Abstract
Sleep spindles are thalamocortical oscillations in non-rapid eye movement (NREM) sleep, that play an important role in sleep-related neuroplasticity and offline information processing. Several studies with full-night sleep recordings have reported a positive association between sleep spindles and fluid intelligence scores, however more recently it has been shown that only few sleep spindle measures correlate with intelligence in females and none in males. Sleep spindle regulation underlies a circadian rhythm, however the association between spindles and intelligence has not been investigated in daytime nap sleep so far. In a sample of 86 healthy male human subjects, we investigated the correlation between fluid intelligence and sleep spindle parameters in an afternoon nap of 100 minutes. Mean sleep spindle length, amplitude and density were computed for each subject and for each derivation for both slow and fast spindles. A positive association was found between intelligence and slow spindle duration, but not any other sleep spindle parameter. As a positive correlation between intelligence and slow sleep spindle duration in full-night polysomnography has only been reported in females but not males, our results suggest that the association between intelligence and sleep spindles is more complex than previously assumed.
Similar content being viewed by others
Introduction
Sleep spindles are thalamocortical oscillations1,2 emerging during NREM sleep with the physiological potential to facilitate neuroplasticity3,4,5. Sleep spindle characteristics such as spindle density, frequency or amplitude are trait-like individual characteristics with genetic and anatomical underpinnings6,7,8. The hypothesis that trait-like electrophysiological characteristics with a function so closely related to cognition may be related to individual cognitive performance were corroborated in early studies which revealed a positive correlation between several sleep spindle parameters and intelligence9,10,11,12,13,14. However, a recent review suggested that reports on a positive association between spindles and intelligence are often inconsistent and mostly rely on small sample sizes and that positive findings might be strongly overrepresented in the literature due to publication bias15. In addition, cognitive performance has been shown to correlate with anatomical properties of the brain in a strongly sex-dependent manner16,17, characterized by a positive correlation with white matter morphometric data in females, but less so in males. Sleep spindle amplitude also heavily relies on the morphology of thalamocortical white matter tracts18. In line with these observations recent findings have confirmed that sleep spindle parameters correlate with intelligence mainly in females, and less so in males15,19.
In the present study, we aimed to corroborate and extend these findings by investigating the correlates of intelligence in a large sample of male subjects recorded during afternoon naps. Nap sleep spindles are typically considered as representative of night spindle activity as they are similarly associated with neuroplasticity processes. However, sleep spindles are also modulated by circadian regulation20. We hypothesized that – in line with recent findings – no significant correlation between intelligence and sleep spindle parameters would be observed in afternoon naps. Results inconsistent with this hypothesis would suggest that the spindle-intelligence association might be different for night sleep and afternoon naps.
Materials and Methods
Participants and Instructions
86 male subjects were recruited at local universities through flyers and email lists and were paid for their participation. Exclusion criteria as assessed in a screening interview included a history of sleep disorders (assessed also via Pittsburgh Sleep Quality Index21, drug abuse, or psychiatric or neurological diseases; further shift work, transmeridian flights within the past month, regular daytime naps, extreme chronotype (assessed via Morningness-Evenings-Questionnaire22, or regular consumption of more than 2 cups of coffee or 5 cigarettes per day. 7 subjects were excluded from the study due to their failure to produce at least one epoch of N2 sleep. The mean age for the remaining 79 subjects was 23.29 years (SD 2.63 years, range 18–30 years). Experimental procedures were approved by the ethics committee of the University of Munich, participants gave written informed consent and all procedures were carried out in accordance with the approved guidelines.
Participants were instructed to follow a regular sleep pattern in the week preceding the sleep recording, which was controlled by sleep diaries. On the morning of the sleep recording, participants were instructed to get up not after 7 a.m. to increase probability of falling asleep during sleep recording. Participants arrived at 13:00 hours at the sleep laboratory. After electrode placement, participants had to complete the German version of Culture Fair Test (CFT 20-R)23, a non-verbal test of fluid intelligence with a high g-loading24. The mean IQ was 116.4 (SD 15.1, range 80–143).
They further completed questionnaires on handedness, vocabulary, and self-assessed creativity (not analyzed here), however did not engage in any memory-related activity to avoid respective confounding effects on sleep spindle activity.
Data Acquisition
After EEG application and behavioral testing, during a 100 minutes lights off period participants underwent polysomnography using a digital recorder (Comlab 32 Digital Sleep Lab, Brainlab V 3.3 Software, Schwarzer GmbH, Munich, Germany) with a sampling rate of 250 Hz with 6 EEG electrodes (F3, F4, C3, C4, O1, O2, all referenced to the contralateral mastoids), EMG, ECG and EOG. Polysomnography recordings were scored by 30 second epochs according to standard criteria25.
