 h after infiltration. (B) No reaction was observed after infiltration of crude protein from E. coli expressing the empty vector (pJexpress 401). (C) Reduced HR-like symptoms caused by S200A-LesA, in which the residue Ser-200 from Xff LesA catalytic triad was replaced by alanine. Wild-type LesA caused the same necrosis shown in A. Pictures of leaves were taken by Hossein Gouran.
LesA is required for virulence of X. fastidiosa in grapevines
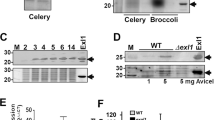
To test whether LesA has a role in Xff virulence, we generated a lesA mutant using homologous recombination and compared the performance of both proteins after inoculation in grapevines. Our Xff lesA mutant lacked lipase and esterase activities (Fig. 6A,B) and expresses no LesA protein (Fig. 6C). Interestingly, the Xff lesA mutant was predominantly found in the biofilm mode of growth when cultivated in liquid media, unlike the wild-type strain (Fig. 6D) and formed large cell aggregates (Fig. 6E). The inability of the lesA mutant to elicit typical PD symptoms was confirmed by significantly reduced symptomatic leaves (50%; p < 0.05) observed in infected grapevines compared with plants inoculated with the parental strain (Fig. 6F).
X. fastidiosa lesA mutants are deficient in virulence.
(A) Lipase activity was analyzed around Xff lesA mutant (lesA-) and WT (Tem1) colonies grown in PD3-agar medium supplemented with 1% trybutirin. (B) Esterase activity of proteins from lesA- and WT by 4-methylumbelliferyl butyrate (4-MUB) assay. Esterase activity is diminished significantly in the lesA mutant. (C) Immunoblot detection of LesA in Xff lesA− and WT total proteins. The LesA protein was not detected in the mutant, confirming the mutagenesis of the lesA gene. (D) Analysis of biofilm formation in liquid culture, showing the predominance of the biofilm growth mode in lesA− when compared to WT. (E) Scanning electron micrographs of Xff culture showing the aggregation of lesA− cells typically found in biofilms. (F) Pierce’s disease symptoms in grapevines. Thompson Seedless infected with the wild-type strain Temecula1, the lesA− strain and uninfected (mock inoculated). Data is shown as the difference from uninfected controls (average of 26 plants). Plants inoculated with Xff lesA− strain have significantly less PD symptoms (*One-Tailed T-test with Welch’s correction and p value of 0.0351) than Temecula1 infected plants. Pictures were taken by Hossein Gouran.
Wild-type Xff and its quorum-sensing mutants have distinct patterns of expression for lesA
Cell–cell signaling plays an important role in the virulence of many plant pathogenic bacteria. Xff produces the quorum-sensing signaling molecule DSF (diffusible signaling factor) that regulates bacterial pathogenesis. In Xanthomonas campestris pv. campestris (Xcc) and Xanthomonas oryzae pv. oryzae (Xoo), DSF-deficient mutants have reduced virulence33,34. Xff rpfF mutants deficient in DSF production possess a hypervirulent phenotype when inoculated into grapevines30. The rpfF gene is required for DSF production in both Xff and Xanthomonas species. Interestingly, Xff rpfC mutants overproduce DSF and have a hyperattachment phenotype, which makes them deficient in virulence and movement in grapevine xylem vessels35. For specific virulence-related genes, messenger RNA accumulation for rpfC and rpfF mutants and the wild type was assessed by RT-PCR. The lesA gene was down regulated in the rpfC mutant (Fig. 7).
LesA is down regulated in an Xff quorum-sensing mutant.
Comparison of RNA accumulation in mutants and wild-type by RT-PCR revealed that lesA is regulated by rpfC. RNA was extracted from Xff cells grown in PD3 medium (three flasks/condition; 107–108 cells/mL) at 28 °C and 120 rpm. The 16S rRNA gene was used as an endogenous control. Unpaired t test with Welch’s correction was used for statistical analysis (one-tailed; *p < 0.01).
Agrobacterium harboring X. fastidiosa LesA is hypervirulent
To assess whether LesA increases A. tumefaciens virulence, we expressed the Xff lesA gene under the control of its own promoter for an in-planta virulence assay (Fig. 8). When inoculated into walnut plants, which are highly susceptible to this pathogen, the infection is usually localized to a region of tumor formation at the site of infection; however, Agrobacterium harboring Xff LesA displayed a systemic necrotic response. The first signs of necrosis appeared in walnut plants inoculated with Agro-A281-LesA at eight weeks post inoculation, but not in plants that were mock-inoculated (PBS) or those that contained an empty vector (Fig. 8A,B). Interestingly, walnut plants inoculated with Agrobacterium expressing Xff LesA were dead at 12 weeks post inoculation (Fig. 8C). When inoculated into grapevines, the same phenotype was not observed (data not shown).
Virulence assay in walnut plants.
Xff lesA gene was expressed in A. tumefaciens under the control of its own promoter. Agrobacterium harboring Xff LesA became hypervirulent. Walnut plants inoculated with agrobacterium expressing Xff LesA (Agro-A281-LesA), but not from the mock inoculation (PBS) and empty vector (A,B), presented the first signs of disease at eight wpi and were dead at 12 wpi (C). Pictures were taken by Hossein Gouran.
Discussion
PD symptom development has long been thought to result from blockage of xylem vessels by Xf biofilm and associated gels and tyloses, which leads to water stress in the distal parts of the infected plant and to PD2. Our results support an alternative mechanism independent of water stress: a phytotoxic effect resulting from the action of a Xff enzyme, the LesA lipase/esterase encoded by the PD1703 locus. Xff LesA is similar to the type II secreted protein LipA, present in all sequenced xanthomonad genomes and in many other Gram-negative bacteria. LipA from the rice pathogen Xanthomonas oryzae pv. oryzae (Xoo) is a 42 kDa α/β hydrolase fold protein with lipase/esterase function and short-chain specificity. Recently, LipA from Xoo was characterized as a cell wall-degrading enzyme with a carbohydrate-binding domain essential to the protein’s virulence function21. In Xanthomonas campestris pv. vesicatoria (Xcv), LipA is expressed from an early stage of tomato leaf infection and is required for wild-type virulence22. Additionally, treatment with Xoo LipA elicited callose deposition and programmed cell death in rice leaves and roots and an active site serine-to-alanine substitution reduced but did not completely suppress virulence21.
