Abstract
Neonatal invasive disease due to Streptococcus agalactiae is life threatening and preventive strategies suitable for resource limited settings are urgently needed. Protective coverage of vaccine candidates based on capsular epitopes will relate to local epidemiology of S. agalactiae serotypes and successful management of critical infections depends on timely therapy with effective antibiotics. This is the first report on serotype distribution and antimicrobial susceptibility of S. agalactiae in pregnant women from a Central African region. Serotypes V, III and Ib accounted for 88/109 (81%) serotypes and all isolates were susceptible to penicillin and clindamycin while 13% showed intermediate susceptibility to erythromycin.
Similar content being viewed by others
Introduction
Neonatal invasive disease due to Streptococcus agalactiae is a life threatening infection. In industrialized countries prenatal screening in pregnant women and intra-partum antibiotic prophylaxis (IAP) have been widely established and successfully reduced the incidence of newborn morbidity and mortality related to S. agalactiae1,2. In low income settings recto-vaginal colonization rates with S. agalactiae in pregnant women are similar3; however, screening and IAP for prevention of invasive neonatal disease is mostly not implemented due to limitations in resources and infrastructure. Vaccination of pregnant women is an alternative prevention strategy that proved effective for other neonatal infectious diseases (i.e. neonatal tetanus) and several S. agalactiae vaccine candidates are currently in pre-clinical and clinical development4. Ten capsular polysaccharides have been identified as important virulence factors of S. agalactiae and differentiate S. agalactiae into ten distinct serotypes. Based on serotype pathogenicity and prevalence, a trivalent polysaccharide-protein conjugate vaccine composed of capsular epitopes from serotypes Ia, Ib and III is in Phase II evaluation (NCT02046148); protective coverage will depend on selected S. agalactiae serotypes and S. agalactiae serotype distribution in target populations. In case of critical infections due to S. agalactiae effective management will depend on timely therapy with antibiotics to which S. agalactiae is sensitive. There is a lack of knowledge on the epidemiology of S. agalactiae in pregnant women from Central sub-Saharan Africa. The aim of this study was to describe serotype distribution and antimicrobial susceptibility of S. agalactiae in pregnant women from Gabon.
Methods
Serotypes and antibiotic susceptibility of S. agalactiae isolates from HIV-negative pregnant women participating in a multicentre randomized controlled clinical trial comparing the safety and efficacy of mefloquine and sulfadoxine-pyrimethamine for intermittent preventive treatment for malaria in pregnancy (IPTp) in Gabon (MIPPAD trial; NCT 00811421, registration date December 18, 2008) were studied. Detailed information on study design, methods, clinical data and outcomes has been published recently5,6; the study protocol was approved by the CERMEL institutional ethics review board and all experiments were carried out in accordance with international regulations including the Declaration of Helsinki . In summary, recto-vaginal swabs were taken at presentation for delivery; nylon flocked swabs were used and submerged into Amies transport medium; Lim Broth was inoculated, incubated at 37 °C and then sub-cultured on Granada agar under aerobic conditions at 37 °C for 24 h; yellow-orange pigmented colonies on Granada agar confirmed growth of S. agalactiae. After storage at −20 °Celsius isolates were re-cultured on 5% sheep blood agar and serotypes of the capsular polysaccharides Ia, Ib, II-IX were determined by latex agglutination (Strep-B-latex kit; Article 54991/Lot La-1-4, Statens Serum Institute, Denmark) according to the manufacturer’s instructions. Isolates were additionally confirmed by S. agalactiae multiplex PCR as described by Creti et al.7. Antibiotic drug susceptibility testing was performed on horse blood agar plates (MHF, BioMérieux®). Disc diffusion test was performed with erythromycin (15 μg), clindamycin (2 μg), cefotaxim (30 μg), vancomycin (5 μg) and linezolid (10 μg). gMIC determination was performed as E-Test (BioMérieux®) for benzylpenicillin, cefalotin, cefotaxim and gentamicin; isolates were screened for the presence of high-level gentamicin resistance (HLGR, MIC > 500 mg/L). Isolates with intermediate susceptibility to erythromycin were investigated for inducible clindamycin resistance by double disc diffusion test (DDT). Disc diffusion tests and gMIC determination were performed and interpreted according to the EUCAST (www.eucast.org) guidelines.