Data Analysis
Polysomnography recordings were analyzed using a methodology similar to our recent studies15,19. N2 and N3 sleep epochs were subjected to automated sleep spindle analysis using the Individual Adjustment Method (IAM)26,27: High-resolution (bin width: 0.0625 Hz) average amplitude spectrum (with zero-padding) of the signal of EEG electrodes and the second-order derivatives of a down-sampled (0.25 Hz) average amplitude spectrum were computed. Two spectral peaks corresponding to slow and fast spindles were identified based on the zero-crossings of the average of the second order derivatives of the spectra of all available EEG derivations. The edges of these spectral peaks were defined on the frequency scale of the high resolution spectra as the individual slow and fast spindle frequency ranges, respectively. EEG data was filtered for these individual sleep spindle frequency ranges and a sleep spindle was detected wherever the envelope of the filtered signal exceeded a derivation-specific amplitude criterion for at least 0.5 seconds. The amplitude criterion was defined as the mean of the high resolution amplitude spectral values of NREM sleep EEG of the given electrode at the boundaries of the individual sleep spindle frequency range, multiplied by the number of bins within the individual frequency range. The individual mean length and mean maximum amplitude (defined by the mean maximum of the envelopes of filtered EEG signals over the detected spindles) as well as sleep spindle density (number of sleep spindles/ minute) was computed for each subject and each derivation for both slow and fast spindles.
We computed Pearson’s point-moment correlations between IQ scores and individual slow and fast spindle parameters (frequency, density, duration and amplitude). In order to control for multiple comparisons we implemented the Benjamini-Hochberg procedure of false discovery rate (FDR) correction28.
Results
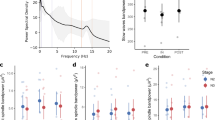
We found a positive correlation between intelligence and slow spindle duration (statistically significant on all electrodes, significant after FDR correction). A tendency for a positive correlation between intelligence and slow spindle density on some electrodes was also seen, which however was not significant after FDR correction. No correlation between intelligence and either sleep spindle amplitude or frequency was found and no correlation between intelligence and any of the fast spindle parameters was seen either. However, correlations with intelligence did not differ significantly between slow and fast spindles. Intelligence did not correlate significantly with any measures of sleep macrostructure. Our results are summarized in Table 1; the strongest results are illustrated as a scatterplot on Fig. 1. Consistent with the narrow age range of our subjects, our results did not change significantly if partial correlations (controlling for age) were calculated.
Scatterplots illustrating the correlation between slow (left panel) and fast (right panel) spindle duration (axis y) and CFT IQ score (axis x) on the electrode F4 where the effect was found to be the strongest.
While the correlation is only significant in case of slow spindle duration, the two correlation coefficients are not statistically different from each other (Fisher’s z = 3.12, p > 0.1).
In order to assess the similarity between nap and night spindle parameters, individual sleep spindle parameter averages obtained on the electrode F4 were compared with the average parameters obtained from the comparable sample of 57 male subjects under 30 years old participating in our earlier night sleep study15, analyzed with the same detection algorithm. Both slow and fast spindle density was higher in night sleep (p < 0.001 in both cases). Slow spindle duration and mean amplitude were not significantly different in naps and night sleep. Fast spindle duration was longer in naps (p < 0.05) while mean amplitude was higher in night sleep (p < 0.001). Both slow and fast spindle middle frequency was lower in night sleep (p < 0.001 in both cases). This is in line with previous comparisons of day and night spindles29, which also found higher frequency and duration (on a frontal electrode), but lower density and amplitude in naps (albeit the density effect was only significant on a parietal electrode and the amplitude effect remained a tendency on both). Sleep macrostructure and spindle parameters are reported in Tables 2, 3, 4, 5, 6; topographic differences in nap sleep spindle parameters are represented on Fig. 2.
Discussion
In a large sample of only male subjects, we did not find any correlation between intelligence and fast spindle parameters and no significant correlation between intelligence and slow spindle density or amplitude. This confirms recent full-night sleep recordings with similar null-findings15. In particular the absence of a positive correlation between fast spindle amplitude and intelligence in males is in line with previous night sleep studies revealing such a correlation only in females both in case of adolescents19 and adults15. In contrast to null-findings in full-night sleep recordings, however, we found a positive correlation between intelligence and slow spindle duration in our male sample, which had previously been reported for females only15.