Is the Xff lipase/esterase secretion associated with the PD pathogenic process? The preponderance of evidence seems to support this hypothesis. The SSP profile identified LesA as the most abundantly secreted protein. The Xff SSP preparations cleaved p-nitrophenol butyrate and tributyrin, but no long chain triacylglycerides (Fig. S2A,B), identical to the action of the type II secreted lipase LipA. The short-chain specificity of LesA indicates that this enzyme has both esterase and lipase activities.
An association is suggested not only by the presence of homologs of PD1703 in Xoo- and Xcv-infected rice21,31,36 and tomato22, respectively, but also by its absence from Xf strain EB92-1, found in elderberry. EB92-1 infects and survives in grapevines for many years but does not cause symptoms and provides effective biocontrol against Xff. A genome draft of the EB92-1 strain revealed that 10 potential pathogenicity effectors were missing, including LesA (PD1703) and another predicted type II secreted enzyme present in our SSP profile, the serine protease PD095637,38. On the other hand, lipase/esterase genes of Xff are present in the genome of an Xf virulent strain responsible for citrus variegated chlorosis (CVC). The CVC 9a5c strain genes XF0357, XF0358 and XF2151 are apparent homologs of the Xff Temecula1 genes PD1703 (LesA), PD1702 and PD1211, respectively. In a previous study, the corresponding CVC proteins were not reported in the Xff 9a5c secretome, possibly because the secreted protein fraction was underrepresented due to depletion by the cell washing procedure used16,39. We cannot eliminate the possibility of different secreted virulence factors among various Xf subspecies; however, LesA could also play a role in Xff 9a5c pathogenesis.
Our proteomic analysis (Table 3) revealed that LesA is one of six Xff proteins identified in infected grapevine leaves, clearly indicating that LesA is abundantly secreted by Xff during plant infection/colonization in addition to in liquid culture (Tables 1,2). If LesA is partially responsible for PD symptoms, there should be a spatial association of LesA with the leaf symptom pattern of radial transition from green tissue around the point of petiole attachment to scorching that begins at the leaf margins (Fig. 4A). Previous findings32 showed a gradual decrease in Xff titer proceeding from the leaf blade center to the leaf margin, counter to the pattern of symptom development. We have demonstrated that on a per-Xff-cell basis, LesA accumulates most abundantly near the leaf margins in a distribution positively correlated with the development of scorch symptoms (Fig. 4D).
As a direct demonstration of the ability of LesA to induce symptoms, LesA was produced in E. coli and a crude extract was pressure-infiltrated into grapevine leaf blade, inducing local necrosis. The empty-vector control did not induce necrosis and the catalytic triad control, S200A-LesA, induced only minute regions of necrosis (Fig. 5), showing further similarity to Xoo LipA21. The weak reaction of grapevine leaf to the extract from E. coli expressing Xff S200A-LesA may result from residual lipase/esterase activity. Another serine esterase retained a few percent of the wild-type activity in an active site serine-to-alanine replacement mutant40.
The co-localization of LesA and leaf marginal necrosis at a distance from the central leaf region of greatest Xff cell accumulation raised questions about LesA secretion and translocation. Two broad hypotheses were considered: i) secretion and transport of LesA as a soluble protein, or ii) entrapment of LesA in OMVs for transport toward the leaf margins. OMVs are small spherical structures that allow interaction of Gram-negative bacteria with their environment by releasing the vesicular contents adjacent to prokaryotic and eukaryotic cells. OMVs are a vehicle by which Gram-negative pathogens communicate with and intoxicate host cells24. The first hypothesis is fragile because if LesA is a phytotoxin, necrotic lesions should occur everywhere, including in proximity to bacterial aggregates in the central part of the leaf. But necrosis appears first at the leaf margins, favoring the second hypothesis of OMV-facilitated transport of LesA toward the leaf margin with subsequent release of the OMV cargo.
OMVs are formed by outer membrane (OM) entrapment of periplasmic constituents as OMV cargo, including soluble proteins, phospholipids, lipopolysaccharides (LPSs) and DNA23,41,42. Many pathogenic bacteria use OMVs to release virulence factors43,44,45,46,47, quorum sensing signals48, pathogen-associated molecular patterns (PAMPs)49,50 and other OM components like surface-exposed adhesins51. Xff releases OMVs carrying the autotransporter XatA25. The complete OMV protein cargo was not determined here. XatA impacts migration, colonization and biofilm formation, but a direct relationship between PD symptoms and XatA was not observed25.
The presence of LesA was highly concentrated in the leaf margins and its concentration gradually decreased toward the petiole, where the bacteria are typically found as a biofilm network. SEM and TEM imaging (Fig. 3) identified a major filamentous network formed in the presence of LesA, but at low concentration and well distributed. Biofilm behavior has been well studied in Xff2. Biofilms represent mainly dead cells and are down regulated for genes that are associated with disease. In contrast, the planktonic bioform is motile, spreads long distances and expresses genes associated with disease2. Biofilm behavior and its relation to PD have been studied using two specific mutations, rpfF and rpfC. The rpfF gene is responsible for synthesis of diffusible signal factor (DSF). DSF is an unsaturated fatty acid that modulates Xff virulence and biofilm formation by cell–cell signaling. Xff rpfF mutants are deficient in DSF production, possess a hypervirulent phenotype and are found mainly in a planktonic bioform30. Xff rpfC mutants exhibit a hyperattachment phenotype (increased biofilm formation) associated with their inability to migrate in xylem vessels and to cause PD35. Interestingly, gene expression analysis of lesA in both rpfF and rpfC quorum-sensing mutants found that lesA is highly down regulated in the non-pathogenic rpfC mutant (Fig. 7). We hypothesize that lesA provides specific nutrients needed to sustain planktonic growth through its cell wall degrading lipase/esterase activity, much like that observed for the pectin degrading activity of pglA17. As a consequence, lesA mutants that do not make LesA display a biofilm mode of growth similar to rpfC mutants. We believe that the inability of Xff rpfC mutants to cause PD symptoms in grapevine may be related in part to decreased expression of LesA and other potential virulence factors regulated by rpfC, as observed in Xcc rpfC mutants. Xcc rpfC mutants are deficient in the production of virulence factors such as extracellular polysaccharide (EPS) and in the secretion of extracellular enzymes52. Formation of OMVs by Xff is significantly higher in the rpfF mutant, which is disrupted for DSF53. This hypervirulent mutant also displayed XadA on the OMV’s surface, correlating with our proteomic data showing XadA abundantly present in Xff OMVs (Table 2). If LesA is delivered by OMVs, then increased OMV production may be partially responsible for increased rpfF mutant virulence. We propose that the release of LesA through OMVs to distant sites, without biofilm formation, may be a major cause of tissue destruction and result in relocation of nutrients from the leaf margins toward bacterial colonization sites.