Results and Discussion
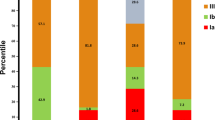
The prevalence of maternal S. agalactiae colonization at delivery has been reported by Capan-Melser et al. and was 19% (106/549; 95% CI 16–23%); S. agalactiae colonization was unrelated to IPTp and illiteracy was the only factor associated with higher colonization rates5. One hundred and nine S. agalactiae isolates were available for serotyping and antibiotic resistance testing. Serotyping by latex agglutination test and PCR was congruent and there was no non-typeable isolate. Almost a third (33/109, 30.3%) of isolates were serotype V; second most prevalent was serotype III (30/109, 27.5%), followed by serotype Ib (25/109, 22.9%), serotype Ia (14/109, 12.8%) and serotype II (7/109, 6.4%) (Table 1). No serotypes IV, VI, VII, XIII or XI were identified.
Serotype distribution varies between countries. Serotype V as most common serotype has been reported from The Gambia8 and Egypt9 as well, while in South Africa and Morocco serotypes Ia and III dominated with around 30–40% and serotype V represented only around 10% of isolates10,11,12. Two studies from Zimbabwe found serotypes III, V and Ia to be the most prevalent serotypes in decreasing order13,14. Importantly, one study from South Africa did not find significant differences in serotype distribution between vaginal and newborn colonizing isolates, but serotype distribution of invasive S. agalactiae isolates were significantly different to that of colonizing isolates11. Therefore, in the light of development of serotype-based vaccines, studies on invasive isolates are crucial. In a systematic review and meta-analysis on S. agalactiae disease in infants aged <3 months no low-income country was represented in the serotype assessment due to lack of data; the most frequently identified serotype in all regions with available data was serotype III (48.9%) followed by serotypes Ia (22.9%), Ib (7.0%), II (6.2%) and V (9.1%) and five serotypes (Ia, Ib, II, III, V) accounted for >85% of all serotypes15. Limited data on molecular characteristics of African GBS isolates suggests that the overall population structure is similar to industrialized countries16,17,18.
In this study, by disc diffusion test evaluation, all isolates were susceptible to clindamycin, cefotaxim, linezolid and vancomycin. Fourteen (13%) isolates showed intermediate susceptibility to erythromycin (Table 2); for these isolates no inducible clindamycin resistance was found by DDT. By E-test, no resistance to benzylpenicilline, cefotaxim and cefalotin and no high level gentamicin resistance were observed (Table 2). Whereas various studies in Asia, Europe and the United States have found a significant association between serotype V and macrolide resistance, it is remarkable that this association was not found in our cohort (Fisher’s Exact Test).
S. agalactiae has been regarded universally susceptible to penicillins and these are the antibiotics of first choice for the treatment of S. agalactiae infections. Data of this study confirm susceptibility of S. agalactiae to penicillins also for this setting; however, evidence of S. agalactiae with reduced penicillin susceptibility and additional resistance to other antibiotic drug classes is increasing19,20,21 and also reported from the African continent22,23 underlining the importance of continuous surveillance to guide empiric treatment. Erythromycin is commonly used in case of allergy to penicillins but varying rates of erythromycin resistance are reported by most studies evaluating its susceptibility. In this study intermediate susceptibility for erythromycin was seen in 13%, a rate that has also been reported for other sub-Saharan African settings22,23. As all isolates were susceptible to clindamycin in this study; clindamycin may therefore be a more suitable second-line antibiotic for S. agalactiae infections than erythromycin in this setting. The absence of high level gentamicin resistance supports the recommendation that neonates with suspicion of sepsis or meningitis should be treated with the combination of ampicillin and gentamicin.