Delta and theta power in naps appear to generally contribute to the same homeostatic processes as night sleep30, but sleep spindles are particularly dependent on circadian regulation20. The main differences between day and night spindles have been observed for spindle frequency and density31, in line with the melatonin-dependent circadian regulation of spindles20,29. However, less prominent day-night differences in spindle duration and amplitude have been reported, not always reaching statistical significance29,31. Several reports suggest that day sleep spindles recorded during nap sleep periods are indeed involved in neural plasticity processes as night spindles are, supporting the consolidation of memories in both children32 and adults33. Sleep spindles recorded in afternoon naps could thus be considered as representative of night spindle activity and therefore good candidate markers of IQ. In the light of our current results, nap spindles might even be a more sensitive marker of cognitive processing than night sleep spindles as evidenced by a positive association between intelligence and slow spindle duration, previously only seen in females15. Sleep spindles preferentially occur during the up-states of cortical slow oscillations1 and durations most likely reflect the length of such up-states. Previous reports34 found a positive correlation between slow wave upstate length and memory consolidation, as well as the coupling strength of sleep spindles to slow oscillations and intelligence14, suggesting that the coupling of these oscillations may be functionally important for cognitive functioning and intelligence.
It must be noted, however, that i) the correlations we found in young napping males were still weaker in effect size than what we previously found in a much more heterogeneous female subsample15 and ii) we were unable to reproduce this finding in a re-analysis of 57 night sleep EEG recordings from our previous study15 including only male subjects below 30 years of age: in this subsample, no correlation between IQ scores and slow spindle duration was seen (see Table 2). Therefore, while some significant positive correlations were found in our male-only napping sample, the weak effect size of our positive findings about the correlation between intelligence and nap slow spindle duration do not firmly ascertain whether such an association in males is specific for nap sleep. Also, since there was a lack of a significant difference between slow and fast spindle correlation coefficients (see Fig. 2), it cannot be claimed with certainty that this association is specific for slow spindles.
Overall, our results confirm that sleep spindle amplitude is not correlated with intelligence in males, supporting the view of a sexual dimorphism of the neural mechanisms behind intelligence16,17 with a triangular relationship between white matter morphology, sleep spindle amplitude and intelligence only in females15,17,18. It further confirms our recent null findings regarding other spindle parameters, which had been reported before in studies with smaller sample sizes15. Since many previous studies reported on a large number of sleep spindle variables with little consistency in their methodology and because null findings are less likely to be published15, the spindle-intelligence association might have been overestimated due to publication bias. Further research into the possible relationship between sleep spindling and intelligence is required, including possible interactions with circadian rhythms and the inclusion of female participants in order to reproduce the positive correlation between sleep spindle amplitude and intelligence also in nap sleep. A direct study of the association between nap and night spindle activity and intelligence in the same subjects would help clarify whether some or all spindle correlates of intelligence are specific for nap vs. full-night sleep.
Additional Information
How to cite this article: Ujma, P. P. et al. Nap sleep spindle correlates of intelligence. Sci. Rep. 5, 17159; doi: 10.1038/srep17159 (2015).
References
Steriade, M. The corticothalamic system in sleep. Front Biosci 1, d878–899 (2003).
Steriade, M. & Deschenes, M. The thalamus as a neuronal oscillator. Brain Res 320, 1–63 (1984).
Buzsaki, G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience 31, 551–570 (1989).
Rosanova, M. & Ulrich, D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci 25, 9398–9405 (2005).
Genzel, L., Kroes, M. C., Dresler, M. & Battaglia, F. P. Light sleep versus slow wave sleep in memory consolidation: a question of global versus local processes? Trends Neurosci 37, 10–19 (2014).
De Gennaro, L., Ferrara, M., Vecchio, F., Curcio, G. & Bertini, M. An electroencephalographic fingerprint of human sleep. NeuroImage 26, 114–122 (2005).
De Gennaro, L. et al. The electroencephalographic fingerprint of sleep is genetically determined: a twin study. Ann Neurol 64, 455–460 (2008).
Landolt, H. P. Genetic determination of sleep EEG profiles in healthy humans. Prog Brain Res 193, 51–61 (2011).
Fogel, S. M., Nader, R., Cote, K. A. & Smith, C. T. Sleep spindles and learning potential. Behavioral neuroscience 121, 1–10, doi: 10.1037/0735-7044.121.1.1 (2007).
Geiger, A. et al. The sleep EEG as a marker of intellectual ability in school age children. Sleep 34, 181–189 (2011).
Gruber, R. et al. The association between sleep spindles and IQ in healthy school-age children. International Journal of Psychophysiology 89, 229–240 (2013).
Lustenberger, C., Maric, A., Durr, R., Achermann, P. & Huber, R. Triangular relationship between sleep spindle activity, general cognitive ability and the efficiency of declarative learning. PLoS One 7, 21 (2012).
Schabus, M. et al. Sleep spindle-related activity in the human EEG and its relation to general cognitive and learning abilities. The European journal of neuroscience 23, 1738–1746, doi: 10.1111/j.1460-9568.2006.04694.x (2006).
Bodizs, R. et al. Prediction of general mental ability based on neural oscillation measures of sleep. Journal of sleep research 14, 285–292, doi: 10.1111/j.1365-2869.2005.00472.x (2005).