Further corroboration of LesA participation in Xff pathogenesis has been shown through the two-component system of GacS/GacA. This system is involved in environmental signaling and control of secondary metabolites and production of extracellular enzymes. It regulates the virulence of many pathogenic and environmental bacteria, including Xff54. GacA is a response regulator that controls various physiological processes and pathogenicity factors through transcriptional activation of genes that regulate pathogenesis, e.g., genes involved in quorum-sensing, toxin production, motility, biofilm formation and extracellular polysaccharide production55,56,57,58,59. Several putative pathogenicity-related genes are regulated by gacA in Xff, including the secreted lipase-esterase LesA. Interestingly, Xff gacA mutants expressed less LesA and developed significantly less severe disease symptoms when inoculated into grapevines54. Xff gacA mutants may be deficient in pathogenesis due to low expression of virulence factors such as LesA.
To confirm the correlation between LesA and Xff pathogenicity, we generated a lesA mutant, which induced less severe PD symptoms than the wild-type strain. The lack of virulence of our Xff lesA mutant, similar to those of Xff rpfC, gacA, Xoo, and Xcv lipA mutants21,22, supports a significant role for LesA in Xff pathogenicity. In addition, expression of lesA under its own promoter in Agrobacterium tumefaciens propagated a systemic cell death response in inoculated walnut plants. These findings strongly suggest that LesA is part of the Xff repertoire of secreted virulence factors and is directly associated with bacterial pathogenesis.
We propose a model for Xf pathogenesis in PD based on the secretion, movement and accumulation of the lipase/esterase LesA in infected grapevine leaves, leading to leaf scorching and chlorosis. In our preferred symptom-inducing mechanism, planktonic forms of Xff secrete LesA by means of OMVs. These vesicles increasingly accumulate in the leaf margins and release their cargo, including LesA, initiating PD symptom development.
Material and Methods
Xff strains and growth conditions
The WT Temecula1 strain of Xylella fastidiosa subspecies fastidiosa (Xff; ATCC 700964) and Xff lesA mutant were grown in PD3 medium60 with aeration (120 rpm) at 28 °C. Plate cultures were prepared in the same medium with the addition of 1.5% agar. PD3 medium was supplemented with kanamycin (5 μg/mL) for selective growth of the lesA mutant. The rpfF and rpfC strains used in this study were kindly provided by Prof. Steven E. Lindow.
Isolation of secreted proteins and outer membrane vesicles from culture supernatants
Xff cells were harvested by centrifugation at 8000 × g for 15 min at 4 °C. The culture supernatant was transferred to 38.5 mL tubes and centrifuged at 38,000 × g for 1 h at 4 °C in a SW28 rotor (Beckman Coulter, USA). The supernatant was collected (the remaining pellet was discarded), transferred to 12 mL tubes and centrifuged at 150,000 × g for 3 h at 4 °C (SW41 Ti rotor, Beckman Coulter). The supernatant, containing SSPs, was concentrated 75 to 100× using Amicon Ultra-15 3 K filters units (Millipore). The pellet, containing OMVs, was resuspended in 300 μL PBS (pH 7.4) for subsequent SDS-PAGE analysis. For electron microscopy analysis, the vesicle pellet was resuspended in 50 mM HEPES buffer (pH 6.8).
Outer membrane and total protein extraction
Outer membrane protein extraction was performed as described27. To prepare total protein extracts, bacteria from a 2 mL culture (four to six days old) were harvested by centrifugation at 8000 × g for 15 min at 4 °C and washed three times with 1 mL washing buffer containing 10 mM Tris-HCl (pH 8.8), 3 mM KCl, 50 mM NaCl, 5 mM EDTA and 1 mM PMSF and were centrifuged for 2 min at 3000 × g. The pelleted cells were then lysed with 200 μL 10 mM Tris (pH 8.8) with 0.5% w/v SDS, 5 mM EDTA and 1 mM PMSF. After adding DTT to 100 mM, the sample was boiled 3 min and stored at −80 °C.
Bacterial surface digestion
Xff cells were harvested by centrifugation at 8000 × g for 15 min at 4 °C and washed three times with 1 mL PBS. A total of 4 × 108 cells were resuspended in 100 μL filtered and sterilized 50 mM ammonium carbonate buffer (pH 7.5). Digestions were carried out with 20 μg sequencing grade modified trypsin (Promega) in the presence of 5 mM DTT for 15 min at 37 °C. The digestion mixture was centrifuged at 3500 × g for 10 min at 4 °C and the supernatant (containing the peptides) was collected. The trypsin reaction was stopped by adding formic acid to 0.1%. The reaction was filtered using Amicon Ultra Microcon 3 K filters units (Millipore) and the flow-through peptides were held at −20 °C for later analysis.