In conclusion, S. agalactiae isolates colonizing pregnant women in Gabon showed a serotype distribution in line with other reports from Africa and a susceptibility profile suggesting penicillins as first-line and clindamycin as alternative antibiotic for infections due to S. agalactiae.
Additional Information
How to cite this article: Belard, S. et al. Streptococcus agalactiae Serotype Distribution and Antimicrobial Susceptibility in Pregnant Women in Gabon, Central Africa. Sci. Rep. 5, 17281; doi: 10.1038/srep17281 (2015).
References
CDC. Prevention of Perinatal Group B Streptococcal Disease, Revised Guidelines (2010). Available at: http://www.cdc.gov/mmwr/pdf/rr/rr5910.pdf. (Accessed: 04.02.2015)
Lopez Sastre, J. B., Fernandez Colomer, B., Coto Cotallo, G. D. & Ramos Aparicio, A. Trends in the epidemiology of neonatal sepsis of vertical transmission in the era of group B streptococcal prevention. Acta Paediatr 94 (4), 451 (2005).
Capan, M. et al. Epidemiology and management of group B streptococcal colonization during pregnancy in Africa. Wien Klin Wochenschr 124 Suppl 3, 14 (2012).
Chen, V. L., Avci, F. Y. & Kasper, D. L. A maternal vaccine against group B streptococcus: past, present and future. Vaccine 31 Suppl 4, D13 (2013).
Capan-Melser, M. et al. Evaluation of intermittent preventive treatment of malaria against group B streptococcus colonization in pregnant women: a nested analysis of a randomized controlled clinical trial of sulfadoxine/pyrimethamine versus mefloquine. J Antimicrob Chemother pii: dkv041. [Epub ahead of print] (2015).
Gonzalez, R. et al. Intermittent preventive treatment of malaria in pregnancy with mefloquine in HIV-negative women: a multicentre randomized controlled trial. PLoS Med 11 (9), e1001733 (2014).
Creti, R., Fabretti, F., Orefici, G. & von Hunolstein, C. Multiplex PCR assay for direct identification of group B streptococcal alpha-protein-like protein genes. J Clin Microbiol 42 (3), 1326 (2004).
Suara, R. O. et al. Carriage of group B streptococci in pregnant Gambian mothers and their infants. J Infect Dis 170 (5), 1316 (1994).
Shabayek, S., Abdalla, S. & Abouzeid, A. M. Serotype and surface protein gene distribution of colonizing group B streptococcus in women in Egypt. Epidemiol Infect 142 (1), 208 (2014).
Kwatra, G. et al. Serotype-specific acquisition and loss of group B streptococcus recto-vaginal colonization in late pregnancy. PLoS One 9 (6), e98778 (2014).
Madzivhandila, M. et al. Serotype distribution and invasive potential of group B streptococcus isolates causing disease in infants and colonizing maternal-newborn dyads. PLoS One 6 (3), e17861 (2011).
Aitmhand, R., Moustaoui, N., Belabbes, H., Elmdaghri, N. & Benbachir, M. Serotypes and antimicrobial susceptibility of group B streptococcus isolated from neonates in Casablanca. Scand J Infect Dis 32 (3), 339 (2000).
Moyo, S. R., Mudzori, J., Tswana, S. A. & Maeland, J. A. Prevalence, capsular type distribution, anthropometric and obstetric factors of group B streptococcus (Streptococcus agalactiae) colonization in pregnancy. Cent Afr J Med 46 (5), 115 (2000).
Mavenyengwa, R. T., Maeland, J. A. & Moyo, S. R. Serotype markers in a Streptococcus agalactiae strain collection from Zimbabwe. Indian J Med Microbiol 28 (4), 313 (2010).
Edmond, K. M. et al. Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet 379 (9815), 547 (2012).
Brochet, M., Couve, E., Bercion, R., Sire, J. M. & Glaser, P. Population structure of human isolates of Streptococcus agalactiae from Dakar and Bangui. J Clin Microbiol 47 (3), 800 (2009).