Ujma, P. P. et al. Sleep spindles and intelligence: evidence for a sexual dimorphism. J Neurosci 34, 16358–16368 (2014).
Gur, R. C. et al. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci 19, 4065–4072 (1999).
Haier, R. J., Jung, R. E., Yeo, R. A., Head, K. & Alkire, M. T. The neuroanatomy of general intelligence: sex matters. NeuroImage 25, 320–327 (2005).
Piantoni, G. et al. Individual differences in white matter diffusion affect sleep oscillations. J Neurosci 33, 227–233 (2013).
Bódizs, R., Gombos, F., Ujma, P. P. & Kovács, I. Sleep spindling and fluid intelligence across adolescent development: sex matters. Frontiers in Human Neuroscience 8, doi: 10.3389/fnhum.2014.00952 (2014).
Dijk, D. J. et al. Melatonin effect on daytime sleep in men: suppression of EEG low frequency activity and enhancement of spindle frequency activity. Neurosci Lett 201, 13–16 (1995).
Buysse, D. J., Reynolds, C. F., 3rd, Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28, 193–213 (1989).
Horne, J. A. & Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 4, 97–110 (1976).
Weiss, R. & Weiss, B. CFT-20R Grundintelligenzstest Skala 2 - Revision. (Hogrefe Verlag GmbH & Co. KG, 2006).
Duncan, J. et al. A Neural Basis for General Intelligence. Science 289, 457–460, doi: 10.1126/science.289.5478.457 (2000).
Iber, C., Ancoli-Israel, S., Chesson, A. & Quan, S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. 1st edn, (American Academy of Sleep Medicine, 2007).
Bódizs, R., Körmendi, J., Rigó, P. & Lázár, A. S. The individual adjustment method of sleep spindle analysis: Methodological improvements and roots in the fingerprint paradigm. Journal of Neuroscience Methods 178, 205–213 (2009).
Ujma, P. P. et al. A comparison of two sleep spindle detection methods based on all night averages: individually adjusted versus fixed frequencies. Frontiers in Human Neuroscience 9, doi: 10.3389/fnhum.2015.00052 (2015).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 57, 289–300 (1995).
Knoblauch, V., Martens, W., Wirz-Justice, A., Krauchi, K. & Cajochen, C. Regional differences in the circadian modulation of human sleep spindle characteristics. The European journal of neuroscience 18, 155–163 (2003).
Dijk, D. J., Beersma, D. G. & Daan, S. EEG power density during nap sleep: reflection of an hourglass measuring the duration of prior wakefulness. J Biol Rhythms 2, 207–219 (1987).
Knoblauch, V. et al. Age-related changes in the circadian modulation of sleep-spindle frequency during nap sleep. Sleep 28, 1093–1101 (2005).
Kurdziel, L., Duclos, K. & Spencer, R. M. C. Sleep spindles in midday naps enhance learning in preschool children. Proceedings of the National Academy of Sciences 110, 17267–17272, doi: 10.1073/pnas.1306418110 (2013).
Cox, R., Hofman, W. F. & Talamini, L. M. Involvement of spindles in memory consolidation is slow wave sleep-specific. Learning & Memory 19, 264–267 (2012).
Heib, D. P. J. et al. Slow Oscillation Amplitudes and Up-State Lengths Relate to Memory Improvement. PLoS One 8, e82049, doi: 10.1371/journal.pone.0082049 (2013).
Acknowledgements
We would like to thank Cynthia Marisch, Johanna Pömmerl and Fee Stremmel for their assistance in this study. Péter Ujma was supported by the Hungarian Brain Research Program (KTIA_ NAP_13-1-2013-0001). Martin Dresler was supported by the Volkswagen Foundation.
Author information
Authors and Affiliations
Contributions
P.P.U., R.B. and M.D. designed research; J.S., B.N.K. and M.D. performed research; P.P.U., R.B., F.G. and M.D. analyzed data; P.P.U., R.B., L.G., A.S. and M.D. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ujma, P., Bódizs, R., Gombos, F. et al. Nap sleep spindle correlates of intelligence. Sci Rep 5, 17159 (2015). https://doi.org/10.1038/srep17159
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep17159
This article is cited by
-
The relationship between cognitive ability and BOLD activation across sleep–wake states
Brain Imaging and Behavior (2022)
-
Massive online data annotation, crowdsourcing to generate high quality sleep spindle annotations from EEG data
Scientific Data (2020)
-
Individual spindle detection and analysis in high-density recordings across the night and in thalamic stroke
Scientific Reports (2018)
-
Effect of spike-timing-dependent plasticity on stochastic burst synchronization in a scale-free neuronal network
Cognitive Neurodynamics (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.