Grapevine leaf protein extraction
Grapevine (Vitis vinifera L. cv. ‘Thompson Seedless’) leaves from Xff-infected (n = 5) and non-infected (n = 5) plants were collected about one meter above the point of inoculation (12 wpi). Plant tissues were flash frozen in liquid nitrogen, lyophilized and kept at -80°C. Proteins were extracted using a phenol extraction procedure as described61. Each leaf segment (Fig. 4A) was ground in liquid nitrogen using a pestle and mortar containing 1% (w/w) PVPP. One hundred mg plant material was resuspended in 600 μL extraction buffer (0.7 M sucrose, 0.1 M KCl, 0.5 M Tris-HCl pH7.5, 0.5 M EDTA, 1 mM PMSF and 2% β-mercaptoethanol). The suspension was homogenized three times (1 min each) using a MM300 TissueLyser (Qiagen). An equal volume of UltraPure buffer-saturated phenol (Life Technologies, USA) was added and the mixture was rehomogenized as described above. After centrifugation at 12,000 × g for 15 min at 4°C, the upper phenol phase was removed and the remaining pellet used for re-extraction in extraction buffer. Proteins were precipitated from the phenol phase using five volumes of 100 mM ammonium acetate in methanol overnight at -20°C followed by centrifugation at 12,000 × g for 15 min at 4°C. Protein pellets were washed four times with 4 mL 100 mM ammonium acetate in methanol and dried 10 min in the hood. Proteins were solubilized with urea buffer (7 M urea, 2 M thiourea, 40 mM Tris, 2% Chaps and 18 mM DTT). The protein concentration was determined according to Bradford’s method using BSA as the standard.
Protein preparation and mass spectrometry analysis
Xff SSPs and OMV proteins were resolved by SDS-PAGE prior to in-gel digestion to reduce the amount of non-protein contaminants in the samples. Peptides from the cell shaving (surfaceome) procedure were desalted using Aspire RP30 desalting tips (Thermo-Fisher Scientific) and subjected directly to LC/MSMS analysis. For the in-gel digestion used for SSPs and OMV proteome analysis, gel pieces were washed twice with 100 to 150 μL 50 mM ammonium bicarbonate (AmBic; pH 8.0), followed by dehydration with acetonitrile (ACN; three to four times the total volume of gel pieces) for 10 to 15 min. Proteins were reduced for 30 min at 56°C in a solution of 10 mM DTT and 50 mM AmBic. Gel pieces were dehydrated again, followed by replacement of ACN by 55 mM iodoacetamide in 50 mM AmBic. Gel pieces were incubated 20 min in the dark at room temperature, followed by two washes with 150 to 200 μL of 50 mM AmBic for 15 min each. Gel pieces were dehydrated with ACN, dried by speed vacuum centrifugation and subjected to tryptic digestion overnight. Peptides were extracted by adding 60% ACN and 0.1% trifluoroacetic acid (TFA) in water to the gel pieces, followed by sonication for 10 min. The solution containing the peptides was mixed with the supernatant resulting from the tryptic digestion, followed by speed vacuum centrifugation. Digested peptides were then desalted using Aspire RP30 desalting tips and resuspended in loading buffer.
The digested peptides were analyzed using a QExactive mass spectrometer (Thermo Fisher Scientific) coupled with an Easy-LC (Thermo Fisher Scientific) and a nanospray ionization source. The peptides were loaded onto a trap (100 micron, C18 100 Å 5U) and desalted online before separation using a reverse phased column (75 micron, C18 200 Å 3U). The gradient duration for separation of peptides was 60 min using 0.1% formic acid and 100% ACN for solvents A and B respectively. Data was acquired using a data-dependent ms/ms method with a full scan range of 300 to 1600 Da and a resolution of 70,000. The ms/ms method’s resolution was 17,500 with an isolation width of 2 m/z with normalized collision energy of 27. The nanospray source was operated using 2.2 KV spray voltage and a heated transfer capillary temperature of 250°C. Raw data was analyzed using X!Tandem and visualized using Scaffold Proteome Software (Version 3.01). Samples were searched against Uniprot databases appended with the cRAP database, which recognizes common laboratory contaminants. Reverse decoy databases were also applied to the database prior to the X!Tandem searches.
For the grapevine proteomic analysis, leaf proteins were precipitated using a ProteoExtract protein precipitation kit (Calbiochem) followed by dehydration overnight in a sterile fume hood. The protein pellet was resuspended in 50 mM AmBic (pH 8.0) and subjected to an in-solution tryptic digestion. Digested peptides were then desalted and subjected to LC/MSMS as described above.
Electron microscopy analysis of outer membrane vesicles
Outer membrane vesicles were resuspended in 50 mM HEPES buffer (pH 6.8) and fixed with 4% paraformaldehyde in 1 M Sorenson’s phosphate buffer (pH 7.4). Copper grids (400 mesh) supported with formvar coating were used for electron microscopy. Ten μL fixed OMVs were placed in the grids and allowed to settle for 10 min. The excess sample was removed with filter paper, followed by quickly staining with 1% ammonium molybdate. Grids were air-dried completely before visualization in a Philips CM120 (FEI/Philips Inc.) electron microscope at 80 KV.
Western-blot analysis
To detect LesA, anti-LesA antibody was diluted in PBS-M 1% (PBS plus 1% non-fat dried milk; 1:1000), followed by detection using HRP-conjugated goat anti-rabbit antibody (1:2000 for grapevines and 1:4000 for Xff samples) (Life Technologies, USA). Blocking and washing steps were performed with PBS-M 5% (PBS plus 5% non-fat dried milk) and PBS-T 0.1% (PBS plus 0.1% Tween 20), respectively. Developments were carried out using ECL Plus western blotting detection reagents (GE Life Sciences, USA). To detect MopB and EF-Tu in Xff samples, polyclonal antibodies (dilutions of 1:20,000 for MopB and 1:10,000 for EF-Tu) were used, followed by detection using HRP-conjugated goat anti-rabbit antibody (1:20,000). Blocking and washing steps were carried out as described for LesA. Blots were developed using SuperSignal West Dura Chemiluminescent Substrate (Thermo Scientific, USA). The anti-LesA polyclonal antibody was generated by immunizing rabbits with LesA immunogenic synthetic peptides (GeneScript, USA). The anti-MopB27 and anti-EF-Tu polyclonal antibodies were kindly provided by George Bruening (Plant Pathology, UC Davis).