Florindo, C. et al. Molecular epidemiology of group B streptococcal meningitis in children beyond the neonatal period from Angola. J Med Microbiol 60 (Pt 9), 1276 (2011).
Huber, C. A., McOdimba, F., Pflueger, V., Daubenberger, C. A. & Revathi, G. Characterization of invasive and colonizing isolates of Streptococcus agalactiae in East African adults. J Clin Microbiol 49 (10), 3652 (2011).
Andrews, J. I. et al. Group B streptococci causing neonatal bloodstream infection: antimicrobial susceptibility and serotyping results from SENTRY centers in the Western Hemisphere. Am J Obstet Gynecol 183 (4), 859 (2000).
Kimura, K. et al. High frequency of fluoroquinolone- and macrolide-resistant streptococci among clinically isolated group B streptococci with reduced penicillin susceptibility. J Antimicrob Chemother 68 (3), 539 (2013).
Crespo-Ortiz Mdel, P., Castaneda-Ramirez, C. R., Recalde-Bolanos, M. & Velez-Londono, J. D. Emerging trends in invasive and noninvasive isolates of Streptococcus agalactiae in a Latin American hospital: a 17-year study. BMC Infect Dis 14, 428 (2014).
Moyo, S. R., Maeland, J. A. & Munemo, E. S. Susceptibility of Zimbabwean Streptococcus agalactiae (group B streptococcus; GBS) isolates to four different antibiotics. Cent Afr J Med 47 (9–10), 226 (2001).
Joachim, A., Matee, M. I., Massawe, F. A. & Lyamuya, E. F. Maternal and neonatal colonisation of group B streptococcus at Muhimbili National Hospital in Dar es Salaam, Tanzania: prevalence, risk factors and antimicrobial resistance. BMC Public Health 9, 437 (2009).
Acknowledgements
This work was part of a clinical trial (MIPPAD) supported by EDCTP (EDCTPIP.07.31080.002), BMBF (FKZ 01KA0803) and the Malaria in Pregnancy Consortium, which is funded through a grant from the Bill and Melinda Gates Foundation to the Liverpool School of Tropical Medicine and the Federal Ministry of Education and Research (BMBF FKZ: da01KA0803), Germany. SB is a participant in the Charité Clinical Scientist Program founded by the Charité – Universitätsmedizin Berlin and the Berlin Institute of Health. We thank all pregnant women for their willingness to participate in this study. We are grateful to Uta Falke for her support in the laboratory.
Author information
Authors and Affiliations
Contributions
M.R., S.B., R.B., N.T., R.G., C.M. and P.K. conceived the study. M.C., G.M., R.Z., M.G., P.M., S.A. and A.A. performed the clinical study. M.C., S.B. and N.T. performed micobiological studies. S.B. and R.B. wrote the main manuscript text and prepared the Tables 1 and 2. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Belard, S., Toepfner, N., Capan-Melser, M. et al. Streptococcus agalactiae Serotype Distribution and Antimicrobial Susceptibility in Pregnant Women in Gabon, Central Africa. Sci Rep 5, 17281 (2015). https://doi.org/10.1038/srep17281
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep17281
This article is cited by
-
Epidemiology of co-infections in pregnant women living with human immunodeficiency virus 1 in rural Gabon: a cross-sectional study
Infectious Diseases of Poverty (2023)
-
Prevalence and molecular characterization of group B streptococcus in pregnant women from hospitals in Ohangwena and Oshikoto regions of Namibia
BMC Microbiology (2021)
-
Novel Chimeric Multiepitope Vaccine for Streptococcosis Disease in Nile Tilapia (Oreochromis niloticus Linn.)
Scientific Reports (2020)
-
Group B Streptococcus colonization rate and serotype distribution among pregnant women and their newborns at Adama Hospital Medical College, Ethiopia
Scientific Reports (2020)
-
Streptococcus agalactiae maternal colonization, antibiotic resistance and serotype profiles in Africa: a meta-analysis
Annals of Clinical Microbiology and Antimicrobials (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.