Immunogold electron microscopy
Immunogold electron microscopy (IEM) was performed using fresh cultures of Xff. The sample containing Xff cells and the filamentous network secreted in the culture supernatant were fixed with 4% paraformaldehyde in 1 M Sorenson’s phosphate buffer (pH 7.4). The fixed sample was embedded in LR White resin as described62. Ultra-thin sections were cut and placed onto coated grids (200 mesh; treated with glow-discharge), followed by blocking with 1% fish gelatin for 30 min. Grids were blotted with anti-LesA (1:500) antibody for 1 h at RT and washed with PBS. The primary antibody was detected using anti-rabbit (1:50) antibody coupled to 10 nm gold particles. Unbound conjugate was removed using a sequence of washing steps with PBS. The preparation was negatively stained with 1% ammonium molybdate, air-dried and visualized in a Philips CM120 (FEI/Philips Inc.) electron microscope at 80 KV.
Lipase and esterase activity assays
Tributyrin (C4), tricaprin (C10) and a mixture of tryglicerides (C2 to C10) (Sigma-Aldrich, USA) were used as substrates for LesA activity in a plate assay21,63. The triglyceride substrates (0.5%; v/v) were prepared in a buffer containing 100 mM Tris-HCl (pH 8.0), 25 mM CaCl2, sonicated at 30 W for three min to emulsify the substrates, mixed with an equal volume of 2% agarose solution and solidified in Petri plates. Fifty μL Xff soluble supernatant proteins (700 ng/μL) were added to the wells and assayed for a zone of clearance for 24 to 48 h at RT. The culture medium PD3 was used as a negative control. For the Xff lesA mutant lipase activity assay, tributyrin (3%; v/v) was emulsified in PD3 agar medium using a Polytron PT 3100 prior to autoclaving. Esterase activity was also determined using 4-methylumbelliferyl butyrate (4-MUB) substrate as previously described64. For the SSPs esterase activity assay, pNP butyrate (pNP-C4) was used as the substrate in a spectrophotometric assay, which was performed at A405 after a 10 min incubation of pNP-C4 (1 to 5 mM) with Xff soluble supernatant proteins (1 μg/well) in 50 mM Tris-HCl (pH 7.5) at 37 °C.
LesA detection in grapevine leaves by ELISA
To detect LesA in grapevine (Vitis vinifera L. c.v. ‘Thompson Seedless’) by ELISA, leaves from Xff-infected (12 wpi) and non-infected plants were collected and divided in three sections (inside, middle and outside; ∼2 cm radial dimension each). Ninety mg leaf tissue was homogenized in 900 μL coating buffer (0.1 M sodium carbonate buffer, pH 9.6) for three min using a MM300 TissueLyser (Qiagen, USA). The number of Xff cells present in each homogenized sample was determined using a double-antibody sandwich (DAS)-ELISA assay (Agdia Inc., USA) following manufacturer’s instructions. For LesA detection, the homogenized solution was used to coat (100 μL/well) a ninety-six-well Maxisorp microtiter plate (NUNC, USA) for two h at RT. The wells were washed two times with PBS-T 0.1% (PBS plus 0.1% Tween 20) and blocked for one h at RT with PBS-M 5%. The plate was washed three times with PBS-T 0.1%, followed by incubation with anti-LesA (1:1000) antibody in PBS-M 1% for one h at 37 °C. The plate was washed three times with PBS-T 0.1% followed by incubation with HRP-conjugated anti-rabbit (1:1000) in PBS-M 1% for one h at 37 °C. The plate was washed four times with PBS-T 0.1% and developed with TMB (3,3′,5,5′-tetramethylbenzidine). One-way ANOVA and Mann-Whitney tests were carried out using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA). A value of p < 0.05 was considered statistically significant.
Hypersensitive response assay in grapevine leaves
The heterologous expression of wild type LesA and its mutated version, S200A-LesA, in which the amino acid Ser-200 of the active triad was replaced by alanine, was accessed after cloning both genes in the pJexpress 401 (DNA2.0, USA) vector for expression under the T5 promoter. The insertion of cloned genes was verified by PCR using primers for sites flanking LesA genes, followed by transformation of ElectroMAX DH5α competent cells (Life Technologies, USA). For heterologous expression, cells were grown in LB medium supplemented with kanamycin (50 μg/mL) at 37 °C and 120 rpm to an A600 of 0.8. IPTG (1 mM) was added to the culture, followed by incubation for three h at 30 °C and 120 rpm. Total protein was extracted from E. coli cells using a CellLytic B Plus dit (Sigma-Aldrich, USA). The protein concentration was determined according to Bradford’s method using BSA as the standard. Total proteins from LesA, S200A-LesA and empty vector-expressing E. coli were used for syringe infiltration in greenhouse-grown grapevine (Vitis vinifera L. c.v. ‘Thompson Seedless’) leaves. One hundred μg/spot of protein (1 μg/μL) was infiltrated into each leaf spot. HR-like lesions in the infiltrated area were photographed 24 h after inoculation.
Isolation of the lesA mutant strain
The mutagenesis cassette was chemically synthesized (GenScript, USA) after insertion of a kanamycin resistance gene within Ser-200, the first amino acid of the active triad. The entire open reading frame of PD1703 (494 bp at 5′ and 670 bp at 3′) was chosen as flanking homology regions in this cassette. The synthesized pUC57-PD1703::kan was electroporated into Xff WT Temecula1 as described previously65. Primers outside of PD1703 were used to confirm double crossover events in transformants using gel electrophoresis and sequencing.
Grapevine infection and disease quantification
Grapevines (‘Thompson Seedless’) were inoculated with 20 μL Xff suspension containing ∼2 × 107 cells. The plants were inoculated with 10 μL on the first day and reinoculated with 10 μL on the second day, with an independently grown Xff culture used for each inoculation. The bacteria were introduced into each plant at three to four inches above the soil using a number 0 insect pin. Twenty-five plants were inoculated with Xff wild type strain, 26 plants with lesA mutant and 26 plants with PBS (mock inoculation). Quantification of symptoms was performed using 18 randomly selected plants/treatment at 14 wpi. We show the average percent scorching of the vine with standard error, with nodes without leaves and petioles being excluded from this analysis. Leaf Point System: 0-24% scorching of leaf = 0 points, 25-49% = 1 pt, 50-74% = 2 pts, 75-100% = 3 pts. Values for individual leaves were then summed and divided by the total possible scorching (3 * number of leaves) to give one value per vine. One-Tailed T-test with Welch’s correction was carried out using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA). A value of p < 0.05 was considered statistically significant.
RNA extraction and real-time RT-PCR
Xff 3A2 (WT), rpfC and rpfF cells were grown in 50 mL PD3 liquid medium (three flasks/condition) until the cultures reached 107 to 108 cells/mL. RNA was extracted using the miRNeasy Mini Kit (Qiagen, USA) following manufacture’s instructions. cDNA was synthesized using SuperScript III First-Strand Synthesis SuperMix (Life Technologies, USA). RT-PCR reactions were performed using TaqMan Universal PCR Master Mix (Life Technologies, USA) on the StepOne Real-Time PCR System (PE Applied Biosystems, USA). The level of gene transcription was normalized to 16S rRNA and expressed as a relative difference. The statistical analysis was performed using GraphPad Prism software, version 5 (Graph-Pad Software, San Diego, USA), using the Unpaired t test with Welch’s correction. The data was considered significant when p < 0.05.
Agrobacterium virulence assay in walnut plants
BPROM, a bacterial sigma70 promoter recognition program, was used to predict the potential promoter for lesA. This software predicted one promoter region starting at 229 bps 5′ of the lesA start codon. The lesA open reading frame and the 500 bps 5′ of the intergenic space, which was predicted to include the promoter region for lesA, were cloned into the binary vector pDU97.1005. Expression of LesA was tested by detecting protein activity on a tributyrin agar plate. The presence of LesA activity ensured that the predicted 500 bps 5′ of this gene contain the active lesA promoter region, since no other promoter was present in the binary vector. Subsequently, positive colonies with LesA activity were transformed into a disarmed Agrobacterium strain (EHA101-PCH32) by electroporation. The lesA region and empty vector containing Agrobacterium strains were infected into walnut (Chandler) by creating an incision in the bark on the main stem. Ten μL resuspended bacterial culture (108 cells in PBS) was placed on the incision and wrapped with parafilm. The symptoms were observed at eight and 12 weeks post inoculation.
Additional Information
How to cite this article: Nascimento, R. et al. The Type II Secreted Lipase/Esterase LesA is a Key Virulence Factor Required for Xylella fastidiosa Pathogenesis in Grapevines. Sci. Rep. 6, 18598; doi: 10.1038/srep18598 (2016).
Change history
25 February 2016
A correction has been published and is appended to both the HTML and PDF versions of this paper. The error has not been fixed in the paper.
References
Davis, M. J., Purcell, A. H. & Thomson, S. V. Pierce’s disease of grapevines: isolation of the causal bacterium. Science 199, 75–77 (1978).
Chatterjee, S., Almeida, R. P. & Lindow, S. Living in two worlds: the plant and insect lifestyles of Xylella fastidiosa. Annual review of phytopathology 46, 243–271 (2008).
Purcell, A. H. & Hopkins, D. L. Fastidious xylem-limited bacterial plant pathogens. Annual review of phytopathology 34, 131–151 (1996).
Redak, R. A. et al. The biology of xylem fluid-feeding insect vectors of Xylella fastidiosa and their relation to disease epidemiology. Annual review of entomology 49, 243–270 (2004).
Hopkins, D. L. Xylella-Fastidiosa - Xylem-Limited Bacterial Pathogen of Plants. Annual review of phytopathology 27, 271–290 (1989).
Esau, K. Anatomic effects of the viruses of Pierce’s disease and phony peach. Hilgarida 18, 423–482 (1948).
Alves, E., Marucci, C. R., Lopes, J. R. S. & Leite, B. Leaf symptoms on plum, coffee and citrus and the relationship with the extent of xylem vessels colonized by Xylella fastidiosa. J Phytopathol 152, 291–297 (2004).
Baccari, C. & Lindow, S. E. Assessment of the Process of Movement of Xylella fastidiosa Within Susceptible and Resistant Grape Cultivars. Phytopathology 101, 77–84 (2011).
Chatelet, D. S., Wistrom, C. M., Purcell, A. H., Rost, T. L. & Matthews, M. A. Xylem structure of four grape varieties and 12 alternative hosts to the xylem-limited bacterium Xylella fastidious. Ann Bot-London 108, 73–85 (2011).
Fogaca, A. C. et al. Effects of the antimicrobial peptide gomesin on the global gene expression profile, virulence and biofilm formation of Xylella fastidiosa. Fems Microbiol Lett 306, 152–159 (2010).
Fritschi, F. B., Lin, H. & Walker, M. A. Scanning electron Microscopy reveals different response pattern of four Vitis genotypes to Xylella fastidiosa infection. Plant Dis 92, 276–286 (2008).
Goodwin, P. H., Devay, J. E. & Meredith, C. P. Roles of Water-Stress and Phytotoxins in the Development of Pierces Disease of the Grapevine. Physiol Mol Plant P 32, 1–15 (1988).
Daugherty, M. P., Lopes, J. R. S. & Almeida, R. P. P. Strain-specific alfalfa water stress induced by Xylella fastidiosa. Eur J Plant Pathol 127, 333–340 (2010).
Van Sluys, M. A. et al. Comparative analyses of the complete genome sequences of Pierce’s disease and citrus variegated chlorosis strains of Xylella fastidiosa. J Bacteriol 185, 1018–1026 (2003).
Jha, G., Rajeshwari, R. & Sonti, R. V. Bacterial type two secretion system secreted proteins: double-edged swords for plant pathogens. Mol Plant Microbe Interact 18, 891–898 (2005).
Simpson, A. J. et al. The genome sequence of the plant pathogen Xylella fastidiosa. The Xylella fastidiosa Consortium of the Organization for Nucleotide Sequencing and Analysis. Nature 406, 151–159 (2000).
Roper, M. C., Greve, L. C., Warren, J. G., Labavitch, J. M. & Kirkpatrick, B. C. Xylella fastidiosa requires polygalacturonase for colonization and pathogenicity in Vitis vinifera grapevines. Mol Plant Microbe Interact 20, 411–419 (2007).
Darvill, A. G. & Albersheim, P. Phytoalexins and Their Elicitors - a Defense against Microbial Infection in Plants. Annu Rev Plant Phys 35, 243–275 (1984).
Ryan, C. A. & Farmer, E. E. Oligosaccharide Signals in Plants - a Current Assessment. Annu Rev Plant Phys 42, 651–674 (1991).
Braun, E. J. & Rodrigues, C. A. Purification and Properties of an Endoxylanase from a Corn Stalk Rot Strain of Erwinia-Chrysanthemi. Phytopathology 83, 332–338 (1993).
Aparna, G., Chatterjee, A., Sonti, R. V. & Sankaranarayanan, R. A cell wall-degrading esterase of Xanthomonas oryzae requires a unique substrate recognition module for pathogenesis on rice. Plant Cell 21, 1860–1873 (2009).
Tamir-Ariel, D., Rosenberg, T., Navon, N. & Burdman, S. A secreted lipolytic enzyme from Xanthomonas campestris pv. vesicatoria is expressed in planta and contributes to its virulence. Mol Plant Pathol 13, 556–567 (2012).
Kulp, A. & Kuehn, M. J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol 64, 163–184 (2010).
Kuehn, M. J. & Kesty, N. C. Bacterial outer membrane vesicles and the host-pathogen interaction. Gene Dev 19, 2645–2655 (2005).
Matsumoto, A., Huston, S. L., Killiny, N. & Igo, M. M. XatA, an AT-1 autotransporter important for the virulence of Xylella fastidiosa Temecula1. MicrobiologyOpen 1, 33–45 (2012).
Sidhu, V. K., Vorholter, F. J., Niehaus, K. & Watt, S. A. Analysis of outer membrane vesicle associated proteins isolated from the plant pathogenic bacterium Xanthomonas campestris pv. campestris. BMC microbiology 8, 87 (2008).
Dandekar, A. M. et al. An engineered innate immune defense protects grapevines from Pierce disease. Proc Natl Acad Sci USA 109, 3721–3725 (2012).
Kunze, G. et al. The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 16, 3496–3507 (2004).
Zipfel, C. et al. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125, 749–760 (2006).
Newman, K. L., Almeida, R. P., Purcell, A. H. & Lindow, S. E. Cell-cell signaling controls Xylella fastidiosa interactions with both insects and plants. Proc Natl Acad Sci USA 101, 1737–1742 (2004).
Jha, G., Rajeshwari, R. & Sonti, R. V. Functional interplay between two Xanthomonas oryzae pv. oryzae secretion systems in modulating virulence on rice. Mol Plant Microbe In 20, 31–40 (2007).
Gambetta, G. A., Fei, J., Rost, T. L. & Matthews, M. A. Leaf scorch symptoms are not correlated with bacterial populations during Pierce’s disease. J Exp Bot 58, 4037–4046 (2007).
Chatterjee, S. & Sonti, R. V. rpfF mutants of Xanthomonas oryzae pv. oryzae are deficient for virulence and growth under low iron conditions. Mol Plant Microbe Interact 15, 463–471 (2002).
Barber, C. E. et al. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Molecular microbiology 24, 555–566 (1997).
Chatterjee, S., Wistrom, C. & Lindow, S. E. A cell-cell signaling sensor is required for virulence and insect transmission of Xylella fastidiosa. Proc Natl Acad Sci USA 105, 2670–2675 (2008).
Rajeshwari, R., Jha, G. & Sonti, R. V. Role of an in planta-expressed xylanase of Xanthomonas oryzae pv. oryzae in promoting virulence on rice. Molecular plant-microbe interactions : MPMI 18, 830–837 (2005).
Zhang, S. et al. The Xylella fastidiosa biocontrol strain EB92-1 genome is very similar and syntenic to Pierce’s disease strains. J Bacteriol 193, 5576–5577 (2011).
Zhang, S. et al. Three New Pierce’s Disease Pathogenicity Effectors Identified Using Xylella fastidiosa Biocontrol Strain EB92-1. PLoS One 10, e0133796 (2015).
Smolka, M. B. et al. Proteome analysis of the plant pathogen Xylella fastidiosa reveals major cellular and extracellular proteins and a peculiar codon bias distribution. Proteomics 3, 224–237 (2003).
Thamwiriyasati, N., Powthongchin, B., Kittiworakarn, J., Katzenmeier, G. & Angsuthanasombat, C. Esterase activity of Bordetella pertussis CyaC-acyltransferase against synthetic substrates: implications for catalytic mechanism in vivo. Fems Microbiol Lett 304, 183–190 (2010).
Deatherage, B. L. et al. Biogenesis of bacterial membrane vesicles. Molecular microbiology 72, 1395–1407 (2009).
Renelli, M., Matias, V., Lo, R. Y. & Beveridge, T. J. DNA-containing membrane vesicles of Pseudomonas aeruginosa PAO1 and their genetic transformation potential. Microbiology 150, 2161–2169 (2004).
Wai, S. N. et al. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 115, 25–35 (2003).
Kato, S., Kowashi, Y. & Demuth, D. R. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microbial pathogenesis 32, 1–13 (2002).
Kadurugamuwa, J. L. & Beveridge, T. J. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. Journal of bacteriology 178, 2767–2774 (1996).
Kadurugamuwa, J. L. & Beveridge, T. J. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. Journal of bacteriology 177, 3998–4008 (1995).
Kolling, G. L. & Matthews, K. R. Export of virulence genes and Shiga toxin by membrane vesicles of Escherichia coli O157:H7. Appl Environ Microb 65, 1843–1848 (1999).
Mashburn, L. M. & Whiteley, M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437, 422–425 (2005).
Bauman, S. J. & Kuehn, M. J. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect 8, 2400–2408 (2006).
Fritz, J. H., Ferrero, R. L., Philpott, D. J. & Girardin, S. E. Nod-like proteins in immunity, inflammation and disease. Nat Immunol 7, 1250–1257 (2006).
Tavano, R. et al. The membrane expression of Neisseria meningitidis adhesin A (NadA) increases the proimmune effects of MenB OMVs on human macrophages, compared with NadA(-) OMVs, without further stimulating their proinflammatory activity on circulating monocytes. J Leukocyte Biol 86, 143–153 (2009).
Slater, H., Alvarez-Morales, A., Barber, C. E., Daniels, M. J. & Dow, J. M. A two-component system involving an HD-GYP domain protein links cell-cell signalling to pathogenicity gene expression in Xanthomonas campestris. Molecular microbiology 38, 986–1003 (2000).
Ionescu, M. et al. Xylella fastidiosa outer membrane vesicles modulate plant colonization by blocking attachment to surfaces. Proc Natl Acad Sci USA 111, E3910–3918 (2014).
Shi, X. Y., Dumenyo, C. K., Hernandez-Martinez, R., Azad, H. & Cooksey, D. A. Characterization of regulatory pathways in Xylella fastidiosa: genes and phenotypes controlled by gacA. Applied and environmental microbiology 75, 2275–2283 (2009).
Heeb, S. & Haas, D. Regulatory roles of the GacS/GacA two-component system in plant-associated and other gram-negative bacteria. Mol Plant Microbe Interact 14, 1351–1363 (2001).
De la Torre-Zavala, S. et al. Gene expression of Pht cluster genes and a putative non-ribosomal peptide synthetase required for phaseolotoxin production is regulated by GacS/GacA in Pseudomonas syringae pv. phaseolicola. Research in microbiology 162, 488–498 (2011).
Brencic, A. et al. The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. Molecular microbiology 73, 434–445 (2009).
Yan, Q., Wu, X. G., Wei, H. L., Wang, H. M. & Zhang, L. Q. Differential control of the PcoI/PcoR quorum-sensing system in Pseudomonas fluorescens 2P24 by sigma factor RpoS and the GacS/GacA two-component regulatory system. Microbiological research 164, 18–26 (2009).
Reimmann, C., Valverde, C., Kay, E. & Haas, D. Posttranscriptional repression of GacS/GacA-controlled genes by the RNA-binding protein RsmE acting together with RsmA in the biocontrol strain Pseudomonas fluorescens CHA0. J Bacteriol 187, 276–285 (2005).
Almeida, R. P., Mann, R. & Purcell, A. H. Xylella fastidiosa cultivation on a minimal solid defined medium. Current microbiology 48, 368–372 (2004).
Schuster, A. M. & Davies, E. Ribonucleic Acid and Protein Metabolism in Pea Epicotyls : II. Response to Wounding in Aged Tissue. Plant physiology 73, 817–821 (1983).
Erickson, P. A., Anderson, D. H. & Fisher, S. K. Use of Uranyl Acetate En-Bloc to Improve Tissue Preservation and Labeling for Postembedding Immunoelectron Microscopy. J Electron Micr Tech 5, 303–314 (1987).
Smeltzer, M. S., Hart, M. E. & Iandolo, J. J. Quantitative spectrophotometric assay for staphylococcal lipase. Appl Environ Microb 58, 2815–2819 (1992).
Vaneechoutte, M., Verschraegen, G. & Claeys, G. & Flamen, P. Rapid identification of Branhamella catarrhalis with 4-methylumbelliferyl butyrate. Journal of clinical microbiology 26, 1227–1228 (1988).
Matsumoto, A., Young, G. M. & Igo, M. M. Chromosome-based genetic complementation system for Xylella fastidiosa. Appl Environ Microb 75, 1679–1687 (2009).
Acknowledgements
The authors acknowledge research support obtained from the Citrus Research Board and the CDFA PD Board of California. RN is grateful to Brazilian agency CAPES for scholarship’s support. We thank David Dolan for his contributions to experimental procedures in the greenhouse. We express our gratitude to Grete Adamson and Patricia Kysar (Electron Microscopy Laboratory, School of Medicine, University of California at Davis) for technical support.
Author information
Authors and Affiliations
Contributions
R.N., H.G., H.W.G., G.B., L.R.G. and A.M.D. conceived and designed the experiments; R.N., H.G., H.W.G., H.O.A., A.T. and P.A.F. performed the experiments; S.C. performed the in silico analysis; B.J.R. supervised the work of S.C.; R.N., H.G., H.W.G., G.B., L.R.G. and A.M.D. wrote the paper with contributions from all authors.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Nascimento, R., Gouran, H., Chakraborty, S. et al. The Type II Secreted Lipase/Esterase LesA is a Key Virulence Factor Required for Xylella fastidiosa Pathogenesis in Grapevines. Sci Rep 6, 18598 (2016). https://doi.org/10.1038/srep18598
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep18598
This article is cited by
-
Molecular and functional characterization of two RGA type genes in the PdR1b locus for Pierce’s disease resistance in Vitis arizonica/candicans
Plant Cell, Tissue and Organ Culture (PCTOC) (2022)
-
Controlled spatial organization of bacterial growth reveals key role of cell filamentation preceding Xylella fastidiosa biofilm formation
npj Biofilms and Microbiomes (2021)
-
Identification and analysis of seven effector protein families with different adaptive and evolutionary histories in plant-associated members of the Xanthomonadaceae
Scientific Reports (2017)
-
The Secreted Protease PrtA Controls Cell Growth, Biofilm Formation and Pathogenicity in Xylella fastidiosa
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.